Published online Dec 21, 2018. doi: 10.3748/wjg.v24.i47.5366
Peer-review started: August 9, 2018
First decision: October 5, 2018
Revised: November 26, 2018
Accepted: November 30, 2018
Article in press: November 30, 2018
Published online: December 21, 2018
Processing time: 136 Days and 15 Hours
To investigate whether duodenal lesions induced by major venous occlusions can be attenuated by BPC 157 regardless nitric oxide (NO) system involvement.
Male Wistar rats underwent superior anterior pancreaticoduodenal vein (SAPDV)-ligation and were treated with a bath at the ligated SAPDV site (BPC 157 10 μg, 10 ng/kg per 1 mL bath/rat; L-NAME 5 mg/kg per 1 mL bath/rat; L-arginine 100 mg/kg per 1 mL bath/rat, alone and/or together; or BPC 157 10 μg/kg instilled into the rat stomach, at 1 min ligation-time). We recorded the vessel presentation (filled/appearance or emptied/disappearance) between the 5 arcade vessels arising from the SAPDV on the ventral duodenum side, the inferior anterior pancreaticoduodenal vein (IAPDV) and superior mesenteric vein (SMV) as bypassing vascular pathway to document the duodenal lesions presentation; increased NO- and oxidative stress [malondialdehyde (MDA)]-levels in duodenum.
Unlike the severe course in the SAPDV-ligated controls, after BPC 157 application, the rats exhibited strong attenuation of the mucosal lesions and serosal congestion, improved vessel presentation, increased interconnections, increased branching by more than 60% from the initial value, the IAPDV and SMV were not congested. Interestingly, after 5 min and 30 min of L-NAME and L-arginine treatment alone, decreased mucosal and serosal duodenal lesions were observed; their effect was worsened at 24 h, and no effect on the collateral vessels and branching was seen. Together, L-NAME+L-arginine antagonized each other’s response, and thus, there was an NO-related effect. With BPC 157, all SAPDV-ligated rats receiving L-NAME and/or L-arginine appeared similar to the rats treated with BPC 157 alone. Also, BPC 157 in SAPDV-ligated rats normalized levels of NO and MDA, two oxidative stress markers, in duodenal tissues.
BPC 157, rapidly bypassing occlusion, rescued the original duodenal flow through IAPDV to SMV flow, an effect related to the NO system and reduction of free radical formation.
Core tip: Up to now, application of prototype cytoprotective agent BPC 157 induced bypassing of occlusion, in rats underwent vessels occlusions, through rapid collaterals presentation, and lesions and whole consequent syndrome (ischemic colitis; deep vein thrombosis, Virchow triad) were attenuated. In rats underwent superior anterior pancreaticoduodenal vein-ligation, medication was with BPC 157, L-NAME; L-arginine, saline given alone and/or together at 1 min ligation time. Unlike severe course in controls, BPC 157 rats commonly exhibited strong attenuation of mucosal lesion and serosal congestion, since BPC 157 rescued original duodenal flow through inferior anterior pancreaticoduodenal vein to superior mesenteric vein flow.
- Citation: Amic F, Drmic D, Bilic Z, Krezic I, Zizek H, Peklic M, Klicek R, Pajtak A, Amic E, Vidovic T, Rakic M, Milkovic Perisa M, Horvat Pavlov K, Kokot A, Tvrdeic A, Boban Blagaic A, Zovak M, Seiwerth S, Sikiric P. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol 2018; 24(47): 5366-5378
- URL: https://www.wjgnet.com/1007-9327/full/v24/i47/5366.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i47.5366
We focused on major venous occlusions and duodenal lesions in the rat and treatment with the stable gastric pentadecapeptide BPC 157, which is a prototype cytoprotective agent used in ulcerative colitis and multiple sclerosis trials (LD1 not achieved) and is known to counteract duodenal lesions[1-10]. Rat duodenal lesion research is mostly based on cysteamine and acetic acid models[11-14]; investigations are sparse on the impact of major venous obstruction and whether recruitment of blood vessels to bypass obstruction may occur quickly, and if so, whether it may be facilitated by a suitable therapy. On the other hand, in rats that underwent other vessel occlusions (i.e., left colic artery and vein, infrarenal inferior caval vein[15,16]), after application of stable gastric pentadecapeptide BPC 157, the bypassing occurs through a rapid collateral presentation, and the lesions and consequent syndrome were largely attenuated. Rapidly reestablished arcade vessel interconnections upon BPC 157 application mitigated the harm in two ligations of the left colic artery and vein and consequently alleviated ischemic colitis as well[15]. In rats with infrarenal inferior caval vein occlusion, the therapeutic effect occurs through the immediate presentation of the left ovaric vein (LOV) and other veins, bypassing the obstruction, and counteracting all the symptoms of Virchow’s triad[16]. The normalized levels of two oxidative stress markers, nitric oxide (NO) and malondialdehyde (MDA), in tissues as well as the reduced L-NAME and L-arginine effects showed BPC 157 effectiveness over the NO-system background[15,16].
These findings were related[15,16] to the cytoprotective agents, to the effects originally noted in the stomach, which is the rapid endothelial protection. Using BPC 157 as a prototype cytoprotective agent[15,16], these mechanisms can prevent and resolve adjacent ischemic mucosal lesions[17-21] providing activation of the collateral circulation[15,16]. This activation is specific and can circumvent obstructions[15,16]. That factor had not been considered in vascular studies of ischemic colitis or deep vein thrombosis[22-25]. As a result exerted within the immediate post-injury time in vascular studies of ischemic colitis or deep vein thrombosis[15,16], it reestablishes the continuity of blood flow, in particular[15,16]. Also, this effect was shown to be a long-lasting effect[15,16]. It was also applicable in the later period, even after additional colon or major vein obstruction had occurred[15,16].
This will also resolve mucosal lesions that would appear with disturbed duodenal circulation, if the cytoprotection/endothelium protection is essential[15,16] to quickly restore blood supply to the ischemic area, and if the application of BPC 157 may offer a fundamental treatment with the rapid and successful recruitment of blood vessels during harmful events. In support, various harmful conditions, all overwhelmed, such as vascular obstruction, short-lasting blood deprivation, reperfusion, long-lasting blood deprivation and additional bowel obstruction, verify that BCP 157 can rapidly activate collaterals as we suggested[15,16].
We treated occluded superior anterior pancreaticoduodenal veins (SPDAVs) and duodenal lesions (that would otherwise rapidly appear and sustainably progress) with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. Namely, interaction between BPC 157 and NO-system goes in different systems and species. This was also demonstrated using both L-NAME and L-arginine as individual agents or in combination[1-5] in cytoprotection studies. Furthermore, venous obstruction may be more damaging to the intestine than corresponding artery occlusions and rapidly induces mucosal lesions (i.e., within 5 min in the case of the superior mesenteric vein)[26]. Thus, bypassing one or more of the vascular obstructions and thereby achieving a therapeutic effect[15,16] appears, if BPC 157 rapidly activates collaterals due to its particular direct and rapid effect on vessel presentation. The main focus of the intervention was parallel with the findings in the colon and venous tissues[15,16]. Levels of oxidative stress MDA and NO were also assessed in duodenal tissue, and the results showed NO- and MDA- duodenal tissue levels and relative to the ligation course or therapeutic effects.
We used male Albino Wistar rats, 200 g b.w., randomly assigned to groups with at least 7 rats per group. Local ethics committee approved all of the experiments. Rats had access to food and water ad libitum before the procedure and until the end of the experiment. The surgical procedure was performed as well as the assessments were performed by a blinded observer.
We used the pentadecapeptide Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, M.W. 1419, named BPC 157 (Diagen, Ljubljana, Slovenia), freely soluble in water at pH 7.0 and in saline, prepared as previously described[1-10]. L-NAME (Sigma, United States) and L-arginine (Sigma, United States) were used as previously described[1-10].
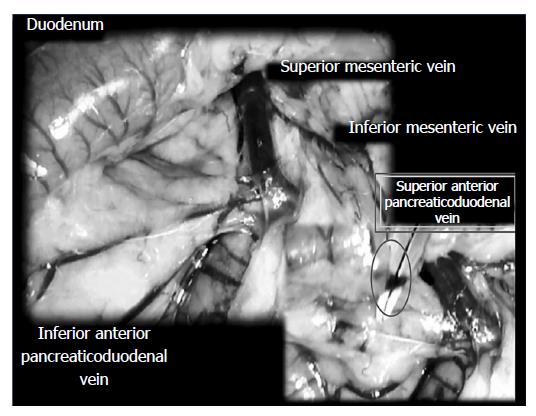
Surgery: The surgery was conducted in deeply anesthetized rats. The SAPDV was occluded by ligation (Premilene 7/0, Braun) of 5 arcade vessels at the duodenal serosa within the SAPDV branches. The 30-mm blood-flow affecting a duodenum segment was marked (Figure 1).
Medication: We used two regimens of BPC 157 application: 10 μg, 10 ng/kg per 1 mL bath/rat or 10 μg/kg instilled into the rat stomach, at 1 min ligation-time. To investigate the effect of NO-agents [L-NAME (NO-system-blockade), L-arginine (NO-system over-stimulation); L-NAME+L-arginine (NO-system immobilization)] we used L-NAME 5 mg/kg per 1 mL bath/rat; L-arginine 100 mg/kg per 1 mL bath/rat, alone and/or together. Controls received a saline bath of equal volume at 1 min of ligation time or an equal volume of saline instilled into the stomach of the SAPDV-ligated rats.
Assessment: Considering the point immediately before therapy (as 100%) [see the assessment shown in Figure 1 (SAPDV rats)], we scored the vessel presentation {recorded filled/appearance or emptied/disappearance [camera attached to a USB microscope (Veho discovery VMS-004 deluxe)]} between the 5 arcade vessels arising from the SAPDV on the ventral duodenum side (as described before[15]), the IAPDV, SMV as bypassing vascular pathway. Scoring 0-3 was as follows: 0, presentation similar to healthy rats; 1, mild congestion; 2, moderate congestion; and 3, severe congestion, after 5 min; then, at 30 min, at 24 h. Likewise, upon the duodenum opening and after sacrifice, we assessed the congested hemorrhagic areas as the sum of the largest lesion diameters[21,27-29]. As described previously[1-10,15,30], we processed the representative tissue sections for further histological analysis.
As described before[15,16,31,32], at the end of the experiment and at 5 min, 30 min and 24 h of ligation time in SAPDV-ligated rats, we assessed the oxidative stress in the collected tissue samples by quantifying thiobarbituric acid-reactive species (TBARS) as MDA equivalents (results expressed in nmol per mg of protein). Briefly, we homogenized the tissue samples [in PBS (pH 7.4) containing 0.1 mmol/L butylated hydroxytoluene (BHT) (TissueRuptor, Qiagen, United States)] and sonicated [for 30 s in an ice bath (Ultrasonic bath, Branson, United States)], added trichloroacetic acid (TCA, 10%) to the homogenate, centrifuged the mixture (at 3000 rpm for 5 min), and collected the supernatant. Then, we added 1% TBA, boiled the samples (95 °C, 60 min), and kept the tubes on ice for 10 min. Following centrifugation (14000 rpm, 10 min), we determined the absorbance of the mixture at the wavelength of 532 nm. We assessed the concentration of MDA from a standard calibration curve plotted using 1,1,3,3’-tetraethoxypropane (TEP). We express the extent of lipid peroxidation as MDA using a molar extinction coefficient for MDA of 1.56 × 105 mol/L per cm and determined protein concentration using a commercial kit.
In SAPDV-ligated rats, as described before[15,16,31,32], at the end of the experiment and at 5 min, 30 min and 24 h ligation time, we determined the NO levels (μmol/mg protein) in the duodenum tissue samples using the Griess reaction (Griess Reagent System, Promega, United States). Briefly, we added sulfanilamide to the homogenized tissue; we incubated the mixture, and added N-1-naphthylethylenediamine dihydrochloride. We measured absorbance at 540 nm using a sodium nitrite solution as a standard. We determined protein concentrations using a commercial kit (BioRad Protein DR Assay Reagent Kit, United States).
We used parametric one-way ANOVA with a post hoc Newman-Keuls test, nonparametric Kruskal-Wallis and subsequent Mann-Whitney U-test to compare the groups. We considered P < 0.05 to be statistically relevant.
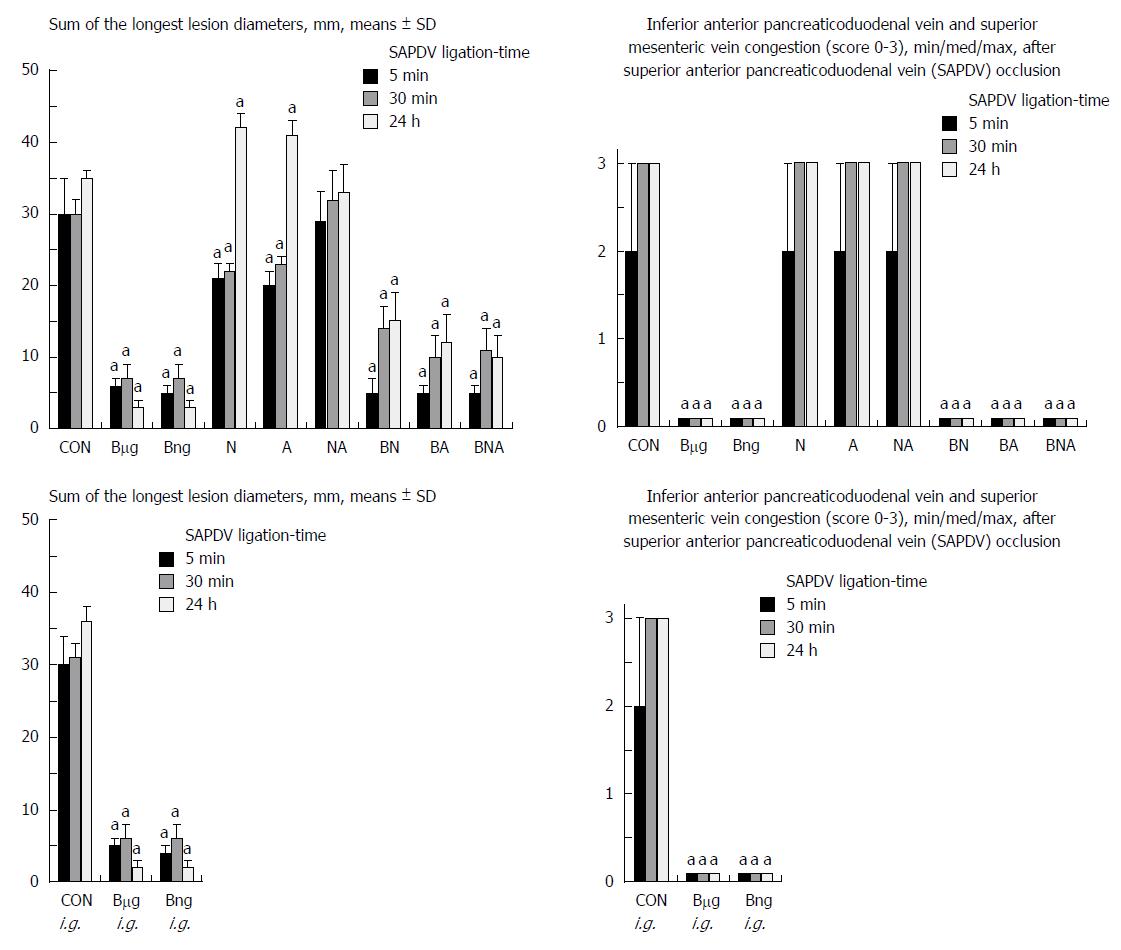
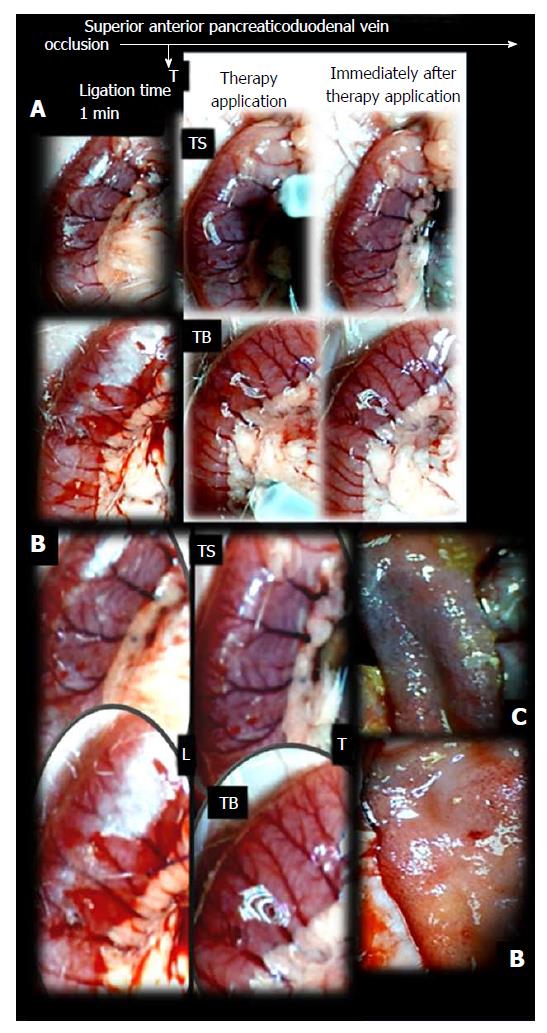
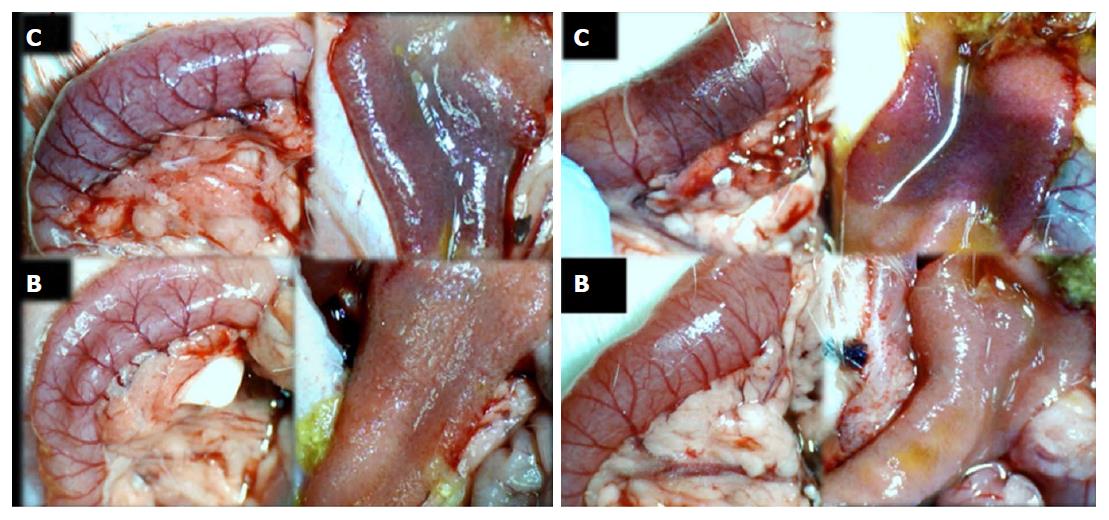
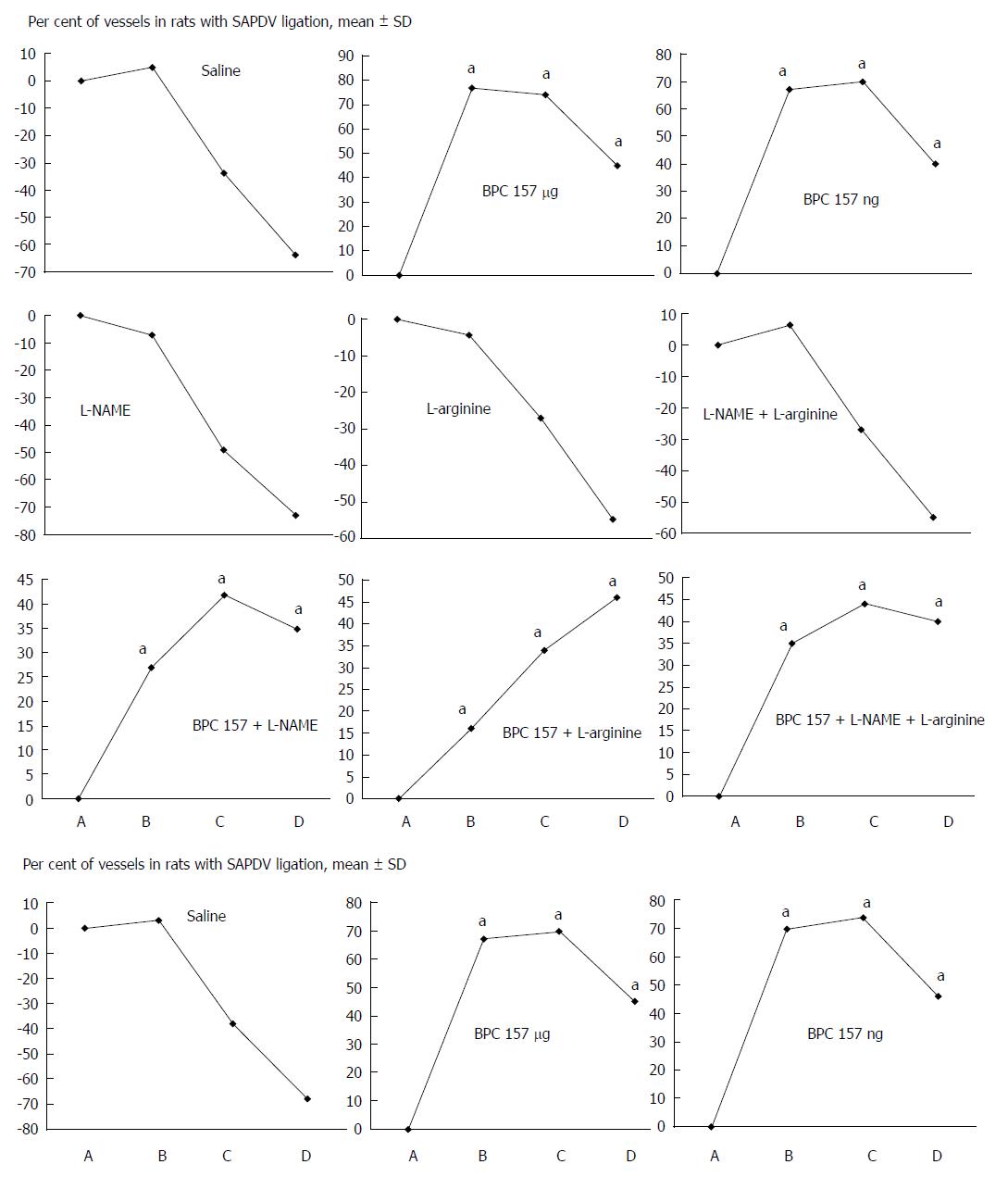
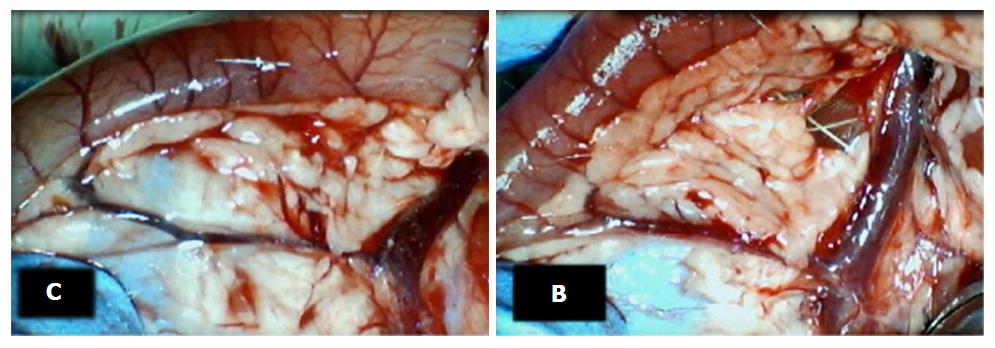
Failure progressed in control SAPDV-ligated rats (5-30 min - 24 h). Lesions were more than 30 mm in diameter and included hemorrhagic mucosal lesions, serosal congestion (Figures 2-4) with deteriorating vessels, losing collaterals, and branching was present in 30% or less of the initial value at the end of the experiment (Figure 5). IAPDV and SMV were both congested (Figures 2 and 6).
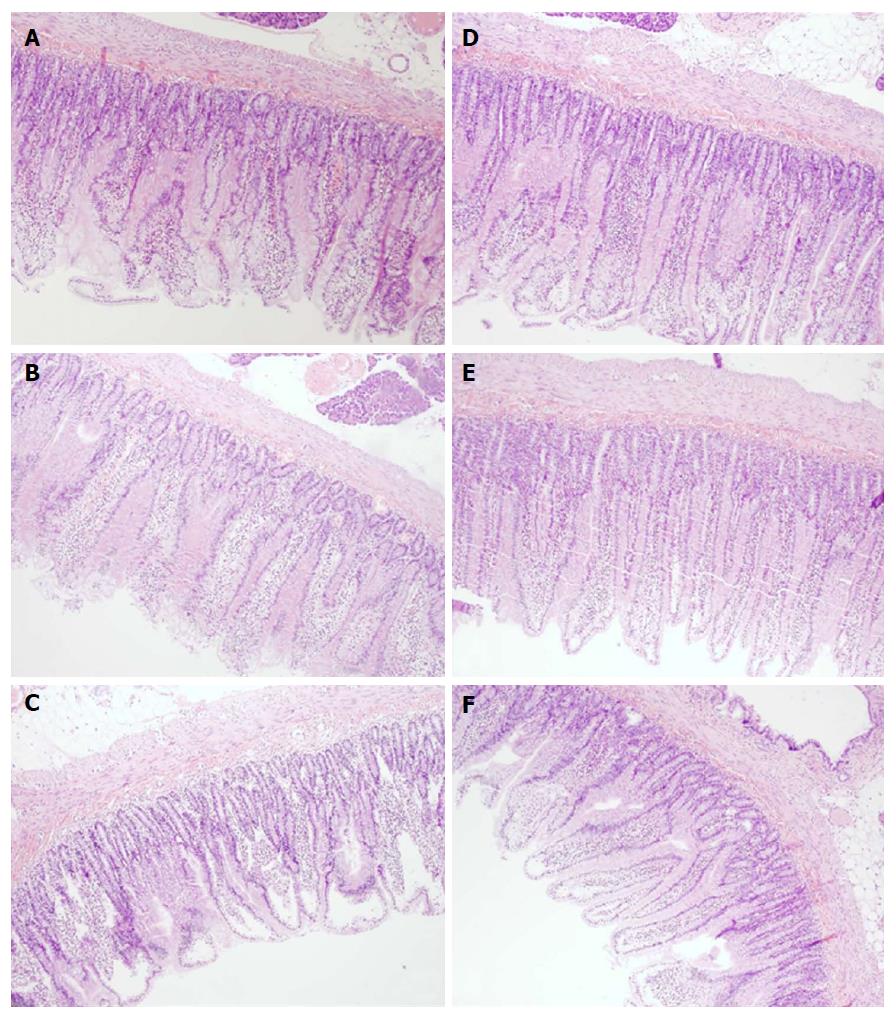
Microscopically (Figure 7), the lesions progressed from mild villous edema with mild lymphocytic infiltrate (5 min ligation time) toward denuded villous tops with marked villous edema and submucosal capillary congestion (30 min ligation time) to the substantial subepithelial space with abundant lifting of epithelial layer from lamina propira extending down sides of villi, villous edema with capillary congestion, submucosal congestion and lymphocytic infiltrate (24 h ligation time) (Figure 7).
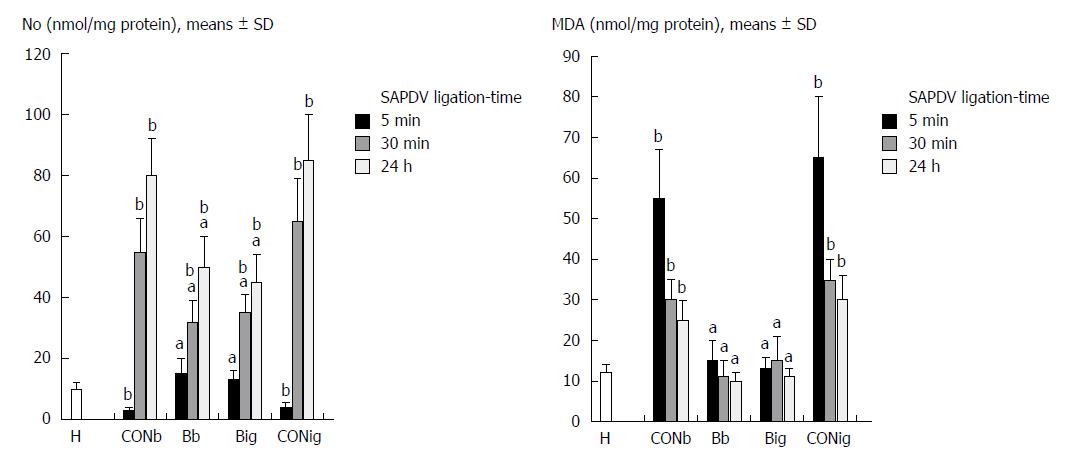
The negative chain of events was first characterized by decreased NO levels and increased MDA levels in the duodenum tissue, followed by excessive NO release and increased MDA levels in the duodenum tissue (Figure 8).
In contrast, after BPC 157 therapy (applied directly to the duodenum as a bath, or instilled into the stomach), only a few treated rats exhibited more than 10 mm-diameter mucosal lesions and serosal congestion (Figures 2-4). Frequently, vessel presentation was markedly improved with increased interconnections, and branching increased more than 60% from the initial value (Figure 5). IAPDV and SMV were both non-congested (Figures 2 and 6). Thereby, the treatment rescued the original duodenal flow through IAPDV to SMV flow, and the duodenal lesions largely diminished.
Microscopically, BPC 157 rats exhibited intestinal preservation with only mild villous edema and mild lymphocytic infiltrates. The elevation of the epithelium from the lamina propria was found only on the apical portion of the villi (after 24 h of ligation) (Figure 7).
Oxidative stress and NO determination in the duodenum tissue with BPC 157 revealed an increase in the duodenal NO values up to the normal values, but normal MDA-values compared to the excessively increased values seen in the controls (Figure 8).
L-NAME and L-arginine treatment alone decreased mucosal and serosal duodenal lesions at 5 min and 30 min. Their effects were less at 24 h, but they did not further influence the loss of the collateral vessels and branching. Together, L-NAME+L-arginine antagonized each other’s response at any treatment point, and thus, NO-related effects were seen. When treated with BPC 157, all SAPDV-ligated rats receiving L-NAME and/or L-arginine responded similarly to the rats treated with BPC 157 alone (Figures 2, 5 and 6).
Thus, it seems that BPC 157 rescues SAPDV failure and duodenum lesions, and its effect is related to the NO system.
We attempted to alleviate major venous obstruction and duodenal lesions in rats with stable gastric pentadecapeptide BPC 157 treatment and to investigate its relation to the NO system[1-10]. In duodenal lesion development, the occlusion of the SAPDV appears as the key failure (before other vessel occlusions: i.e., left colic artery and vein ligation, and inferior caval vein ligation)[15,16] that could never be spontaneously alleviated, even though well-placed vessels or additional therapy with NO agents [NOS-over-activation (L-arginine) and/or NOS-blockade (L-NAME)]. On the other hand, BPC 157 therapy resulted in promptly alleviated vascular presentation and then alleviated a perilous course of duodenal lesions in rats with SAPDV occlusions. We documented again “running” toward the bypassing vessel occlusion(s)[15,16] and that BPC 157 after SAPDV occlusion quickly restores the blood supply to the ischemically injured area. BPC 157 rapidly activates collaterals much like a fundamental treatment that counteracted the injurious course (ischemic colitis[15]; the syndrome resulting from inferior caval vein infrarenal-ligation[16]) and this effect involves the NO system and reduction of free radical formation[15,16].
The SAPDV-ligated rats treated with BPC 157 exhibited a complete reduction of the SAPDV-occlusion syndrome: improved vessel presentation, increased interconnections, duodenal arcade branching increased up to 60% from the initial value, and uncongested IAPDV and SMV. Thereby, BCP 157 treatment rescued the original duodenal flow through IAPDV to SMV flow and largely mitigated the duodenal lesions (Figures 2-6). The results provide consistent evidence for the beneficial effects of a rapid and sustained BPC 157 μg and ng regimen.
In addition, we clarified the distinction between BPC 157 and NO-agent activity. We assumed that a prolonged occlusion would result in a more severe lesion and that a persisting and powerful defensive process would oppose these injuries and assure long-lasting protection. Thus, BPC 157 therapy, along with the findings mentioned before[15,16], exhibited a special “bypassing” effect (through arcade vessels and/or minor and major vessels[15,16]) making vascular occlusion harmless and reorganizing the blood flow to counteract the duodenal lesions in rats with SAPDV-ligation. Similar to occlusion of the other vessels[15,16], this study indicates the early positive outcome (“bypassing” effect) of BPC 157 that may be crucial for its persisting beneficial effect, even with continuous occlusions. The effect of BPC 157 in rats with SAPDV-ligation and rats with left colic artery and vein ligations may indicate that the action of BPC 157 overlaps in the duodenum and colon[15,16]. BPC 157 plays an important role in with respect to the “bypassing” effect that maintains duodenum and colon mucosal integrity[15,16] and interacts with the NO system (L-NAME and L-arginine exhibited parallel effects (lesion worsening), which mitigated each other) in SAPDV, left colic artery and vein circulation, and duodenal and colon lesions[15,16]. Together, the “bypassing” effect combined with duodenum (and colon) mucosal integrity as the revealed phenomenon could be quite complex[15,16] as it was seen with respect to the L-NAME (NOS blockade) and L-arginine (NOS over-activity) treatments, which given alone and/or together (NO-system immobilized) never exhibited the “bypassing” effect and did not provide any mucosal protection[15,16]. However, when given together with BPC 157 (L-NAME+BC 157; L-arginine+BPC 157; L-NAME+L-arginine+BPC 157), with either SAPDV-ligation or left colic artery and vein ligation, the BPC 157 beneficial effect was observed, compensating either the L-NAME and L-arginine effects, while mucosal protection was seen at a higher level.
It is possible that the environment created by vessel(s) occlusion is responsible for the “bypassing” effect (SAPDV-duodenal arcade vessel interconnections-IAPDV-SMV required for adequate compensation and duodenal mucosal protection consistently obtained by BPC 157 administration) and remains outside the regular L-NAME or L-arginine influence on blood vessels but inside BPC 157 influence on NO-agent effects. L-NAME and L-arginine were given in doses necessary to instantly induce hypertension or hypotension[33], while both effects were counteracted by BPC 157 administration[33]. Providing a complex beneficial effect, BPC 157 did not affect normal blood pressure[33]; in addition to counteracting L-NAME induced hypertension and L-arginine induced hypotension[33], when BPC 157 counteracted arterial[34] or venous hypertension[16] or systemic hypotension[16], it also counteracted other disturbances, which may be related to blood pressure disturbances (i.e., potassium-overload induced arrhythmias[34]; Virchow’s triad[16]; chronic heart failure induced by doxorubicin in hypotensive rats and mice[35]; venous hypertension in systemically hypotensive rats with inferior caval vein occlusion[16]). Therefore, in SAPDV-ligated rats, venous occlusion alone causes severe congestion and increased intravascular pressure[26]; the vessels hampered with occlusions would fail to respond to either NO agent. On the other hand, this complete failure is not present in left colic artery and vein ligated rats[15] (arterial occlusion results only in reduced inflow perfusion with normal outflow[26], arteriovenous occlusion balances inflow and outflow alteration). Thus, the arteriovenous occlusion, intravascular pressure and tone equilibrium[26] (left colic artery and vein ligated rats[15]) still permits NO-agents activity, unlike the venous occlusion, severe congestion and increased intravascular pressure[26] (SAPDV-ligated rats). Syndrome in left colic artery and vein ligated rats[15] [the decrease (L-NAME) or increase (L-arginine) in vessels], but without the vessel interconnection and bypassing effect, worsening the mucosal lesions (even after the initial short-lasting protective effect) accords with the more severe syndrome in SAPDV-ligated rats (vessels unresponsive to NO-agents) (Figure 5).
The common failure to heal SAPDV or left colic artery and vein occlusions[15], parallelism much like in other models[15,35,36], substantiates particular aspect of a NO system dual (L-NAME vs L-arginine) role (vs combination) (for review, see[1-5]). Parallel L-NAME/L-arginine activity, which was noted as parallel outcomes, as a specific point (much like before[15,35,36], L-NAME and L-arginine regularly attenuated or antagonized each other’s responses, presenting values comparable to the control), also appeared with two pharmacologically distinct mechanisms with opposing effects on the same signaling pathway (for review, see[1-5]). In SAPDV-ligated rats, this parallelism is constant with also distinctive effects, lesion attenuation (early intervals) vs lesion worsening (late interval), unless BPC 157 had been administered and the beneficial effect always attenuated the lesion[15,35,36]. Likewise, if the NO system immobilized (mutual actions of the combined L-NAME and L-arginine), it still produces the severe pathology in the L-NAME+L-arginine animals. If this remained severe pathology is further attenuated, it means that the other system(s) (likely cholinergic and BPC 157) functioned along with. In this view, the particular role of BPC 157 reestablished effectiveness (L-NAME+L-arginine → L-NAME+L-arginine+BPC 157). It means that BPC 157 is effective (L-NAME+L-arginine+BPC 157; L-arginine+BPC 157; L-NAME+ BPC 157) with all of the NO-system presentations [inactivated (L-NAME + L-arginine); overstimulated (L-arginine); or blocked (L-NAME)][15,35,36]. Thus, the three distinct NO-endpoints (NO-immobilization; -over-activity; -blockade) should be overwhelmed to achieve vessel presentation seen in rats that underwent BPC 157 treatment[15,35,36]. BPC 157 may consolidate the NO system to produce more effective healing (i.e., the stimulatory and inhibitory effects of the NO system promoted the interconnection of arcade vessels to bypass major obstructions) (a result not observed with L-NAME or L-arginine treatment)[15].
This special BPC 157 role seems to be supported by the NO and MDA values seen in the duodenal tissues. The beneficial effects promptly counteract the full negative syndrome. Otherwise, after SAPDV-ligation, sudden decrease of blood supply appears. NO-levels decrease in the duodenum tissue. Heavy loss of endothelial cells occurs immediately from the vascular wall[37]. A lower eNOS production ability[37], oxidative stress appears as a result of the lysis of endothelial cells[38,39]; and excessive NO release generated by the inducible isozyme damaging the vascular wall and other tissues cells, especially in combination with reactive oxygen intermediates, and failing endothelial production[32,40,41]. The positive chain of events includes a BPC 157 treatment restored endothelial integrity and reversed most of the oxidative damage[15,16,31,32] while interacting with the NO system[1-5]. The positive endothelium syndrome (i.e., increased duodenal NO-values, but normal MDA-values, indicative of adequate eNOS system function[1-5]) appears with general significance, and thereby, restored endothelial integrity and reversed most of the oxidative damage. With BPC 157, this recovery appears even during reperfusion (occurring while vessel occlusion is still present) much like during exaggerated reperfusion (occurring when vessel occlusion is removed)[15].
There is evidence that BPC 157 also affects several other molecular pathways[16,42-47], and thereby, the rapid recruitment of existing blood vessels following ongoing harmful events instead otherwise poor response to increased demands. Combined, these findings may substantiate the effects of which were seen at the later time point. In addition, this may accentuate the original cytoprotection understanding (endothelium maintenance → epithelium maintenance)[17-21] to the complexity of reestablishing original flow by bypassing occlusion[15,16]. And thereby, we documented recovery of SAPDV-occlusion by AIPDV → SMV, or ICV-occlusion by LOV and other veins[16], or even two obstructions, the proximal and distal ligations in the left colic artery and vein through arcades within the ligations in rats with colitis[15]. Furthermore, its subsequent strong angiogenic effect and its healing effects[9,43-49]may be consequence of the specific activation of the collateral circulation that can circumvent obstructions and reestablish the continuity of blood flow[15,16]. And thereby, its angiogenic effect and its healing effects[9,43-49] may overcome those of standard anti-ulcer agents[48]. Also, these effects are likely to be further extended. BPC 157 accelerates the blood flow recovery and vessel number in rats with hindlimb ischemia[43]. BPC 157 upregulates VEGFR2 expression in rats with hindlimb ischemia and endothelial cell cultures and promotes VEGFR2 internalization in association with VEGFR2-Akt-eNOS activation[43]. As mentioned, shared limitation of activity[17-21,50], only prophylactic effectiveness of standard cytoprotective agents, is avoided. With both prophylactic and therapeutic abilities[1-10], native and stable in human gastric juice BPC 157 as a novel mediator of Robert’s cytoprotection[1-10] maintains gastrointestinal mucosal integrity[1-10]. In addition, the comparative effectiveness of BPC 157 when instilled into the stomach supports this contention of a prototype of a more effective class of cytoprotective agents.
In conclusion, this study provides evidence for a “bypassing” effect combined with mucosal integrity (i.e., duodenum and colon) or major vessel obstruction made harmless[15,16] as observed after BPC 157 treatment, which can be regarded as an implementation of the original cytoprotection concept in vascular occlusion therapy.
The research background was our recent claim that treatment with the prototype cytoprotective agent, stable gastric pentadecapeptide BPC 157, induced bypassing of occlusions in rats that underwent vessel occlusions through the rapid presentation of collaterals. In this study, we focused on the resolving of the duodenal lesions induced by major venous occlusions. These lesions can be counteracted by BPC 157 regardless of the involvement of the nitric oxide (NO) system while recruitment of blood vessels to bypass obstruction may occur quickly.
Research motivation was to resolve the major venous occlusions and duodenal lesions in the rat with the use of the stable gastric pentadecapeptide BPC 157 and/or NO-agents, L-NAME (NOS-blocker) and L-arginine (NOS-substrate). BPC 157 is a prototype cytoprotective agent used in ulcerative colitis and multiple sclerosis trials (LD1 not achieved) and is known to counteract duodenal lesions. Rat duodenal lesion research is mostly based on cysteamine and acetic acid models. Investigations are sparse on the impact of major venous obstruction and whether recruitment of blood vessels to bypass obstruction may occur quickly, and if so, whether it may be facilitated by a suitable therapy.
Research objectives were the occluded superior anterior pancreaticoduodenal vein (SAPDV) and duodenal lesions (congestion) and the recovering effect (rapidly activated collaterals bypassing vascular occlusion, absent lesions), the therapy effect of stable gastric pentadecapeptide BPC 157 vs harmful effect of NO-agents [L-NAME (NO-system-blockade), L-arginine (NO-system over-stimulation); L-NAME+L-arginine (NO-system immobilization)]. We recorded the vessel presentation (filled/appearance or emptied/disappearance) between the 5 arcade vessels arising from the SAPDV on the ventral duodenum side, the inferior anterior pancreaticoduodenal vein (IAPDV), superior mesenteric vein (SMV) as bypassing vascular pathway to document the duodenal lesions presentation. In duodenum, BPC 157 normalizes NO-levels and counteracts increased oxidative stress [malondialdehyde (MDA)]-levels.
For research methods, we used rats with the occluded SAPDV and duodenal lesions (congestion) to reveal the recovering effect (rapidly activated collaterals bypassing vascular occlusion, absent lesions), at 5 min, 30 min and 24 h ligation time. Therapy effect of the stable gastric pentadecapeptide BPC 157 was achieved with two regimens (10 μg, 10 ng/kg per 1 mL bath/rat or 10 μg/kg instilled into the rat stomach), at 1 min ligation-time. Harmful effect of NO-agents [L-NAME (NO-system-blockade), L-arginine (NO-system over-stimulation); L-NAME+L-arginine (NO-system immobilization)] goes with L-NAME 5 mg/kg per 1 mL bath/rat; L-arginine 100 mg/kg per 1 mL bath/rat, alone and/or together. Considering the point immediately before therapy (as 100%), we scored the vessel presentation [recorded filled/appearance or emptied/disappearance (camera attached to a USB microscope)] between the 5 arcade vessels arising from the SAPDV on the ventral duodenum side, the IAPDV, SMV as bypassing vascular pathway. Upon the duodenum opening and after sacrifice, we assessed the congested hemorrhagic areas as the sum of the largest lesion diameters. In the collected duodenal tissue samples, we assessed NO-levels (Griess reaction) and oxidative stress [by quantifying thiobarbituric acid-reactive species (TBARS) as MDA equivalents].
For research results, we attempted to alleviate major venous obstruction and duodenal lesions in rats with stable gastric pentadecapeptide BPC 157 treatment and to investigate its relation to the NO system. In duodenal lesion development, the occlusion of the SAPDV appears as the key failure (before other vessel occlusions: i.e., left colic artery and vein ligation, and inferior caval vein ligation) that could never be spontaneously alleviated, even though well-placed vessels or additional therapy with NO agents [NOS-over-activation (L-arginine) and/or NOS-blockade (L-NAME)]. On the other hand, BPC 157 therapy resulted in promptly alleviated vascular presentation and then alleviated a perilous course of duodenal lesions in rats with SAPDV occlusions. We documented again “running” toward the bypassing vessel occlusion(s) and that BPC 157 after SAPDV occlusion quickly restores the blood supply to the ischemically injured area. BPC 157 rapidly activates collaterals much like a fundamental treatment that and counteracted the injurious course (ischemic colitis; the syndrome resulting from inferior caval vein infrarenal-ligation) and this effect involves the NO system and reduction of free radical formation.
The new phenomena that were found through experiments in this study of the duodenal lesions with an obstructed major vein (SAPDV) offered is the early positive outcome (“bypassing” effect) of BPC 157. The hypotheses that were confirmed through experiments in this study are related to the cytoprotective agents, to the effects originally noted in the stomach, which is the rapid endothelial protection. Using BPC 157 as a prototype cytoprotective agent, these mechanisms can prevent and resolve adjacent ischemic mucosal lesions providing activation of the collateral circulation. This activation is specific and can circumvent obstructions. That effect may be crucial for its persisting beneficial effect, even with continuous occlusions. The effect of BPC 157 in rats with SAPDV-ligation and previously, in rats with left colic artery and vein ligations may indicate that the action of BPC 157 overlaps in the duodenum and colon. BPC 157 plays an important role in with respect to the “bypassing” effect that maintains duodenum and colon mucosal integrity and interacts with the NO system [L-NAME and L-arginine exhibited parallel effects (lesion worsening), which mitigated each other] in SAPDV, left colic artery and vein circulation, and duodenal and colon lesions. Thus, these findings can be regarded as an implementation of the original cytoprotection concept in vascular occlusion therapy.
For research perspectives, we attempted to alleviate major venous obstruction and duodenal lesions in rats with stable gastric pentadecapeptide BPC 157 treatment and to investigate its relation to the NO system. In duodenal lesion development, the SAPDV occlusion appears as the key failure that could never be spontaneously alleviated, even though well-placed vessels or additional therapy with NO agents [NOS-overactivation (L-arginine) and/or NOS-blockade (L-NAME)]. Finally, BPC 157 therapy results in “bypassing” effect combined with mucosal integrity, or major vessel obstruction made harmless. These BPC 157 effects can be an implementation of the original cytoprotection concept in vascular occlusion therapy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fu TL, Vukojević J S- Editor: Ma RY L- Editor: A E- Editor: Huang Y
| 1. | Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, Sever M, Zoricic I, Zoricic Z, Batelja L. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des. 2017;23:4012-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, Grgic T, Strbe S, Zukanovic G, Crvenkovic D. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr Neuropharmacol. 2016;14:857-865. [PubMed] |
| 3. | Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, Zenko A, Drmic D, Rucman R, Sikiric P. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121-1125. [PubMed] |
| 4. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126-1135. [PubMed] |
| 5. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76-83. [PubMed] |
| 6. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126-132. [PubMed] |
| 7. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Sikiric P, Seiwerth S, Brcic L, Blagaic AB, Zoricic I, Sever M, Klicek R, Radic B, Keller N, Sipos K. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006;14:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Sikirić P, Petek M, Rucman R, Seiwerth S, Grabarević Z, Rotkvić I, Turković B, Jagić V, Mildner B, Duvnjak M. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J Physiol Paris. 1993;87:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Szabo S, Vincze A, Sandor Z, Jadus M, Gombos Z, Pedram A, Levin E, Hagar J, Iaquinto G. Vascular approach to gastroduodenal ulceration: new studies with endothelins and VEGF. Dig Dis Sci. 1998;43:40S-45S. [PubMed] |
| 12. | Szabo S. Pathogenesis of duodenal ulcer disease. Lab Invest. 1984;51:121-147. [PubMed] |
| 14. | Okabe S, Amagase K. An overview of acetic acid ulcer models--the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321-1341. [PubMed] |
| 15. | Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, Gojkovic S, Krezic I, Vidovic T, Bilic Z. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J Gastroenterol. 2017;23:8465-8488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Vukojević J, Siroglavić M, Kašnik K, Kralj T, Stanćić D, Kokot A, Kolarić D, Drmić D, Sever AZ, Barišić I. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol. 2018;106:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Szabo S. Mechanisms of Mucosal Protection. Gastric Cytoprotection. Boston: Springer 1989; 49-73. |
| 18. | Szabo S, Trier J. Pathogenesis of acute gastric mucosal injury: Sulfhydrils as a protector, adrenal cortex as a modulator, and vascular endothelium as a target. Mechanism of mucosal protection in the upper gastrointestinal tract. New York: Raven 1984; 387-393. |
| 19. | Trier JS, Szabo S, Allan CH. Ethanol-induced damage to mucosal capillaries of rat stomach. Ultrastructural features and effects of prostaglandin F2 beta and cysteamine. Gastroenterology. 1987;92:13-22. [PubMed] |
| 20. | Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228-236. [PubMed] |
| 21. | Sikiric P, Seiwerth S, Grabarevic Z, Petek M, Rucman R, Turkovic B, Rotkvic I, Jagic V, Duvnjak M, Mise S. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994;54:PL63-PL68. [PubMed] |
| 22. | Turhan A, Konerding MA, Tsuda A, Ravnic DJ, Hanidziar D, Lin M, Mentzer SJ. Bridging mucosal vessels associated with rhythmically oscillating blood flow in murine colitis. Anat Rec (Hoboken). 2008;291:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Konerding MA, Turhan A, Ravnic DJ, Lin M, Fuchs C, Secomb TW, Tsuda A, Mentzer SJ. Inflammation-induced intussusceptive angiogenesis in murine colitis. Anat Rec (Hoboken). 2010;293:849-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Ravnic DJ, Konerding MA, Tsuda A, Huss HT, Wolloscheck T, Pratt JP, Mentzer SJ. Structural adaptations in the murine colon microcirculation associated with hapten-induced inflammation. Gut. 2007;56:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Albadawi H, Witting AA, Pershad Y, Wallace A, Fleck AR, Hoang P, Khademhosseini A, Oklu R. Animal models of venous thrombosis. Cardiovasc Diagn Ther. 2017;7:S197-S206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Guzmán-de la Garza FJ, Cámara-Lemarroy CR, Alarcón-Galván G, Cordero-Pérez P, Muñoz-Espinosa LE, Fernández-Garza NE. Different patterns of intestinal response to injury after arterial, venous or arteriovenous occlusion in rats. World J Gastroenterol. 2009;15:3901-3907. [PubMed] |
| 27. | Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, Sever M, Holjevac J, Radic B, Turudic T. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64:597-612. [PubMed] |
| 28. | Mise S, Tonkic A, Pesutic V, Tonkic M, Mise S, Capkun V, Batelja L, Blagaic AB, Kokic N, Zoricic I. The presentation and organization of adaptive cytoprotection in the rat stomach, duodenum, and colon. Dedicated to André Robert the founder of the concept of cytoprotection and adaptive cytoprotection. Med Sci Monit. 2006;12:BR146-BR153. [PubMed] |
| 29. | Bedekovic V, Mise S, Anic T, Staresinic M, Gjurasin M, Kopljar M, Kalogjera L, Drvis P, Boban Blagaic A, Batelja L. Different effect of antiulcer agents on rat cysteamine-induced duodenal ulcer after sialoadenectomy, but not gastrectomy. Eur J Pharmacol. 2003;477:73-80. [PubMed] |
| 30. | Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, Zoricic I, Sever M, Seiwerth S, Sikiric P. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC 157 on gastrointestinal, liver and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 hour-short-bowel rats. PLoS One. 2016;11:e0162590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Belosic Halle Z, Vlainic J, Drmic D, Strinic D, Luetic K, Sucic M, Medvidovic-Grubisic M, Pavelic Turudic T, Petrovic I, Seiwerth S. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Luetic K, Sucic M, Vlainic J, Halle ZB, Strinic D, Vidovic T, Luetic F, Marusic M, Gulic S, Pavelic TT. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology. 2017;25:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, Turković B, Rotkvić I, Mise S, Zoricić I. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol. 1997;332:23-33. [PubMed] |
| 34. | Barisic I, Balenovic D, Klicek R, Radic B, Nikitovic B, Drmic D, Udovicic M, Strinic D, Bardak D, Berkopic L. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept. 2013;181:50-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Lovric-Bencic M, Sikiric P, Hanzevacki JS, Seiwerth S, Rogic D, Kusec V, Aralica G, Konjevoda P, Batelja L, Blagaic AB. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J Pharmacol Sci. 2004;95:19-26. [PubMed] |
| 36. | Medvidovic-Grubisic M, Stambolija V, Kolenc D, Katancic J, Murselovic T, Plestina-Borjan I, Strbe S, Drmic D, Barisic I, Sindic A. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology. 2017;25:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 37. | Berra-Romani R, Avelino-Cruz JE, Raqeeb A, Della Corte A, Cinelli M, Montagnani S, Guerra G, Moccia F, Tanzi F. Ca2+-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13 Suppl 2:S40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Schiller HJ, Reilly PM, Bulkley GB. Tissue perfusion in critical illnesses. Antioxidant therapy. Crit Care Med. 1993;21:S92-102. [PubMed] |
| 39. | Rangan U, Bulkley GB. Prospects for treatment of free radical-mediated tissue injury. Br Med Bull. 1993;49:700-718. [PubMed] |
| 40. | Matthys KE, Bult H. Nitric oxide function in atherosclerosis. Mediators Inflamm. 1997;6:3-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Yousefipour Z, Ranganna K, Newaz MA, Milton SG. Mechanism of acrolein-induced vascular toxicity. J Physiol Pharmacol. 2005;56:337-353. [PubMed] |
| 42. | Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, Brcic L, Rokotov DS, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JS. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl). 2017;95:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, Mu N, Gu J, Zhang W, Wang Y. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485-2499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066-19077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985). 2011;110:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Sikiric P, Separovic J, Anic T, Buljat G, Mikus D, Seiwerth S, Grabarevic Z, Stancic-Rokotov D, Pigac B, Hanzevacki M. The effect of pentadecapeptide BPC 157, H2-blockers, omeprazole and sucralfate on new vessels and new granulation tissue formation. J Physiol Paris. 1999;93:479-485. [PubMed] |
| 49. | Brcic L, Brcic I, Staresinic M, Novinscak T, Sikiric P, Seiwerth S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J Physiol Pharmacol. 2009;60 Suppl 7:191-196. [PubMed] |
| 50. | Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761-767. [PubMed] |