Published online Feb 21, 2018. doi: 10.3748/wjg.v24.i7.862
Peer-review started: December 11, 2017
First decision: December 20, 2017
Revised: December 25, 2017
Accepted: January 16, 2018
Article in press: January 16, 2018
Published online: February 21, 2018
Processing time: 60 Days and 4.7 Hours
To evaluate toxicity and treatment outcome of high-dose radiotherapy (RT) for cervical esophageal cancer (CEC).
We reviewed a total of 62 consecutive patients who received definitive RT for stage I to III cervical esophageal cancer between 2001 and 2015. Patients who received < 45 Gy, treated for lesions below sternal notch, treated with palliative aim, treated with subsequent surgical resection, or diagnosed with synchronous hypopharyngeal cancer were excluded. Treatment failures were divided into local (occurring within the RT field), outfield-esophageal, and regional [occurring in regional lymph node(s)] failures. Factors predictive of esophageal stenosis requiring endoscopic dilation were analyzed.
Grade 1, 2, and 3 esophagitis occurred in 19 (30.6%), 39 (62.9%), and 4 patients (6.5%), respectively, without grade ≥ 4 toxicities. Sixteen patients (25.8%) developed post-RT stenosis, of which 7 cases (43.8%) were malignant. Four patients (6.5%) developed tracheoesophageal fistula (TEF), of which 3 (75%) cases were malignant. Factors significantly correlated with post-RT stenosis were stage T3/4 (P = 0.001), complete circumference involvement (P < 0.0001), stenosis at diagnosis (P = 0.024), and endoscopic complete response (P = 0.017) in univariate analysis, while complete circumference involvement was significant in multivariate analysis (P = 0.003). A higher dose (≥ 60 Gy) was not associated with occurrence of post-RT stenosis or TEF. With a median follow-up of 24.3 (range, 3.4-152) mo, the 2 y local control, outfield esophageal control, progression-free survival, and overall survival (OS) rates were 78.9%, 90.2%, 49.6%, and 57.3%, respectively. Factors significantly correlated with OS were complete circumference involvement (P = 0.023), stenosis at diagnosis (P < 0.0001), and occurrence of post-RT stenosis or TEF (P < 0.001) in univariate analysis, while stenosis at diagnosis (P = 0.004) and occurrence of post-RT stenosis or TEF (P = 0.023) were significant in multivariate analysis.
Chemoradiation for CEC was well tolerated, and a higher dose was not associated with stenosis. Patients with complete circumferential involvement require close follow-up.
Core tip: This study reports the outcome and toxicity of high dose (median 63 Gy) radiotherapy for cervical esophageal cancer. Post-RT stenosis and tracheoesophageal fistula rates were 26% and 6.5%, respectively. Stenosis at diagnosis and post-RT stenosis/fistula was significantly associated with overall survival. Complete circumference involvement was significantly associated with post-RT stenosis but dose higher than 60 Gy was not.
- Citation: Kim JW, Kim TH, Kim JH, Lee IJ. Predictors of post-treatment stenosis in cervical esophageal cancer undergoing high-dose radiotherapy. World J Gastroenterol 2018; 24(7): 862-869
- URL: https://www.wjgnet.com/1007-9327/full/v24/i7/862.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i7.862
Carcinoma of the cervical esophagus is uncommon and accounts for 2%-10% of all esophageal carcinomas[1]. Squamous cell carcinoma (SCC) is predominant in the proximal esophagus, and the highest rates of SCC are found in East Asia and Southern Africa[2]. There are no prospective clinical trials to establish the standard care for cervical esophageal cancer (CEC). Because CEC is located between the cricopharyngeal muscle and the sternal notch, the surgical procedure to CEC is extensive, such as pharyngo-laryngo-esophagectomy[3], resulting in permanent tracheostomy and significant deterioration of quality of life[4]. Concurrent chemoradiotherapy (CRT) has emerged as the preferred treatment modality for CEC[5]. Common toxic effects of definitive CRT for CEC include dysphagia, dehydration, mucositis, esophagitis, dermatitis, and fatigue[6]. Late toxic effects, such as stricture and fistulas may also occur[7,8].
Because of its anatomical proximity to the hypopharynx, CRT protocols for CEC are somewhat analogous to those for hypopharyngeal cancer[6]. However, unlike locally advanced SCC of the hypopharynx which requires 70 Gy in 35 fractions for definitive CRT[9], the standard dose of CRT for esophageal cancer remains 50 Gy[5,10]. Although a higher-than-standard dose of 50 Gy is suggested for CEC[5], the increased dose to the esophagus may lead to a higher incidence of severe toxicities, including ulcer, perforation, and stenosis[11]. Organs at risk for RT planning depend on the site of treatment. Radiation pneumonitis and fibrosis are of major concern when planning for the thoracic esophagus but are of less importance for CEC. Esophageal toxicity information from hypopharyngeal cancer treatment is of limited value; the radiation field for hypopharyngeal cancer includes only a small segment of the cervical esophagus, while RT for CEC includes a large segment of the esophagus because of expansion of the craniocaudal margins from the gross tumor and the entire esophageal circumference. Reports on the high-dose radiation-induced toxicity of CEC are scarce, although information can be inferred from the retrospective data on head and neck cancer patients experiencing toxicities of the proximal esophagus[12]. A toxicity evaluation is required before the administration of dose-escalated protocols.
We report the outcome and toxicity of definitive radiotherapy (RT) for CEC, with an emphasis on the identification of clinical variables associated with the occurrence of post-RT esophageal stenosis and tracheoesophageal fistulas (TEF).
We retrospectively reviewed a total of 62 consecutive patients who received definitive RT for pathologically confirmed stage I-III (American Joint Committee on Cancer 7th edition) CEC between 2001 and 2015. CEC was defined as a tumor of the esophagus located between the inferior border of the cricoid cartilage and the thoracic inlet (suprasternal notch). Tumors with the epicenter located below the sternal notch were generally considered non-CEC for this study. Patients treated with a palliative aim, those who received < 45 Gy, those treated with subsequent surgical resection, or those diagnosed with synchronous hypopharyngeal cancer were excluded. All procedures were performed in accordance with the Helsinki Declaration of 1975, as revised in 1983. This study was approved by the institutional review board (4-2017-0027).
All patients were treated according to institutional protocols, consisting of platinum-based concurrent CRT or RT alone if patients could not tolerate chemotherapy. The gross tumor volume (GTV) was defined as a visible tumor in the esophagus and gross regional lymph node metastasis. The initial clinical target volume (CTV1) was defined by expansion of the GTV by 4 cm craniocaudally and 1-2 cm laterally, as well as bilateral supraclavicular lymph nodes inclusion for elective nodal irradiation. The initial planning target volume (PTV1) was defined as CTV1 plus a 0.5 cm margin in all directions, and 36-45 Gy in a conventional daily fractionation of 1.8 Gy was prescribed for PTV1. The boost CTV (CTV2) included the GTV plus a 3-4 cm craniocaudal margin and a 1-2 cm lateral margin, excluding the elective nodal field. PTV2 was obtained by adding a 0.5 cm margin to CTV2 in all directions and received a total dose of up to 50.4 Gy, while limiting the maximum spinal cord dose under 45 Gy. For patients receiving a total dose higher than 50.4 Gy, an additional boost dose was delivered to PTV3, which comprised the GTV with a narrow margin of 0.5-1 cm in all directions. Cisplatin (or carboplatin) and 5-fluorouracil (5-FU) based chemotherapy was used. Two cycles of chemotherapy were administered concurrently with RT, followed by 1-6 cycles of consolidation chemotherapy[13].
Upon completion of concurrent CRT, patients were evaluated every 3 mo for the first year and every 6 mo thereafter with a physical examination, toxicity assessment, upper gastrointestinal endoscopy, computed tomography (CT) scans of the neck, chest, and abdomen, and, when necessary, positron emission tomography-CT. Acute and late toxicity was assessed using the Radiation Therapy Oncology Group (RTOG) criteria. Esophageal stenosis was evaluated using esophagography or endoscopy, and significant stenosis was defined as symptomatic stenosis requiring endoscopic dilatation and/or stent insertion. Endoscopic complete remission (CR) of the primary tumor was defined when all visible tumors disappeared on endoscopy and a negative biopsy was conducted, with these outcomes lasting for more than 4 wk. Treatment failures were divided into local (occurring within the RT field), outfield-esophageal, and regional [occurring in regional lymph node(s)] failures. Factors predictive of esophageal stenosis requiring endoscopic dilation and TEF were analyzed.
Survival time was measured from the date of diagnosis to the date of the first event or the date of death. Survival curves were estimated using the Kaplan-Meier method, and multivariate analysis was performed using the Cox proportional hazard model. Correlation between clinical variables and post-RT occurrence of esophageal stenosis/TEF was performed using the χ2 test. A P value < 0.05 was indicative of statistical significance.
The median age was 66 (range, 29-86) years, and SCC was predominant (90.3%). The numbers of patients with T1, T2, T3, and T4 disease were 14 (22.6%), 6 (9.7%), 30 (48.4%), and 12 (19.3%), respectively. The median length of tumor involvement of the esophagus was 5.0 (range, 1-14) cm, and 22 patients had a tumor involving 100% of the esophageal circumference. The median biologically equivalent RT dose in 1.8-Gy fraction was 63 (range, 45-90) Gy. Two of the patients received a total dose of 81 Gy and 90 Gy each because a boost RT (18-27 Gy) was delivered to the residual tumor 1-2 mo after 63 Gy. Sixty patients (96.8%) were treated with concurrent chemotherapy (Table 1).
| Characteristics | n (%) | |
| Sex | Female:Male | 4:58 (6.5:93.5) |
| Age | Median 66 yr (range 29-86) | |
| Pathology | Squamous cell carcinoma | 56 (90.3) |
| Adenocarcinoma | 2 (3.2) | |
| Other | 4 (6.5) | |
| T stage | T1 | 14 (22.6) |
| T2 | 6 (9.7) | |
| T3 | 30 (48.4) | |
| T4 | 12 (19.3) | |
| Regional node metastasis | N0 | 14 (22.6) |
| N+ | 48 (77.4) | |
| Tumor length | Median 5.0 cm (range 1-14) | |
| Total length of skip lesions | Median 5.0 cm (range 1-20) | |
| Involved circumference | < 100% | 40 (64.5) |
| 100% | 22 (35.5) | |
| Stenosis at diagnosis | No | 45 (72.6) |
| Yes | 17 (27.4) | |
| Radiation dose (EQD1.8) | Median 63 Gy (range 45-90) | |
| Concurrent chemotherapy | Yes | 60 (96.8) |
| No | 2 (3.2) | |
| Endoscopic response | CR | 39 (62.9) |
| < CR | 23 (37.1) |
Grade 1, 2, and 3 esophagitis occurred in 19 (30.6%), 39 (62.9%), and 4 patients (6.5%), respectively, without grade 4 or 5 toxicities. Sixteen patients (25.8%) developed stenosis requiring dilation within a median of 5.5 mo (range 1.1-22.5) after RT, among which 7 cases (11.3%) were malignant strictures. Four patients (6.5%) developed TEF within a median of 2.6 mo (range 1.8-5.8) after RT, 3 (4.8%) of which were malignant fistulas (Table 2). Factors showing a significant correlation with post-RT stenosis requiring dilation were T3/4 disease (vs T1/2) (P = 0.001), 100% circumference involvement (vs < 100%) (P < 0.0001), stenosis at diagnosis (vs none) (P = 0.024), and endoscopic CR (vs < CR) (P = 0.017) in univariate analysis. A higher dose (≥ 60 Gy) was not associated with post-RT stenosis (P = 0.515). Only 100% circumference involvement was significantly associated with stenosis in multivariate analysis (P = 0.003) (Table 3). Factors showing significant correlation with either post-RT stenosis requiring dilatation or TEF were T3/4 (vs T1/2) (P < 0.0001), 100% circumference involvement (vs < 100%) (P < 0.0001), stenosis at diagnosis (vs none) (P = 0.023), and endoscopic CR (vs < CR) (P = 0.001) in univariate analysis. Higher dose (≥ 60 Gy) was not associated with post-RT stenosis or TEF (P = 0.259). Both 100% circumference involvement (P = 0.002) and endoscopic CR (P = 0.035) were significantly associated with the occurrence of post-RT stenosis or TEF in multivariate analysis (Supplementary Table 1). Table 4 summarizes the clinical variables and treatment outcomes among the 19 patients who developed post-RT stenosis requiring dilatation or TEF. Nine of these patients had endoscopic findings of total esophageal obstruction at the time of diagnosis and 10 patients had dysphagia symptoms only. Seven of the 8 patients (87.5%) who developed non-malignant post-RT stenosis and 5 of the 7 patients (71.4%) who developed malignant post-RT stenosis initially had 100% circumferential esophageal involvement by the tumor. Four patients with post-RT TEF showed a CR or partial response (PR) and developed fistulas within 6 mo after completion of RT. Of the 17 patients who initially had endoscopic finding of total obstruction at the time of diagnosis, post-RT stenosis requiring dilatation was reported in 8 patients (3 malignant stenosis) and malignant TEF in 2 patients (Supplementary Table 2).
| Toxicity | n (%) | RT-event interval (mo) | |
| Esophagitis | RTOG Gr 1 | 19 (30.6) | |
| RTOG Gr 2 | 39 (62.9) | ||
| RTOG Gr 3 | 4 (6.5) | ||
| RT stenosis1 | All | 16 (25.8) | Median 5.5 (range 1.1-22.5) |
| Malignant | 7 (11.3) | ||
| T-E fistula | All | 4 (6.5) | Median 2.6 (range 1.8-5.8) |
| Malignant | 3 (4.8) |
| Characteristics | No. of Patients | Stenosis | P value | ||
| No | Yes | Univariate | Multivariate | ||
| Age | |||||
| ≤ 65 | 30 | 24 (80) | 6 (20) | 0.236 | |
| > 65 | 32 | 22 (69) | 10 (31) | ||
| T stage | |||||
| T1/2 | 20 | 20 (100) | 0 | 0.001 | 0.998 |
| T3/4 | 42 | 26 (62) | 16 (38) | ||
| Involved circumference | |||||
| < 100% | 40 | 37 (93) | 3 (7) | < 0.0001 | 0.003 |
| 100% | 22 | 9 (41) | 13 (59) | ||
| Total length | |||||
| < 5.0 | 28 | 22 (79) | 6 (921) | 0.338 | |
| ≥ 5.0 | 34 | 24 (71) | 10 (29) | ||
| Stenosis at diagnosis | |||||
| No | 45 | 37 (82) | 8 (18) | 0.024 | 0.995 |
| Yes | 17 | 9 (53) | 8 (47) | ||
| Dysphagia at diagnosis | |||||
| ≤ 1 mo | 36 | 30 (83) | 6 (17) | 0.051 | |
| > 1 mo | 26 | 16 (62) | 10 (38) | ||
| RT dose | |||||
| ≥ 60 Gy | 37 | 27 (73) | 10 (27) | 0.515 | |
| < 60 Gy | 25 | 19 (76) | 6 (24) | ||
| Endoscopic response | |||||
| CR | 39 | 33 (85) | 6 (15) | 0.017 | 0.740 |
| < CR | 23 | 13 (56) | 10 (44) | ||
| Age/Sex | T stage | Involve circumf | Initial stenosis/ management | RT (Gy) | Response | Toxicity (onset, mo) | Outcome (mo) |
| 29/F | T3 | 100% | Dysphagia only | 59.4 | CR | Stenosis (20) | NED, alive (152) |
| 70/M | T3 | 100% | Total obst/none | 59.4 | PR | Stenosis (5) | DOOC (12) |
| 64/M | T4a | 100% | Dysphagia only | 63.0 | PR | Stenosis (1) | DM (5), DOD (7) |
| 68/F | T3 | 100% | Dysphagia only | 63.0 | CR | Stenosis (1) | NED, alive (21) |
| 75/M | T3 | 100% | Total obst/none | 63.0 | PR | Stenosis (2) | DOOC (22) |
| 60/F | T4b | 100% | Total obst/stent | 70.0 | CR | Stenosis (4) | NED, alive (23) |
| 68/M | T3 | 100% | Dysphagia only | 70.2 | SD | Stenosis (6) | InF (8), DOD (14) |
| 73/M | T4b | 75% | Total obst/stent | 90.01 | PR | Stenosis (11) | DM (13), DOD (17) |
| 64/M | T3 | 100% | Dysphagia only | 50.4 | PR | Stenosis2 (9) | InF (17), DOD (20) |
| 69/M | T4b | 100% | Total obst/stent | 50.4 | SD | Stenosis2 (2) | DOOC (5) |
| 64/M | T4a | 100% | Dysphagia only | 59.4 | PR | Stenosis2 (7) | DM (3)/InF (9), DOD (11) |
| 68/M | T3 | 100% | Total obst/none | 63.0 | PR | Stenosis2 (2) | DM (3), DOD (5) |
| 73/M | T3 | 100% | Total obst/PEG | 63.0 | PR | Stenosis2 (22) | InF (16), DOD (31) |
| 75/M | T3 | 40% | Dysphagia only | 57.6 | CR | Stenosis2 (14) | InF (7), DOD (31) |
| 74/M | T3 | 50% | Dysphagia only | 81.01 | CR | Stenosis2 (9) | InF (9), DOD (16) |
| 57/M | T3 | 100% | Dysphagia only | 63.0 | PR | TEF (3) | OutF (8), DOD (9) |
| 57/M | T3 | 100% | Total obst/stent | 60.0 | CR | Stenosis (1)/TEF2 (6) | InF (8), DOD (14) |
| 72/M | T3 | 100% | Total obst/stent | 63.0 | PR | TEF2 (2) | DOOC (5) |
| 51/M | T3 | 40% | Dysphagia only | 63.0 | PR | TEF2 (2) | RF (4), DOD (6) |
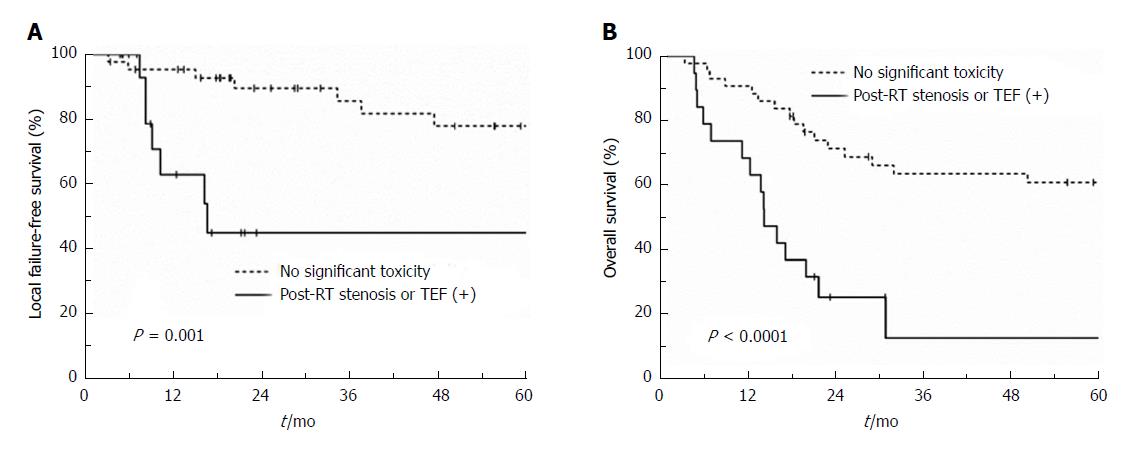
Twenty-seven patients were alive at the time of diagnosis. The median follow-up was 24.3 (range, 3.4-152) mo for all patients and 67.8 (range, 17.8-152) mo for surviving patients. An endoscopic CR was achieved in 39 patients (62.9%). A total of 34 patients experienced treatment failures: 7 local, 3 outfield esophageal, 6 regional, 11 distant, 2 concurrent local and regional, 1 concurrent outfield esophageal and regional, 3 concurrent local and distant, and 1 concurrent outfield esophageal, regional, and distant failure (Supplementary figure 1). The 2-year local failure-free (LFFS), outfield esophageal failure-free, regional failure-free, distant metastasis-free, progression-free, and overall survival (OS) rates were 78.9%, 90.2%, 79.5%, 72.7%, 49.6%, and 57.3%, respectively. T3/4 stage (P = 0.050), stenosis at diagnosis (P = 0.025), and RT stenosis or TEF (P = 0.001) showed a correlation with LFFS in univariate analysis. Only the occurrence of RT stenosis or TEF showed a trend towards poor LFFS in multivariate analysis (P = 0.066) (Supplementary Table 3 and Figure 1A). Factors showing significant correlations with OS were 100% circumference involvement (P = 0.023), stenosis at diagnosis (P < 0.0001), and occurrence of radiation induced stenosis or TEF (P < 0.001) in univariate analysis. Both stenosis at diagnosis (P = 0.004) and occurrence of RT stenosis or TEF (P = 0.023) were significantly associated with OS in multivariate analysis (Supplementary Table 4 and Figure 1B).
The most common radiation-induced late esophageal toxicity is dysphagia due to dysmotility and esophageal stricture[14], and these complications can result from muscular damage, submucosal fibrosis, and possibly nerve damage[15]. Unlike the lower esophagus, the proximal esophagus is composed predominantly of striated muscles, and conscious, voluntary swallowing is the key function in this part of the organ. Thus, stricture after RT rather than impaired peristalsis and involuntary swallowing may be the main cause of dysphagia in the cervical esophagus.
Toxicity evaluation of higher-than-standard-dose RT for CEC was necessary, and the primary objective of the current study was to determine clinical factors associated with the development of post-RT stenosis and TEF. Although most of the patients (96.8%) were treated with standard cisplatin and 5-FU-based CRT, the radiation dose used for the current study, at a median of 63 (range, 45-90) Gy, was significantly higher than the standard dose of 50 Gy. In the current study, preservation of the esophageal passage, either pre-RT (at diagnosis) stenosis (P = 0.004) or post-RT stenosis/TEF (P = 0.023), was an independent prognostic factor associated with OS, suggesting that resolution of the initial stenosis and prevention of post-treatment stenosis are indeed important in prolonging patients’ survival.
The esophagus is a hollow viscous organ with a tubular structure and it functions in series, such that destruction of the complete circumference of a small volume of esophagus could result in dysfunction of the entire organ. Unlike treating head and neck or lung cancers, full circumferential treatment of the esophagus cannot be avoided when treating tumors originating in the esophagus. Maguire et al[16] observed that patients who received > 80 Gy to any portion of the entire organ circumference had an increased risk of late toxicity in multivariate analysis. Although a higher dose to the esophagus increases the risk of severe complications, the application of more than 50 Gy may improve local control. In the current study, the highest dose we prescribed was 63 Gy except for the 2 patients who received a boost dose of 18 Gy and 27 Gy to the residual tumor. Although this is not a dose-escalation study, 63 Gy may be safely delivered to the cervical esophagus without causing severe toxicities.
It should be noted that, while 16 patients (25.8%) developed post-RT stenosis requiring dilation and 4 patients (6.5%) developed TEF, 7 (44%) and 3 (75%) patients were because of persistent or recurrent malignancy, respectively. Clinically diagnosed post-RT stricture may grossly overestimate the risk of radiation induced stenosis and may be an early sign of tumor recurrence[17]. In fact, only complete circumferential tumor involvement was an independent predictor of post-RT stenosis, while T stage, stenosis at diagnosis, and a higher dose (≥ 60 Gy) were not (Table 3). When both post-RT stenosis and TEF were considered, complete circumference tumor involvement and endoscopic CR were both independent predictors (Supplementary Table 1). Considering that 75% of TEF cases were malignant and occurred within the first 6 mo after completion of RT and after achieving a CR or PR, a rapid response to CRT, rather than a higher radiation dose, may be a contributing factor to the development of post-RT TEF.
A study by Atsumi et al[18] suggested that esophageal stenosis is associated with tumor regression after RT. In this study, 109 patients who achieved a CR after definitive CRT were evaluated with esophagography within 3 mo after completion of RT; and T stage, extent of involved circumference, and wall thickness of the tumor region were significantly correlated with esophageal stenosis in multivariate analysis[18]. Luminal narrowing of the esophagus after RT is largely due to fibrosis and inflammation of the submucosal and muscular layers[19,20]. These processes accompany infiltration of inflammatory cells[21] and probably include accumulation of macrophages with increased local levels of proinflammatory cytokines induced by radiation[22,23]; this, in turn, produces edema and fibrosis in the submucosal and underlying muscular layers. These processes may be much more pronounced in and around the shrinking tumors that respond well to RT[18], which may explain the significant correlation between complete circumferential involvement and post-RT stenosis in our study.
Our study showed that, although pre- and post-RT stenosis was a prognostic factor for patients’ survival, complete circumference involvement rather than a higher radiation dose was the key contributing factor. In clinical practice, physicians are often tempted to prescribe a higher-than-standard dose of 50 Gy for esophageal cancer, especially when it is expected that the patient is unable to undergo surgical resection because of tumor location, poor generalized condition, or patient’s refusal for surgery. Our data suggests that patients with cervical esophageal cancer may undergo radiotherapy of up to 63 Gy without increasing the risk of radiation-induced toxicities. Since prospective data is lacking, our study warrants a prospective trial to investigate toxicity and efficacy of high-dose radiotherapy for cervical esophageal cancer.
In conclusion, CRC for CEC was well tolerated, and a higher dose was not associated with post-RT stenosis. Patients with complete circumferential tumor involvement at diagnosis require close follow-up.
The surgical procedure for cervical esophageal cancer (CEC) is extensive, and concurrent chemoradiotherapy (CRT) is the preferred treatment modality. Although a higher-than-standard dose of 50 Gy is suggested for CEC, the increased dose may lead to a higher incidence of severe toxicities, such as ulcer, perforation and stenosis.
Clinical data on radiotherapy with increased dose for CEC are scarce, and a toxicity evaluation is required before the administration of dose-escalated protocols.
To evaluate toxicity and treatment outcome of high dose radiotherapy for CEC, and to determine the factors associated with post-treatment esophageal stenosis.
In this study, the authors reviewed 62 consecutive patients who received definitive RT for stage I to III cervical esophageal cancer between 2001 and 2015. Patients (received < 45 Gy) treated for lesions below sternal notch, treated with palliative aim and subsequent surgical resection, or diagnosed with synchronous hypopharyngeal cancer were excluded. Treatment failures were divided into local, outfield-esophageal, and regional failures. The factors predictive of esophageal stenosis requiring endoscopic dilation were analyzed.
With a median follow-up of 24.3 (range, 3.4-152) mo, the 2-year local control, outfield esophageal control, progression-free survival, and overall survival (OS) rates were 78.9%, 90.2%, 49.6%, and 57.3%, respectively. Grade 1, 2, and 3 esophagitis occurred in 19 (30.6%), 39 (62.9%), and 4 patients (6.5%), respectively, without grade ≥ 4 toxicities. Sixteen patients developed post-RT stenosis, of which 7 cases were malignant. Four patients developed tracheoesophageal fistula (TEF), of which 3 cases were malignant. Factors significantly correlated with OS were complete circumference involvement, stenosis at diagnosis, and occurrence of post-RT stenosis or TEF in univariate analysis, while stenosis at diagnosis and occurrence of post-RT stenosis or TEF were significant in multivariate analysis. Factors significantly correlated with post-RT stenosis were stage T3/4, complete circumference involvement, stenosis at diagnosis, and endoscopic complete response in univariate analysis, while complete circumference involvement was significant in multivariate analysis. A higher dose (≥ 60 Gy) was not associated with the occurrence of post-RT stenosis or TEF.
This study showed that, although pre- and post-RT stenosis was a prognostic factor for patients’ survival, complete circumference involvement rather than a higher radiation dose was the key contributing factor, and suggesting that CEC can be treated with higher than the current standard dose of 50 Gy. CRT for CEC was well tolerated, and patients with complete circumferential involvement require close follow-up.
The data suggests that patients with CEC may undergo radiotherapy of up to 63 Gy without increasing the risk of radiation-induced toxicities. Since prospective data is lacking, our study warrants a prospective trial to investigate toxicity and efficacy of high-dose radiotherapy for CEC.
This study was selected for a poster presentation at the 59th Annual Meeting of the American Society for Radiation Oncology (ASTRO), San Diego, CA, United States September 2017.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arigami T, Kato H, Ono T S- Editor: Wang JL L- Editor: A E- Editor: Huang Y
| 1. | Lee DJ, Harris A, Gillette A, Munoz L, Kashima H. Carcinoma of the cervical esophagus: diagnosis, management, and results. South Med J. 1984;77:1365-1367. [PubMed] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21368] [Article Influence: 2136.8] [Reference Citation Analysis (3)] |
| 3. | Grass GD, Cooper SL, Armeson K, Garrett-Mayer E, Sharma A. Cervical esophageal cancer: a population-based study. Head Neck. 2015;37:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Archibald S, Young JE, Thoma A. Pharyngo-cervical esophageal reconstruction. Clin Plast Surg. 2005;32:339-346, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Esophageal and esophagogastric junction cancers. NCCN guidelines Version 3. 2017;2017: September 28, 2017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. |
| 6. | Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol. 2016;27:1664-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Gkika E, Gauler T, Eberhardt W, Stahl M, Stuschke M, Pöttgen C. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis Esophagus. 2014;27:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Wang SL, Liao Z, Liu H, Ajani J, Swisher S, Cox JD, Komaki R. Intensity-modulated radiation therapy with concurrent chemotherapy for locally advanced cervical and upper thoracic esophageal cancer. World J Gastroenterol. 2006;12:5501-5508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Head and Neck Cancers. NCCN guidelines Version 2.2017. 2017;September 28, 2017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. |
| 10. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 876] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 11. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [PubMed] |
| 12. | Chen AM, Li BQ, Jennelle RL, Lau DH, Yang CC, Courquin J, Vijayakumar S, Purdy JA. Late esophageal toxicity after radiation therapy for head and neck cancer. Head Neck. 2010;32:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kim HW, Kim JH, Lee IJ, Kim JW, Lee YC, Lee CG, Park JJ, Youn YH, Park H. Local control may be the key in improving treatment outcomes of esophageal squamous cell carcinoma undergoing concurrent chemoradiation. Digestion. 2014;90:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Werner-Wasik M. Treatment-related esophagitis. Semin Oncol. 2005;32:S60-S66. [PubMed] |
| 15. | Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812. [PubMed] |
| 16. | Maguire PD, Sibley GS, Zhou SM, Jamieson TA, Light KL, Antoine PA, Herndon JE 2nd, Anscher MS, Marks LB. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999;45:97-103. [PubMed] |
| 17. | Hazard L, Minsky B. Esophagus. Human Radiation Injury. 1st ed. Philadelphia, PA: Lippincott Williams Wilkins 2011; 403-420. |
| 18. | Atsumi K, Shioyama Y, Arimura H, Terashima K, Matsuki T, Ohga S, Yoshitake T, Nonoshita T, Tsurumaru D, Ohnishi K. Esophageal stenosis associated with tumor regression in radiotherapy for esophageal cancer: frequency and prediction. Int J Radiat Oncol Biol Phys. 2012;82:1973-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Seaman WB, Ackerman LV. The effect of radiation on the esophagus; a clinical and histologic study of the effects produced by the betatron. Radiology. 1957;68:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Berthrong M, Fajardo LF. Radiation injury in surgical pathology. Part II. Alimentary tract. Am J Surg Pathol. 1981;5:153-178. [PubMed] |
| 21. | Papazian A, Capron JP, Ducroix JP, Dupas JL, Quenum C, Besson P. Mucosal bridges of the upper esophagus after radiotherapy for Hodgkin’s disease. Gastroenterology. 1983;84:1028-1031. [PubMed] |
| 22. | Handschel J, Sunderkötter C, Prott FJ, Meyer U, Kruse-Lösler B, Joos U. Increase of RM3/1-positive macrophages in radiation-induced oral mucositis. J Pathol. 2001;193:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36:373-381. [PubMed] |