Published online Dec 28, 2018. doi: 10.3748/wjg.v24.i48.5433
Peer-review started: September 28, 2018
First decision: October 26, 2018
Revised: November 7, 2018
Accepted: November 16, 2018
Article in press: November 16, 2018
Published online: December 28, 2018
Processing time: 90 Days and 12.4 Hours
Checkpoint inhibitors are increasingly being used in clinical practice. They can cause various gastrointestinal, hepatic and pancreatic side effects. As these side effects can be serious, appropriate management is essential. The different checkpoint inhibitors with their mechanisms of action and indications, as well as evaluation and management of gastrointestinal, hepatic and pancreatic side effects, are discussed in this article.
Core tip: Checkpoint inhibitors are a kind of immunotherapy used in the treatment of various malignancies. Nevertheless, they carry the risk of causing different immune-related side effects. Physicians should be vigilant in recognizing and appropriately managing these side effects for a better outcome.
- Citation: Ahmed M. Checkpoint inhibitors: What gastroenterologists need to know. World J Gastroenterol 2018; 24(48): 5433-5438
- URL: https://www.wjgnet.com/1007-9327/full/v24/i48/5433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i48.5433
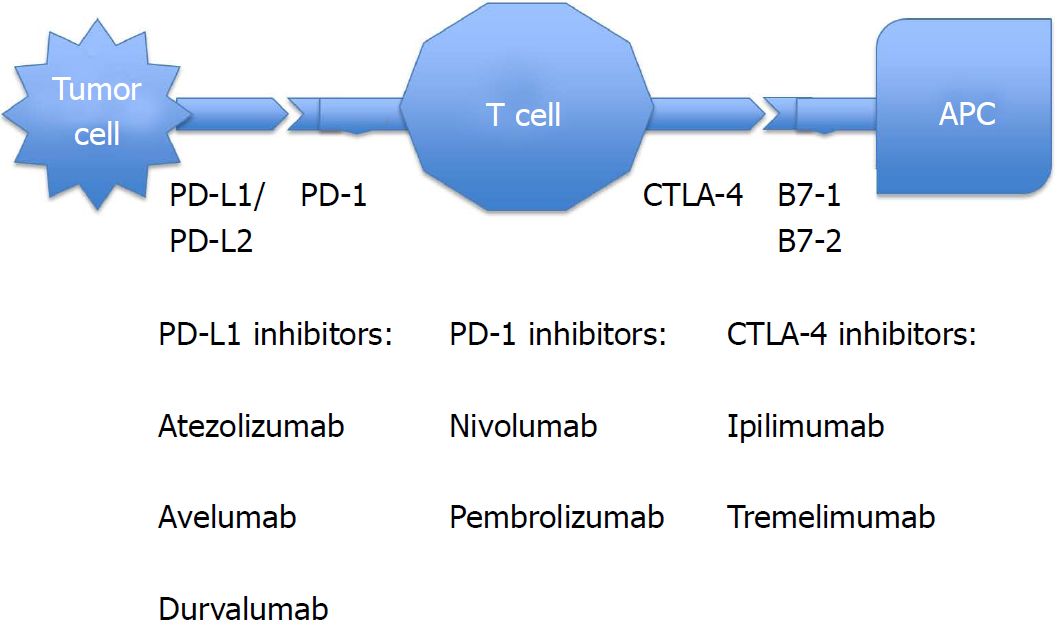
Checkpoint inhibitors have emerged as one of the most promising modalities of anti-cancer therapy[1]. They are monoclonal antibodies that block the checkpoint proteins either on T cells or cancer cells to enhance immune response against tumor cells[2]. Normally, when our body recognizes cancer cells or foreign bodies, our innate immune system (macrophages, dendritic cells, natural killer cells, mast cells, neutrophils, eosinophils and basophils) tries to eliminate them. Then, our adaptive immune system (B lymphocytes and T lymphocytes) starts working via antigen presenting cells. Checkpoint molecules are proteins that control specific cellular processes to prevent errors. Some immune checkpoint proteins help the T cells to remain active, particularly in case of infection, whereas other immune checkpoint proteins regulate the immune system negatively by directing the T cells to switch off. Some cancer cells synthesize high levels of such immune checkpoint proteins, which can switch off the T cells and, as a result, the T cells can neither recognize nor kill the cancer cells.
Some of the common checkpoint proteins include: (1) Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) receptors on CD4 and CD8 T lymphocytes; (2) programmed cell death protein 1 (PD-1) receptors on the surface of T cells, B cells, natural killer (NK) cells, monocytes and dendritic cells; and (3) programmed cell death protein ligand 1 (PD-L1) and programmed cell death protein ligand 2 (PD-L2) proteins on healthy tissues, hematopoietic cells and tumor cells.
When interactions between the PD-1 receptors and PD-L1 (also known as B7-H1) or PD-L2 (also known as B7-H2) occurs, it promotes exhaustion of peripheral effector T cells, conversion of effector T cells to regulatory T (Treg) cells and inhibition of tumor cell apoptosis[3]. Some cancer cells are able to produce PD-L1 and PD-L2 on their surfaces to prevent any immunological attack.
CTLA-4 becomes activated by binding to B7-1 (also known as CD80) and B7-2 (also known as CD86) on antigen presenting cells (APCs), and then inhibits T cell activation at a proximal step in the immune response. On the other hand, PD-1 limits effector T cell function by linking with PD-L1 or PD-L2 in the later stages of the immune response. In the process of carcinogenesis, these immunosuppressive molecules are overexpressed[4]. Checkpoint inhibitors are monoclonal antibodies against PD-1, PD-L1 or CTLA-4 proteins. They act as a form of immunotherapy by blocking the immunosuppressive molecules that otherwise inhibit the immune system from attacking cancer cells. As a consequence, there is an immunological boost against cancer cells[5]. As they target T cells instead of cancer cells, they can be used in various malignancies[6]. A combination of checkpoint inhibitors may give a better anti-tumor response. There was a 23% response rate for metastatic non-small cell lung cancer after administration of durvalumab and tremelimumab[7].
Few checkpoint molecules have been discovered recently. These include TIM-3, LAG3, TIGIT and BTLA.
T cell immunoglobulin and mucin domain 3 (TIM-3) is present on the surface of CD4 T cells, CD8 T cells, regulatory T cells and innate immune cells (dendritic cells, macrophages and natural killer cells). TIM-3 binds to specific ligands: galectin (Gal-9), phosphatidyl serine (PtdSer), high-mobility group box-1 protein (HMGB) and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1). These interactions generate a variety of effects, including effector T cell apoptosis, T cell suppression, suppression of the innate immune response against tumor cells, suppression of anti-tumor activity and promotion of tumor growth[8]. TIM-3 is upregulated in patients with malignancy. In pre-clinical studies, TIM-3 monoclonal antibody monotherapy showed modest anti-tumor activities[9], but combinations of anti-TIM-3 and anti-PD-1/PD-L1 monoclonal antibodies produced significant anti-tumor responses against a variety of malignancies, including colon cancer, lung cancer, ovarian cancer, melanoma, lymphoma, acute myelogenous leukemia and sarcoma[10].
LAG-3 (lymphocyte activation gene-3 protein) is an inhibitory receptor expressed on CD4-positive T-lymphocytes, CD8-positive T-lymphocytes, NK cells and B cells, as well as on plasmacytoid dendritic cells[11-13]. LAG-3 inhibits both activation and proliferation of T cells[14,15]. Anti-LAG3 monoclonal antibodies can bind to the LAG-3 present on tumor infiltrating lymphocytes (TILs), and prevent their binding to MHC (major histocompatibility complex) class II molecules expressed on tumor cells. This may lead to activation of antigen-specific T lymphocytes and cytotoxic T cell-mediated tumor lysis. Clinical trials were done with different types of LAG-3 monoclonal antibodies (IMP321) on various malignancies, such as metastatic renal cell cancer, breast cancer, unresectable pancreatic cancer, as well as advanced and unresectable melanoma[16].
T cell immunoreceptors with Ig and ITIM domains (TIGIT) are inhibitory immunoreceptors present on some T cells (CD4, CD8), NK cells and Treg cells that contain Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domains. TIGIT ligands include CD155 and CD112. In certain malignancies, CD155 and CD112 are highly expressed on macrophages and dendritic cells. TIGIT ligation leads to inhibition of T cell proliferation and suppression of the cytolytic function of NK cells[17]. Anti-tumor activity is suppressed by TIGIT, primarily via Treg cells and not CD8-positive T cells[18]. Anti-TIGIT monoclonal antibodies as a monotherapy or in combination with anti-PD-L-1 antibodies have shown anti-tumor activity[19] in phase I/II trials.
BTLA (a B and T lymphocyte attenuator, also known as CD272) is an inhibitory protein functionally and structurally similar to CTLA-4 and PD-1. It is mainly expressed on immune cells, NK cells, dendritic cells and splenic macrophages. BTLA acts as a ligand for tumor necrosis factor receptor superfamily member 14 (TNFRSF-14), also known as herpes virus entry mediator (HVEM).
BTLA/HVEM complex inhibits T cell activation and proliferation[20]. BTLA is overexpressed in certain malignancies like leukemia and melanoma. In mouse models, BTLA neutralizing antibodies limited tumor growth[21]. Anti-human BTLA monoclonal antibodies are currently in development.
Current checkpoint inhibitors, with their indications and a schematic diagram (Figure 1), are mentioned below[22-28], including CTLA-4 blockers, PD-1 inhibitors, and PD-L1 inhibitors.
Ipilimumab: Indications include melanoma with lymph node involvement, advanced melanoma, non-small cell and small cell lung cancer, advanced renal cell cancer, and hormone refractory prostate cancer. Great success with durable clinical benefit was seen with nivolumab plus ipilimumab combination when given in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer[29].
Tremelimumab: This drug is undergoing human clinical trials for the treatment of various malignancies, but is not yet approved by the United States Food and Drug Administration (FDA).
Nivolumab: Indications are melanoma with lymph node involvement, unresectable or metastatic melanoma, advanced renal cell carcinoma, advanced or metastatic urothelial cancer, metastatic non-small cell lung cancer and small cell lung cancer with progression after platinum-based chemotherapy, refractory classical Hodgkin lymphoma, recurrent or metastatic squamous cell cancer of head and neck, microsatellite instability-high or mismatch repair-deficient colorectal cancer, and hepatocellular carcinoma.
Pembrolizumab: Indications include unresectable or metastatic melanoma, metastatic non-small cell lung cancer, advanced head and neck squamous cell carcinoma, advanced or metastatic gastric or gastroesophageal junction cancer, microsatellite instability-high cancer, locally advanced or metastatic urothelial cancer, recurrent or metastatic cervical cancer, refractory classical Hodgkin lymphoma, and refractory primary mediastinal large B cell lymphoma.
Atezolizumab: Indicated for advanced or metastatic non-small cell lung cancer and advanced or metastatic urothelial cancer.
Avelumab: Indicated for advanced or metastatic urothelial cancer and metastatic Merkel cell cancer.
Durvalumab: Indicated for advanced or metastatic urothelial cancer, as well as unresectable and stage III non-small lung cancer.
Immune-related adverse events (IRAE) can occur due to the use of checkpoint inhibitors. Inflammatory side effects generally involve the skin, gastrointestinal tract, liver and endocrine glands. The cardiovascular, pulmonary, renal, hematological and musculoskeletal system are less commonly involved. IRAE are more severe following administration of CTLA-4 inhibitors in comparison to PD-1 or PD-L1 inhibitors. The time of onset of IRAE is generally 1-6 mo after administration of checkpoint inhibitors[30]. Here, we will be mainly discussing the gastrointestinal, hepatic and pancreatic side effects of checkpoint inhibitors.
Immune-related adverse events are classified according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (AE), version 3.0[31]. Grade 1: Mild AE; Grade 2: Moderate AE; Grade 3: Severe AE; Grade 4: Life-threatening or disabling AE; Grade 5: Death related to AE.
Diarrhea is the most common gastrointestinal side effect after administration of checkpoint inhibitors. Diarrhea occurs in 27%-31% of cases following CTLA-4 inhibitor therapy[32] and less than 4% of cases following anti-PD-1 and anti-PD-L1 therapy[33]. Diarrhea varies from mild to severe in intensity. Immune-mediated mild to severe colitis, colon perforation and even death can occur following checkpoint inhibitor therapy[34,35]. Severe colitis can occur in 5% of cases following CTLA-4 inhibitor therapy and less than 2% of cases following anti-PD-1 therapy. Colitis may mimic Crohn’s colitis or ulcerative colitis, and can be associated with intra-abdominal abscess, anal fissure and fistula[36]. Pembrolizumab-induced collagenous colitis and lymphocytic colitis have also been reported in the literature[37,38].
Mild diarrhea or grade I diarrhea with stool frequency less than 4 times per day can be managed conservatively without discontinuing checkpoint inhibitors. Stool for ova, parasites, giardia antigen, stool culture and C. difficile toxin should be sent to evaluate for any underlying infection. Patient should be given adequate oral hydration and anti-diarrheal agents. If diarrhea still persists for 5-7 d or worsens, patients should be treated similar to cases of moderate diarrhea.
Moderate diarrhea or grade II diarrhea with stool frequency between 4-6 times per day should be managed by discontinuation of checkpoint inhibitors, by ruling out infection by sending stool samples as mentioned above, by giving adequate oral hydration and by administering empiric treatment with oral corticosteroid (prednisone 1 mg/kg per day) with close clinical follow-up[39]. Colonoscopy is not required if the patient responds to the above measures. After 2-5 d control of diarrhea, prednisone should be slowly tapered over a 1-2 mo period of time. Trimethoprim-sulfamethoxazole should be given as a prophylaxis against opportunistic infection during the tapering period[40].
If the patient does not respond to the conservative measures or if the patient has severe diarrhea, i.e., grade III or IV diarrhea, with stool frequency more than 6 times per day with severe and persistent abdominal pain, fever, rectal bleeding or ileus, intravenous hydration and intravenous steroid (methylprednisone 1-2 mg/kg per day) should be started[41]. Antidiarrheal agents like loperamide and lomotil (diphenoxylate/atropine) should be avoided. Abdominal CT (computerized axial tomography) should be done to assess the severity and complications of colitis (perforation and peritonitis). Colonoscopy or flexible sigmoidoscopy should be done not only to evaluate the severity and extent of colitis, but also to take random and targeted colon biopsies to rule out underlying cytomegalovirus (CMV) infection[35]. CMV colitis is diagnosed by characteristic histology (owl’s eye intranuclear inclusion bodies), CMV biopsy PCR (polymerase chain reaction) or CMV biopsy viral culture[42]. Colonoscopic findings may include loss of vascular markings, erythema, congestion, friability, ulcerations and spontaneous bleeding. The severity of diarrhea may not correlate with the colonoscopic or histological findings.
Treatment should be continued until there is significant improvement of diarrhea, i.e., grade 0-1. If there is a clinical response to corticosteroids, it is recommended to continue treatment for a month and then slowly taper. If the patient’s diarrhea is refractory to steroids, or colonoscopy shows severe colitis, multiple colon ulcers or pancolitis, anti-TNF therapy like Infliximab (5 mg/kg every 2 wk) or anti-integrin therapy like Vedolizumab (300 mg 0, 2, 6 wk) should be added[43-45]. Concomitant bacterial, viral or Clostridium difficile infection should be treated simultaneously. Following resolution of symptoms, checkpoint inhibitors can be restarted if the benefits outweigh the risks, and if the daily dose of prednisone can be reduced to less than 10 mg per day without any other immunosuppressive medication.
Diarrhea onset approximately 6 wk after checkpoint inhibitor therapy: assess severity of diarrhea and rule out infection by sending stool samples → Grade I diarrhea: conservative treatment with oral hydration and anti-diarrheal agents → Persistence of diarrhea after 5-7 d → Manage as grade II diarrhea. Grade II diarrhea: stop checkpoint inhibitor, start oral corticosteroid and continue oral hydration: (1) Clinical improvement → 2-5 d after control of diarrhea, start tapering corticosteroid over 1-2 mo plus trimethoprim-sulfamethoxazole as prophylaxis; (2) no clinical improvement → Manage as grade III or IV diarrhea. Grade III or IV diarrhea: hospitalization, parenteral hydration, parenteral corticosteroid, abdominal CT and colonoscopy: (1) Clinical response: continue steroid for a month and taper; (2) no clinical response: anti-TNF therapy.
Checkpoint inhibitors can cause immune-mediated hepatitis in less than 5% of cases receiving these medications[46]. Although this can occur anytime while the patient is on checkpoint inhibitor therapy, it occurs most commonly 6-7 wk after the onset of therapy[47]. Most of the time, patients remain asymptomatic with elevated serum transaminases. Sometimes, the hepatitis can be more severe, with patients presenting with fever, malaise, fatigue, hepatomegaly and hyperbilirubinemia. Acute viral hepatitis (HAV, HBV, HCV, EBV, CMV) and autoimmune hepatitis need to be excluded by serology and liver biopsy[48]. Predominant hepatic parenchymal injury with panlobular hepatitis or predominant bile duct injury with mononuclear cell infiltration around proliferated bile ductules can be seen after checkpoint inhibitor therapy[49]. It is sometimes difficult to distinguish autoimmune hepatitis from drug-induced hepatitis. In autoimmune hepatitis, intra-acinar and portal plasma cells with rosette formation and emperiopolesis are prominent, whereas neutrophilic infiltration is more commonly seen in drug-induced liver injury[50].
Grade 1 immune-mediated hepatitis: patient is asymptomatic or mildly symptomatic, but laboratory studies show AST/ALT: < 2.5 × ULN (upper limit of normal) and total bilirubin: < 1.5 × ULN. Treatment: continue checkpoint inhibitor therapy, but monitor LFT.
Grade 2 immune-mediated hepatitis: patient is symptomatic (fever, malaise, fatigue) and laboratory studies show AST/ALT: 2.5-5 × ULN and total bilirubin: 1.5-3 × ULN. Treatment: (1) hold checkpoint inhibitor therapy; (2) viral hepatitis (HAV, HBV, HCV, HDV, CMV, EBV, HSV, VZV), autoimmune hepatitis and drug-induced hepatitis need to be ruled out[46]; (3) prednisone 1 mg/kg per day, taper the dose when patient’s symptoms improve; and (4) if symptoms do not improve after 48 h, alternate immunosuppressive agents like tacrolimus, mycophenolate mofetil or cyclophosphamide need to be considered.
Grade 3 or 4 immune-mediated hepatitis: patient is symptomatic as mentioned above, and laboratory studies show: AST/ALT: > 5 × ULN; total bilirubin: > 3 × ULN. Treatment: (1) hold checkpoint inhibitors; (2) intravenous solumedrol 2-4 mg/kg per day. Taper the dose when patient’s symptoms improve; and (3) if symptoms do not improve after 5-7 d, tacrolimus 0.10-0.15 mg/kg per day should be added. Alternative agents include mycophenolate mofetil or cyclophosphamide.
Immune-mediated pancreatitis with pancreatic insufficiency has been reported a few months after initiation of checkpoint inhibitor therapy[52]. Asymptomatic elevations of amylase and lipase can occur without fulfilling the diagnostic criteria of acute pancreatitis. As the clinical significance of this lab abnormality is unknown, routine measurement of serum amylase and lipase is not recommended[53]. However, if the patient is symptomatic with abdominal pain or nausea, immune-mediated pancreatitis should be considered by checking amylase and lipase levels and performing imaging studies.
Intravenous methylprednisolone (1 mg/kg per day) for a few days, followed by oral prednisone (1 mg/kg per day). Taper the dose when patient symptoms improve.
Pancreatic enzyme supplementation should be given if there is evidence of pancreatic insufficiency (fecal elastase < 15 μg/g of feces).
Checkpoint inhibitors are novel forms of immunotherapy administered by oncologists. Although they are extremely useful in various advanced and metastatic malignancies, they can cause multiple side effects. Gastroenterologists need to be aware of the various gastrointestinal, hepatic and pancreatic side effects that can be fatal if not managed early. Prompt recognition of these side effects, administration of systemic immunosuppressive therapy and supportive care could improve the clinical outcome without affecting the benefit of checkpoint inhibitors. Multidisciplinary teams should be involved in the management of these side effects. As new checkpoints are being discovered and new checkpoint inhibitors are being developed, patients will be experiencing new IRAE. Management of those IRAE will improve as we gather more experience using new checkpoint inhibitors.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Aykan NF, Caboclo JF, Contini S, Lin JM, Linnebacher M, Tang Y, Wakao H S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Chen Q, Wang C, Chen G, Hu Q, Gu Z. Delivery Strategies for Immune Checkpoint Blockade. Adv Healthc Mater. 2018;7:e1800424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Trivedi MS, Hoffner B, Winkelmann JL, Abbott ME, Hamid O, Carvajal RD. Programmed death 1 immune checkpoint inhibitors. Clin Adv Hematol Oncol. 2015;13:858-868. [PubMed] |
| 3. | Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune Checkpoint Inhibitors: An Innovation in Immunotherapy for the Treatment and Management of Patients with Cancer. Asia Pac J Oncol Nurs. 2017;4:127-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2907] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 5. | Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Met Tre. 2017;3:250-261. [RCA] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 6. | Assarzadegan N, Montgomery E, Anders RA. Immune checkpoint inhibitor colitis: the flip side of the wonder drugs. Virchows Arch. 2018;472:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 509] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 8. | Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol. 2018;11:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 10. | Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1638] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 11. | Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393-1405. [PubMed] |
| 12. | Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885-1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1703] [Cited by in RCA: 1642] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 15. | Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995;25:2718-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 294] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276:80-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 688] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 17. | Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 881] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 18. | Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125:4053-4062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 443] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 19. | Solomon BL, Garrido-Laguna I. TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol Immunother. 2018;67:1659-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Zhang M, Howard K, Winters A, Steavenson S, Anderson S, Smelt S, Doellgast G, Sheelo C, Stevens J, Kim H. Monoclonal antibodies to B and T lymphocyte attenuator (BTLA) have no effect on in vitro B cell proliferation and act to inhibit in vitro T cell proliferation when presented in a cis, but not trans, format relative to the activating stimulus. Clin Exp Immunol. 2011;163:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Sekar D, Govene L, Del Río ML, Sirait-Fischer E, Fink AF, Brüne B, Rodriguez-Barbosa JI, Weigert A. Downregulation of BTLA on NKT Cells Promotes Tumor Immune Control in a Mouse Model of Mammary Carcinoma. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3152] [Article Influence: 450.3] [Reference Citation Analysis (0)] |
| 23. | Medscape. Ipilimumab (Rx). Available from: https://reference.medscape.com/drug/yervoy-ipilimumab-999636. |
| 24. | Medscape. Nivolumab (Rx). Available from: https://reference.medscape.com/drug/opdivo-nivolumab-999989. |
| 25. | Medscape. Pembrolizumab (Rx). Available from: https://reference.medscape.com/drug/keytruda-pembrolizumab-999962. |
| 26. | Medscape. Atezolizumab (Rx). Available from: https://reference.medscape.com/drug/tecentriq-atezolizumab-1000098. |
| 27. | Medscape. Avelumab (Rx). Available from: https://reference.medscape.com/drug/bavencio-avelumab-1000144. |
| 28. | Medscape. Durvalumab (Rx). Available from: https://reference.medscape.com/drug/imfinzi-durvalumab-1000145. |
| 29. | Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1471] [Article Influence: 210.1] [Reference Citation Analysis (0)] |
| 30. | Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, Kähler KC, Loquai C, Reinmuth N, Steins M. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 297] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 31. | Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3. 0. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. |
| 32. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11782] [Article Influence: 785.5] [Reference Citation Analysis (0)] |
| 33. | Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol. 2017;8:730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 34. | Celli R, Kluger HM, Zhang X. Anti-PD-1 Therapy-Associated Perforating Colitis. Case Rep Gastrointest Med. 2018;2018:3406437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Prieux-Klotz C, Dior M, Damotte D, Dreanic J, Brieau B, Brezault C, Abitbol V, Chaussade S, Coriat R. Immune Checkpoint Inhibitor-Induced Colitis: Diagnosis and Management. Target Oncol. 2017;12:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Bertha M, Bellaguara E, Kuzel T, Hanauer S. Checkpoint Inhibitor-Induced Colitis: A New Type of Inflammatory Bowel Disease? ACG Case Rep J. 2017;4:e112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Baroudjian B, Lourenco N, Pagès C, Chami I, Maillet M, Bertheau P, Bagot M, Gornet JM, Lebbé C, Allez M. Anti-PD1-induced collagenous colitis in a melanoma patient. Melanoma Res. 2016;26:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Ahmed M, Francis G. Pembrolizumab-Induced Microscopic Colitis. Am J Gastroenterol. 2018;113:629-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Rudzki JD. Management of adverse events related to checkpoint inhibition therapy. Memo Eur Med Oncol. 2018;11:132-137. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Cooley L, Dendle C, Wolf J, Teh BW, Chen SC, Boutlis C, Thursky KA. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014;44:1350-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Cheng R, Cooper A, Kench J, Watson G, Bye W, McNeil C, Shackel N. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatol. 2015;30:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Goodman AL, Murray CD, Watkins J, Griffiths PD, Webster DP. CMV in the gut: a critical review of CMV detection in the immunocompetent host with colitis. Eur J Clin Microbiol Infect Dis. 2015;34:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Geukes Foppen MH, Rozeman EA, van Wilpe S, Postma C, Snaebjornsson P, van Thienen JV, van Leerdam ME, van den Heuvel M, Blank CU, van Dieren J. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3:e000278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 44. | Hsieh AH, Ferman M, Brown MP, Andrews JM. Vedolizumab: a novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 46. | Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 47. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 48. | Suriawinata AA, Thung SN. Acute and chronic hepatitis. Semin Diagn Pathol. 2006;23:132-148. [PubMed] |
| 49. | Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, Ibrahim N. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 50. | Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, Lucena MI, Castiella A, Lindor K, Björnsson E. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 51. | Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Corrigendum: Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front Pharmacol. 2017;8:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 478] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 53. | Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015;76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |