Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5095
Peer-review started: September 30, 2018
First decision: October 23, 2018
Revised: October 31, 2018
Accepted: November 9, 2018
Article in press: November 9, 2018
Published online: December 7, 2018
Processing time: 67 Days and 19.7 Hours
To identify the effect of hydrogen-rich water (HRW) and electrolyzed-alkaline water (EAW) on high-fat-induced non-alcoholic fatty acid disease in mice.
Mice were divided into four groups: (1) Regular diet (RD)/regular water (RW); (2) high-fat diet (HFD)/RW; (3) RD/EAW; and (4) HFD/EAW. Weight and body composition were measured. After twelve weeks, animals were sacrificed, and livers were processed for histology and reverse-transcriptase polymerase chain reaction. A similar experiment was performed using HRW to determine the influence and importance of molecular hydrogen (H2) in EAW. Finally, we compared the response of hepatocytes isolated from mice drinking HRW or RW to palmitate overload.
EAW had several properties important to the study: (1) pH = 11; (2) oxidation-reduction potential of -495 mV; and (3) H2 = 0.2 mg/L. However, in contrast to other studies, there were no differences between the groups drinking EAW or RW in either the RD or HFD groups. We hypothesized that the null result was due to low H2 concentrations. Therefore, we evaluated the effects of RW and low and high HRW concentrations (L-HRW = 0.3 mg H2/L and H-HRW = 0.8 mg H2/L, respectively) in mice fed an HFD. Compared to RW and L-HRW, H-HRW resulted in a lower increase in fat mass (46% vs 61%), an increase in lean body mass (42% vs 28%), and a decrease in hepatic lipid accumulation (P < 0.01). Lastly, exposure of hepatocytes isolated from mice drinking H-HRW to palmitate overload demonstrated a protective effect from H2 by reducing hepatocyte lipid accumulation in comparison to mice drinking regular water.
H2 is the therapeutic agent in electrolyzed-alkaline water and attenuates HFD-induced nonalcoholic fatty liver disease in mice.
Core tip: In this work, we compared the effects of two functional waters: Electrolyzed alkaline water and Hydrogen-rich water in a high-fat-diet-induced nonalcoholic fatty liver disease (NAFLD) mouse model. Hydrogen-rich water (HRW) has potential for NAFLD treatment by attenuating hepatic lipid accumulation, inflammation, and CD36 expression. However, neither electrolyzed-alkaline water (EAW) nor HRW with a low H2 concentration had protective effects on NAFLD. Additionally, we demonstrated that H2 pretreatment has a protective effect by modifying gene expression. The results demonstrate that H2 has a surprisingly positive impact in preventing NAFLD in mice and is also the key agent responsible in EAW for these benefits.
- Citation: Jackson K, Dressler N, Ben-Shushan RS, Meerson A, LeBaron TW, Tamir S. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet nonalcoholic fatty liver disease mouse model. World J Gastroenterol 2018; 24(45): 5095-5108
- URL: https://www.wjgnet.com/1007-9327/full/v24/i45/5095.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i45.5095
Nonalcoholic fatty liver disease (NAFLD) is considered the hepatic expression of metabolic syndrome, and in most cases, it is associated with obesity, dyslipidemia, diabetes, and insulin resistance[1]. At present, NAFLD is considered the most common liver disease affecting 20%-30% of the Western World’s population[1]. The spectrum of NAFLD ranges from simple, apparently benign, hepatic lipid accumulation (simple steatosis) to nonalcoholic steatohepatitis (NASH)[2]. NASH is characterized by inflammation and collagen deposition (fibrosis)[2] and may progress to hepatic tissue damage (cirrhosis)[3]. Generally, the disease remains in the steatotic stage, characterized by an excessive accumulation of triglycerides in the hepatocytes. However, in about 5% to 10% of cases, the disease will progress to NASH, and from there, 10%-25% will develop cirrhosis with about 1% of those patients progressing to hepatocellular carcinoma[4]. The disease’s pathogenesis is complex and multifactorial and involves genetic and environmental factors, including altered gut microbiome as part of the multistep pathogenic NAFLD model[4]. Insulin resistance is a key factor in the disease onset resulting in increased hepatic de novo lipogenesis (DNL), adipose tissue lipolysis, and inhibition of free fatty acid (FFA) β-oxidation. The reaction of the liver to intracellular lipid buildup results in a cascade of events, including oxidative stress, mitochondrial and endoplasmic reticulum dysfunction, and inflammation[5].
Lifestyle changes that promote weight loss improve disease status; however, this is often difficult to maintain in the long term[6]. At present, there is no specific pharmacological treatment for this disease as most drugs indicated for NAFLD target secondary features of the disease such as obesity, dyslipidemia, and insulin resistance[7]. Due to the high prevalence and increasing NAFLD incidence coupled with the current treatment’s ineffectiveness and health risks, the need for a simple and safe alternative is warranted. Regardless of the factors, NAFLD pathogenesis and progression are linked to excessive oxidative stress and inflammation, the attenuation of which may be a viable approach[8].
Hydrogen-rich and electrolyzed alkaline waters (HRW and EAW, respectively) have been reported as types of functional waters that may ameliorate various disease conditions[9]. EAW is produced via electrolysis of water. At the cathode (equation 1), water is reduced to hydrogen gas/molecular hydrogen (H2) and hydroxide ions (OH-). The OH- ions cause an increase in the resulting water’s pH making it more alkaline. At the anode (equation 2), water is oxidized to oxygen gas (O2) and protons (H+). The increased hydrogen ion (H+) concentration makes the water acidic. EAW units have a membrane that separates the cathode and anode compartments, without which the resulting pH would be neutral (equation 3).
Cathode reaction: 4H2O (l) + 4e- → 2H2 (g) + 4OH- (aq) Equation 1
Anode reaction: 6H2O (l) + 4e- → + O2 (g) + 4H3O+ (aq) Equation 2
Overall reaction: 2H2O (l) → 2H2 (g) + O2 (g) Equation 3
EAW has been reported to have anti-obesity, anti-oxidant, anti-diabetic, and hepatoprotective effects[10-13]. However, the properties of EAW associated with these beneficial effects have been debated[11-14]. EAW exhibits a negative oxidation-reduction potential (ORP) due to the dissolved hydrogen gas and high pH[11], which is expected according to the Nernst equation. Nevertheless, H2 is not recognized by some researchers/commercial companies as the principal therapeutic agent in EAW[15].
H2 has recently been demonstrated to exert therapeutic benefits in animal and human clinical studies in ameliorating excessive inflammation and oxidative stress[16]. It was first confirmed to have therapeutic potential in animals for cancer (hyperbaric chamber)[17] and ischemia-reperfusion (inhalation)[18] in studies published in Science and Nature Medicine, respectively. H2’s potential therapeutic effects have now been confirmed in over 170 different human and animal-disease models and in essentially every organ of the human body[19]. H2 is nonpolar, hydrophobic, and the smallest molecule, thus allowing it to quickly diffuse through cell membranes and reach the mitochondria, nucleus, endoplasmic reticulum, and other subcellular compartments[20]. These properties make it an attractive molecule for NAFLD treatment[21]. Although more research is needed to elucidate the molecular mechanism(s) and optimal dosing for H2, preliminary animal and human studies are promising. Clinical studies of drinking hydrogen-rich water (HRW) have demonstrated beneficial effects in several diseases such as Parkinson’s disease, type II diabetes, rheumatoid arthritis, mitochondrial myopathies, muscle fatigue, metabolic syndrome, hyperlipidemia, liver inflammation (hepatitis B) and others reviewed previously[19,22-30]. Clinical studies involving metabolic and liver conditions further support the potential benefits of hydrogen on NAFLD.
Several animal and cells studies concerning NAFLD and hydrogen therapy have been reported using different models. For example, one study[31] using hydrogen-rich saline in a NAFLD rat model induced with hyperglycemia and hyperlipidemia, found H2 significantly lowered levels of oxidative stress and inflammation[31]. In another study using a methionine-choline-deficient diet-induced NASH model, ingestion of HRW significantly attenuated steatohepatitis to a degree comparable to the drug pioglitazone and suppressed hepatic tumorigenesis in a streptozotocin-induced NASH-related hepatocarcinogenic mouse model[32]. Similar positive effects of H2 have also been reported in other studies[33,34]. In the present study, we used a different NAFLD animal model induced by a high-fat diet (HFD) which is more relevant to human physiology.
We aimed to determine the influence of both types of functional waters, EAW and HRW, on an HFD-induced-NAFLD model, and the importance of H2 as a therapeutic factor in EAW. Furthermore, we investigated if pretreatment with HRW could protect cells from further exposure to a high-fat environment.
Animal protocols were approved by the Israeli National Committee for Animal Ethics and Welfare. In all experiments, animals had ad libitum access to food and water.
Forty-eight C57bl/6J young male mice (three weeks old, approximately 20 g) were obtained from Harlan Laboratories (Israel). The mice were randomly separated into conventional polycarbonate rodent cages (six animals per cage) and fed a regular diet (RD), which was a commercial purified control diet composed of 17% calories from fat, 23% calories from protein, and 60% calories from carbohydrates (TD 120455, Harlan Laboratories, United States). Conventional polycarbonate bottles with a stainless-steel cap were put on the top of each cage. Cages were maintained at 23 °C ± 1 °C and 40%-60% humidity under a 12:12 h light:dark cycle. During this time, animals were weighed twice a week to check that there were no differences in the initial growth rate.
After a 2-week acclimation period, mice were divided into four groups (n = 12) according to the type of diet and water: (1) RD/ Regular Water (RW); (2) RD/EAW; (3) HFD/RW; and (4) HFD/EAW. Mice were individually identified by ear punching. The HFD was a purified commercial diet for inducing obesity (TD 06414, Harlan Laboratories, United States) and was composed of 60.3% calories from fat, 21.3% from carbohydrates, and 18.4% from protein. Formula and specific components of both the RD and HFD are shown in Table 1.
| Ingredients | Ingredient concentration (g/kg) | |
| Regular diet | High fat diet | |
| Casein | 210.0 | 265.0 |
| L-cystine | 3.0 | 4.0 |
| High amylose corn starch | 500.0 | - |
| Maltodextrin | 100.0 | 160.0 |
| Sucrose | 39.14 | 90.0 |
| Anhydrous milkfat | 20.0 | - |
| Lard | 20.0 | 310.0 |
| Soybean oil | 20.0 | 30.0 |
| Cellulose | 35.0 | 65.5 |
| Mineral mix, AIN-93G-MX (94046) (g/kg) | 35.0 | 48.0 |
| Calcium phosphate, dibasic | - | 3.4 |
| Vitamin mix, AIN-93-VX (94047) | 15.0 | 21.0 |
| Choline bitartrate | 2.75 | 3.0 |
| TBHQ, antioxidant | 0.01 | - |
| Protein (% by weight) | 18.6 | 23.5 |
| Carbohydrate (% by weight) | 50.6 | 27.3 |
| Fat (% by weight) | 6.2 | 34.3 |
EAW was prepared with a Water Ionizer Batch System (BTM-3000, BionTech, Korea). Commercial mineral water (initial pH 7.8) was electrolyzed continuously for 3 h, and the water at the cathode was collected (pH 11 ± 0.48, ORP of -495 ± 27 mV, H2 ≈ 0.2 mg/L). The water was transferred to feeding bottles and added to the mice’s cages. Fresh electrolyzed water was prepared twice a week.
Body weight was measured weekly at 9 AM before the morning feeding using an Ohaus Scout Pro 200 g scale (Nänikon Switzerland). Body composition: fat mass (FM), fat-free mass (FFM), and extracellular fluid were measured using time-domain nuclear magnetic resonance (Minispec Analyst AD; Bruker Optics, Silberstreifen, Germany) following a 12 h fast at the end of the acclimation phase and during the experiment at six and 12 wk.
At the end of the experimental period, animals were sacrificed using inhaled isofluorane, and livers were processed for histology and real-time polymerase chain reaction (RT-PCR).
Twenty-Four C57bl/6J males (three weeks old, approximately 20 g) were obtained from Harlan Laboratories (Israel). Mice were separated into appropriate cages and fed a RD for two weeks. After this acclimation period, all animals were fed a HFD ad libitum. Mice were then divided into three groups according to the type of water: (1) Group 1 (control group) received regular water; (2) the second group received hydrogen-rich water at low concentration (L-HRW); and (3) the third group received hydrogen-rich water at high concentration (H-HRW). Mice were individually identified by ear punching. Body weight, food consumption, and fluid intake were measured weekly, and at the end of the experiment, livers were processed for histology, RT-PCR and quantification of total fat were done following the protocol by Roopchand et al[35].
HRW was prepared using a sachet made of a net cloth containing metallic magnesium (Sigma, St. Louis, United States; 98% turnings) and small natural stones. The sachet was immersed in closed glass bottles containing cold water (4 °C) that was previously acidified to pH 2.0 with 37% hydrochloride acid (Sigma). The water was vortexed for 1 h, during which time H2 was produced according to the reaction: Mg + 2H2O → Mg(OH)2 + H2. The final pH of the solution reached ≈ 11.0 and contained dissolved hydrogen at a concentration of 1.2 mg/L. The solution was then diluted with regular water to make high (0.8 mg/L) and low (0.3 mg/L) concentrations of HRW each at a pH of approximately 8.0.
Each solution was transferred to mice-feeding bottles in which a new magnesium sachet was added. Due to the alkaline pH of the water, the amount of H2 produced via the above reaction was minimal (the rate of escape ≈ rate of production) but was enough to maintain the low and high H2 concentration for 24 h. Fresh HRW was produced every other day. The sachets kept in the feeding bottles were rinsed with 5% acetic acid followed by RW every time the water was changed. The H2 concentration in water was measured daily.
The hydrogen concentration in water was determined with H2Blue (H2Sciences Inc., United States), which is a redox titration reagent composed of methylene blue and a colloidal platinum catalyst[36].
After dissection, the liver was cut into small pieces, immersed in 4% paraformaldehyde at room temperature for 24 h, and then stored at 4 °C for 24 h. Samples were dehydrated with ethanol and cleared with xylene and embedded in paraffin. Five-micrometer sections were cut and stained with Hematoxylin and Eosin (HE). Pictures of 4-6 different fields per sample were taken under an Axiovert40-CFL (Zeiss, Germany) microscope equipped with a digital camera (AxioCam MRC, Germany).
Total RNA was extracted using Tri-Reagent (T-9424, SIGMA). One microgram of RNA from each sample was reverse transcribed into cDNA using the Verso cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative RT-PCR was performed in quadruplicate in 384-well plates using the ABI Prism® 7900HT sequence detection system (Applied Biosystems). Each well contained 2 μL of cDNA template (diluted X 20 after the RT reaction), 2.5 μL SYBR green (Bio-Rad, United States), and 0.25 μL each of both forward and reverse gene-specific primers at 10 μmol/L (primers are listed in Table 2). Beta-2 microglobulin (β2m) and β-Actin were used as endogenous reference genes for normalization. The thermal profile was 95 °C for 1 min followed by 40 amplification cycles of 95 °C for 15 s and 60 °C for 30 s. After that melting curve analysis was done. The resulting Ct values were used to determine the relative gene expression.
| Gene marker | Full name | Primer sequence (5'-3') |
| β-Actin | Beta-actin | GCCCTGGACTTCGAGCAAGA |
| TGCCAGGGTACATGGTGGTG | ||
| β-2m | Beta-2 microglobulin | TGCTTGTCTCAC TGACCGGCC |
| TGGGGGTGAATTCAGTGTGAGCC | ||
| SOD2 | Super oxide dismutase 2 | ACAACAGGCCTTATTCCGCT |
| CCCCAGTCATAGTGCTGCAA | ||
| SREBP-1c | Sterol regulatory element Binding protein | CACCCTGTAGGTCACCGTTT |
| AGAACTCCCTGTCTCCGTCA | ||
| ACC1 | Acetyl-CoA | GACAGAGGAAGATGGCGTCC |
| Carboxylase | TACAACTTCTGCTCGCTGGG | |
| TNF-α | Tumor necrosis | CGTCGTAGCAAACCACCAAG |
| Factor-alpha | AGCAAATCGGCTGACGGTG | |
| CRP | C-Reactive Protein | ACTGTGGGGCCAGATGCAAGC |
| GGGGCTGAGTGTCCCACCAAC | ||
| CAT | Catalase | TCACTCAGGTGCGGACATTC |
| TAGTCAGGGTGGACGTCAGT | ||
| IL-6 | Interleukin-6 | TCTATACCACTTCACAAGTCGGA |
| GAATTGCCATTGCACAACTCTTT | ||
| Adipo | Adiponectin | GACGACACCAAAAGGGCTCA |
| GAGGCCATCTCTGCCATCA | ||
| AdipoR2 | Adiponectin | CTCTGACAGGATTTGGGGTCAA |
| Receptor 2 | GTGCCCTTTTCTGAGCCGTA |
Hepatocytes from mice that drank H-HRW or RW were isolated by perfusion to test if consumption of H-HRW had a long-term effect.
Twelve C57bl/6J male mice (three weeks old) were obtained from Harlan Laboratories (Israel). Mice were divided into two groups: (1) One that drank HRW (0.8 mg/L) and (2) the second that drank RW; both groups were fed a regular diet. After four weeks, hepatocytes (one mouse from each group) were isolated using perfusion under anesthesia through the portal vein according to Zhang et al[37].
The primary hepatocytes were isolated through a two-step collagenase perfusion system as described in a previous report[38]. Isolated hepatocytes from each mouse were seeded in 6-well plates (106 cells/wells) for measuring gene expression, and in 12-well plates (200000 cells/well) for the steatosis assay in William’s E medium (Sigma, St. Louis, United States) containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, United States). Cells obtained from each mouse were seeded in a different plate. After 3 h, the medium was replaced with fresh medium without FBS, and the plates were prepared for further processing.
Cells were exposed overnight to 0.3 mmol/L palmitate-BSA conjugate or BSA as a control (in quadruplicate). Preparation of the palmitate stock solution was carried out as described previously[39]. Briefly, 20-mM palmitate (Sodium Palmitate, SIGMA) stock solution was prepared in 0.01 mol/L NaOH by heating at 80 °C. A 2 mmol/L FFA-free BSA (Sigma) solution was prepared in ddH2O and maintained at 37 °C in a water bath. A 4 mmol/L FFA/1% BSA (5:1) solution was obtained by complexing the appropriate amount of palmitate stock solution to the BSA at 50 °C for another 15 min.
After overnight incubation with BSA-palmitate, the medium was removed, and cells were stained with Oil-Red-O to examine fat accumulation (steatosis)[40]. Plates were washed with cold phosphate-buffered saline and fixed in 4% paraformaldehyde for 1 h. After two changes of 70% ethanol, plates were rinsed in distilled water and stained for 30 min by Oil-Red-O and then placed to dry at room temperature. The stain collected by the cells was then extracted with 100% isopropanol. Steatosis was estimated according to the extracted oil red concentration (determined by measuring the absorbance at 520 nm). The steatosis percentage was determined by dividing the average OD value of experimental wells (treated with palmitate) by the average OD value of control cells (treated with BSA only) × 100. In each assay, two plates (HRW or RW) were processed in parallel.
Six-well plates containing 1 × 106 cells were used. The cells were exposed to 0.3 mmol/L bovine serum albumin-palmitate conjugate for 4 h, after which RNA was extracted from cells as described above.
Data are expressed as mean values ± SD. Data were analyzed by Kruskal-Wallis nonparametric test followed by a Mann-Whitney nonparametric test. A P-value ≤ 5% is considered statistically significant. The data were analyzed using the SPSS version 24 (SPSS Inc., Chicago, IL, United States). The statistical methods of this study were reviewed by Ms. Adi Sharabi-Nov of Tel -Hai Academic College.
Water and food intake were measured for the entire group (n = 12) and calculated for each mouse once per week. As expected, mice fed an HFD showed a high-calorie intake when compared to mice fed chow (10.65 kcal/mouse/d vs 8.30 kcal/mouse/d, P < 0.01). Daily water intake was similar in mice drinking RW and EAW (2.49 mL/ mouse).
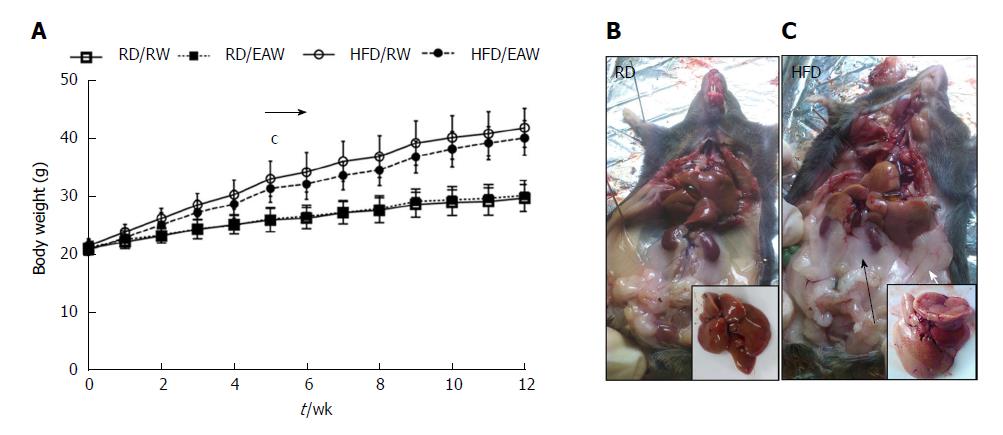
Mice fed an HFD had higher body weights compared to RD mice. Significant body weight differences were observed from the fifth week until the end of the experiment (Figure 1). At the end of 12 wk, the weight was 72% greater in HFD mice than in RD mice (P < 0.001). The differences in final body weight were due to an increase in fat mass. Larger retroperitoneal and epidydimal fat pads were detected in HFD mice. The HFD also affected liver morphology. Livers from mice maintained on a regular diet were dark red/brown and showed a clear and homogeneous texture. Livers from mice fed an HFD were light brown and showed a heterogenic flocculent texture. There was no difference in the weight of mice drinking RW or EAW in either group.
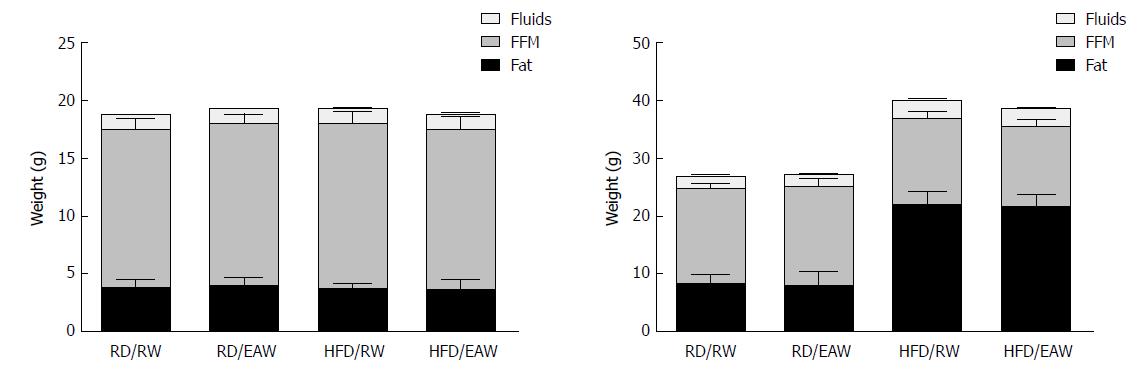
Body composition measurements based on NMR are shown in Figure 2. After 12 wk on different diets, the mice fed a HFD contained more fat (21.7 g ± 2.5 g) than RD mice (8.1 g ± 1.7 g. (P < 0.001). However, FFM was similar in both groups (16.5 g ± 0.8 g and 15.1 g ± 1.2 g in RD and HFD, respectively). EAW did not affect body composition of mice in either the RD or the HFD group.
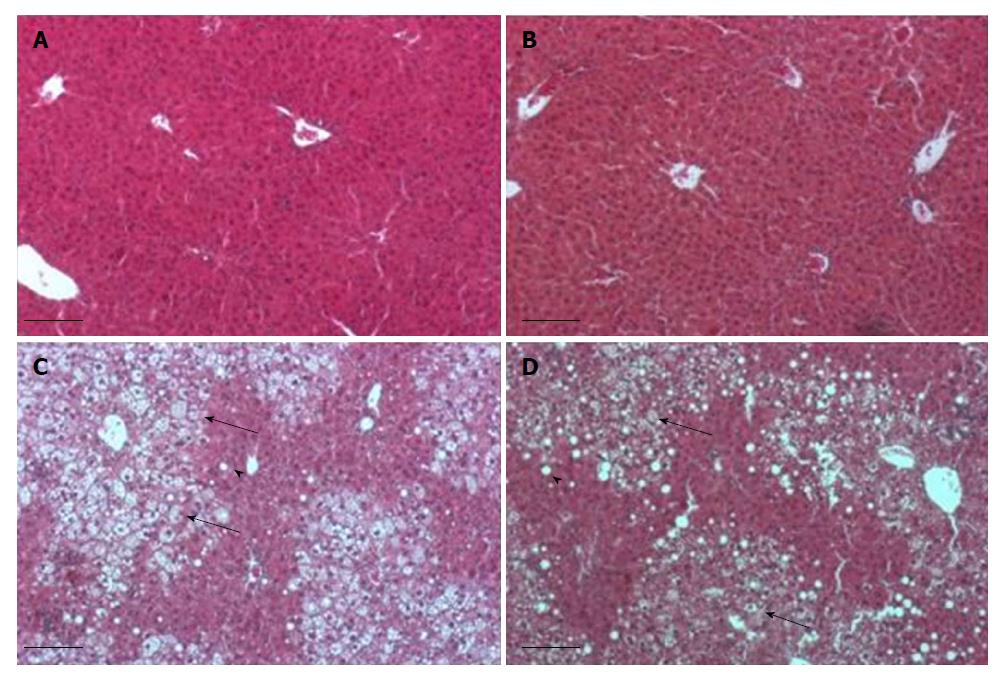
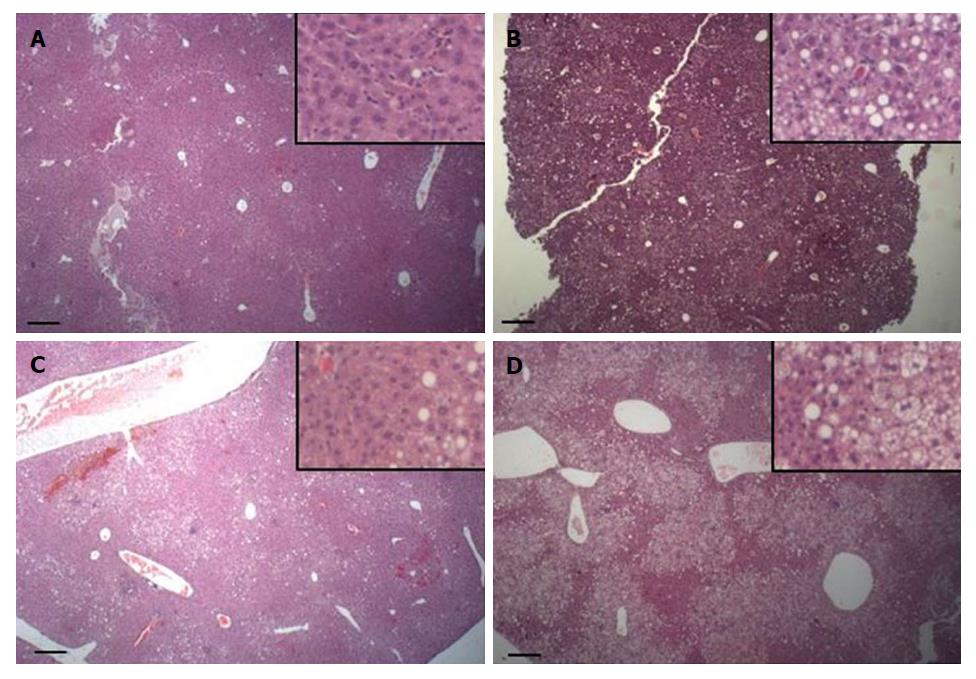
The HE stained sections from livers are shown in Figure 3. Most of the liver parenchyma of HFD mice (Figure 3C and D) was steatotic with many enlarged cells, which were distributed among the remaining normal parenchyma. The enlarged hepatocytes showed micro- and macrovesicular steatosis. RD-fed mice presented liver parenchyma with no signs of steatosis (Figure 3A and B). EAW water did not affect the liver histology in the RD group or the HFD group.
To evaluate the impact of HRW on NAFLD, we used mice fed an HFD only. Mice were separated into three groups according to the type of water. Control group received RW, and experimental groups received HRW at either low (HRW-L, 0.3 mg/L) or high (HRW-H, 0.8 mg/L) concentration. After 12 wk, mice were sacrificed, and liver histology and gene expression were analyzed. Body weight and composition were measured throughout the experiment.
Average water consumption was significantly higher for H-HRW group (8.83 mL/mouse/d) than for L-HRW group (3.66 mL/mouse/d), and control group (3.34 mL/mouse/d, P = 0.012). However, the differences in water consumption did not affect appetite and food consumption. All three groups had the same caloric intake consisting of 9.99 kcal/mouse/day for the control group, 9.38 kcal/mouse/day for L-HRW group, and 10.5 kcal/mouse/day H-HRW group (P = 0.505).
All three groups gained weight during the experiment; however, the H-HRW gained less weight compared to the two other groups (P = 0.0567).
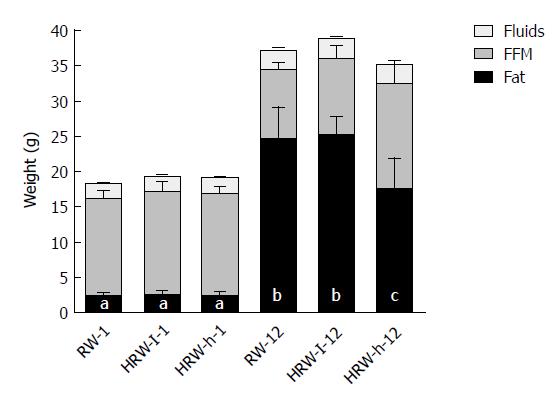
Body composition was significantly affected by HFD. As expected, the total body mass of the three groups increased, mainly due to the addition of fat mass. However, mice in the H-HRW group showed a smaller increase in fat tissue when compared to the other two groups (p = 0.002). At the end of the experiment, mice in the H-HRW group showed a body composition of 46% fat mass and 42% lean body mass, compared to 61% fat mass and 28% lean body mass of the control group. There were also no differences in body water content among the three groups (Figure 4). Since the body mass composition of mice in the L-HRW group was similar to the control group, no further analysis of the L-HRW group was performed.
Mice that drank H-HRW water accumulated significantly (P < 0.01) less hepatic lipids (30.49 mg ± 4.3 mg/300 mg tissue) in comparison to mice that drank RW (48.24 mg ± 6.0 mg/300 mg tissue). This result is corroborated by the histological sections.
Figure 5 shows liver H&E sections of the control group and H-HRW group (two mice from each group, the heaviest and lightest). Each pair of pictures shows the livers of mice of the two different groups that reached a similar total weight. Figure 5A and B show livers of mice in the H-HRW group and control group that achieved final weights of 35.5 g and 35.9 g, respectively. The liver in the control group has visible steatosis, mainly at the periphery of the lobule. In contrast, the liver sections of the H-HRW group hardly developed fat inclusions. Figure 5C and D shows mice livers in the H-HRW group and control group weighing 40.0 g and 40.9 g, respectively. Although both livers are steatotic, the steatosis of the control group is visibly more pronounced. The hepatocytes show microvesicular steatosis at acinar zones 2 and 3; macrovesicular steatosis is most concentrated at zone 1. In contrast, the liver of H-HRW group is characterized by only mild steatosis, mainly at zone 1, and the beginning of microvesicular steatosis at zones 2 and 3.
The effect of HRW on the expression of several genes involved in NAFLD development compared to control is shown in Table 3. Acetyl CoA oxidase (ACOX), a gene related to lipid metabolism, was significantly up-regulated (P < 0.05), while others (such as carnitine palmitoyltransferase 1 (CPT1) and acetyl-CoA carboxylase), did not change significantly. HRW consumption did not alter the expression of the lipogenic enzymes SREBP1-c, fatty acid synthase (FAS) or acetyl-CoA carboxylase (ACC1). The most significant change was in the expression of CD36, which was markedly reduced in the H-HRW group (P = 0.028). Additionally, while the expression of adiponectin receptor 2 (AdipoR2) did not change significantly, a significant increase in adiponectin expression was detected (P = 0.022).
| Gene | RW | HRW | P value | ||
| M | Sd | M | Sd | ||
| IL-6 | 1.10 | 0.46 | 1.17 | 0.26 | 0.26 |
| TNF-α | 1.19a | 0.36 | 0.68b | 0.22 | 0.02 |
| SREBP-1c | 1.04 | 0.19 | 1.02 | 0.30 | 0.87 |
| ACOX | 1.04a | 0.21 | 1.31b | 0.25 | 0.04 |
| CD36 | 1.06a | 0.28 | 0.71b | 0.06 | 0.02 |
| CPT1 | 1.08 | 0.37 | 1.43 | 0.40 | 0.14 |
| ACC2 | 1.12 | 0.50 | 1.05 | 0.23 | 0.64 |
| ACC1 | 1.06 | 0.14 | 1.13 | 0.17 | 0.13 |
| FAS | 1.09 | 0.42 | 1.42 | 0.36 | 0.29 |
| SOD | 1.04 | 0.17 | 0.96 | 0.08 | 0.86 |
| Cat | 1.04 | 0.21 | 1.12 | 0.24 | 0.80 |
| AdipoR2 | 1.01 | 0.22 | 1.14 | 0.28 | 0.23 |
| Adipo | 0.92a | 0.29 | 1.32b | 0.38 | 0.004 |
Genes related to oxidative stress [such as catalase (CAT) and superoxide dismutase (SOD)] did not change significantly; however, the inflammatory gene tumor necrosis factor (TNF)-α, but not interleukin (IL)-6 was significantly down-regulated. However, when the gene expression was measured after overloading with 0.3 mmol/L BSA-palmitate for 4 h, TNF-α and IL-6 were both significantly down-regulated (P = 0.036 and P = 0.002, respectively) as shown in Table 4.
| RW | HRW | P value | |||
| M | Sd | M | Sd | ||
| TNF-α | 9.16a | 1.90 | 5.71b | 4.59 | 0.036 |
| IL-6 | 3.2a | 0.87 | 2.24b | 0.64 | 0.002 |
| CRP | 0.96 | 0.28 | 1.20 | 0.34 | 0.065 |
| SREBP-1c | 0.96 | 0.12 | 1.05 | 0.19 | 0.196 |
| ACC1 | 0.84 | 0.18 | 0.86 | 0.14 | 0.902 |
| SOD | 1.07 | 0.34 | 1.30 | 0.56 | 0.184 |
| AdipoR2 | 0.80 | 0.08 | 0.78 | 0.18 | 1.000 |
| Adipo | 1.00 | 0.30 | 0.79 | 0.30 | 0.097 |
To investigate the long-term effects of HRW on hepatocytes, we extracted cells from different mice drinking either HRW or regular water and compared the fat accumulation after exposing them overnight to 0.3 mmol/L BSA-palmitate in vitro. There was less fat accumulation (P = 0.057) in the hepatocytes from the mice that drank HRW (44.0% ± 18.6%) compared to hepatocytes from mice that drank regular water (52.8% ± 20.9%).
The high prevalence of NAFLD and the lack of safe and effective treatments have resulted in the search for natural alternative methods. The claims of purported functional waters for human health have increased in recent years; however, many of these claims are not supported by the scientific literature. Therefore, our study used mice fed a HFD to determine and compare the potential effects of two claimed functional waters (EAW and HRW). We focused on the early stages of NAFLD, a disease associated with obesity and insulin resistance, which has become the leading cause of liver disease affecting adults and children worldwide.
As expected, mice fed a HFD gained significantly more weight (72%) as fat mass with accompanying liver steatosis compared to mice fed a RD. However, contrary to previous findings, using EAW[13], our results found no differences in body weight or liver histology between the regular water group and the EAW group. EAW was prepared by continuous electrolysis (3 h) to optimize its properties (such as pH, ORP, platinum nanoparticles) according to previous protocols[14,41,42]. The resulting water had a high stable pH (11) and a high negative ORP (-495 mV). However, the H2 concentration in water was only 0.2 mg/L. In this way, mice drinking EAW received high alkaline water with a negative ORP but with only trace amounts of H2. Due to the non-detectable differences between EAW and RW on body weight or liver parenchyma in either the RD or HFD groups, we did not perform any further analysis (such as gene expression or inflammatory markers).
In contrast to the H2 concentration in EAW, our method for HRW preparation allowed us to maintain the H2 concentration. However, L-HRW was also not efficient, but H-HRW was significantly effective at preventing the HFD-induced increase in fat tissue and hepatic lipid accumulation while increasing the amount of lean body mass compared to the control group. We also found that H-HRW significantly abolished the fat inclusions in liver sections, contained fewer triglycerides, and suppressed micro- and macrovesicular steatosis compared to the control. The suppression of fat gain by H-HRW may be attributed to hydrogen’s ability to induce the hepatic hormone fibroblast growth factor-21 (FGF21), which causes an increase in energy expenditure[43]. Indeed, administration of FGF-21 can improve obesity and reverse hepatic steatosis[44]. In their study, H2 water similarly attenuated HFD-induced fatty liver and weight gain in both wildtype and db/db mice. It also reduced hepatic oxidative stress[43].
To understand the mechanism by which H2 affects NAFLD, we measured the expression of several essential genes related to the disease, including those genes related to oxidative stress, lipid metabolism, and inflammation. From the enzymes related to lipid metabolism, only peroxisomal fatty acyl-CoA oxidase (ACOX) showed significant differences. ACOX is one of the first enzymes in the metabolism of lipids and is a rate-limiting enzyme for beta-oxidation of very-long-chain fatty acids in peroxisomes. The participation of ACOX in suppressing NAFLD is essential. Fan et al[45] found that lack of ACOX expression in homozygous (ACOX-/-) mice resulted in liver steatosis, and Kohjima et al[46] found that in humans with NAFLD, its expression was increased two-fold, most likely to compensate for the increase in lipid accumulation. We found that mice that drank HRW showed a significant increase in ACOX expression, which would help enhance fatty acid oxidation, compared to the control group. The increase in ACOX expression is mediated by the transcription factor peroxisome proliferator-activated receptor[46], which can be upregulated by H2[31,43].
Fatty acid translocase-36 (CD36), also known as FAT (fatty acid translocase), is a member of the scavenger receptor family and is involved in low-density lipoprotein, long chain fatty acid, and phospholipid oxidation[47]. CD36 expression in normal livers is relatively low; however, it was demonstrated that an increase in CD36 expression is related to a HFD, liver steatosis, and NAFLD. Wilson et al[48] showed that CD36 deletion in mice fed a HFD reduced their liver lipid contents and induced insulin resistance, while specific induction of CD36 transport in livers led to hepatomegaly and fatty liver[49]. In our study, HRW significantly caused downregulation of CD36 mRNA expression. Suppression of fatty acid uptake and lipid accumulation in Hep G2 cells overloaded with palmitate through CD36 downregulation at the protein level has been reported to have been induced by HRW[33]; however, contrary to our work, the downregulation was only detected at the protein but not the mRNA level[33].
HRW also influenced the expression of other genes related to de novo fatty acid synthesis (such as ACC1, FAS), which are upregulated in NAFLD, and the carnitine palmitoyltransferase 1 gene, which is usually downregulated in NAFLD[46]. Another factor affecting NAFLD is adiponectin, which is a peptide mainly released by adipocytes but also can be released by liver cells[50,51]. When adiponectin binds to its receptor (AipoR2) in the liver, it affects intracellular signaling (resulting in a decrease in de novo lipogenesis) and FFA influx by causing downregulation of CD36 expression and increase in FFA oxidation[52]. In addition to those metabolic effects, adiponectin also has anti-inflammatory actions via inhibition of TNF-α expression through the nuclear factor-κB pathway[53]. In our study, we found that mice that drank HRW showed an increase in the mRNA expression of adiponectin and a decrease in the mRNA expression of TNF-α. The reduction in TNF-α mRNA expression caused by H2 in a similar model has been previously reported[32]. In a double-blinded, human crossover study, drinking HRW for eight weeks also increased adiponectin levels[23].
We did not detect an increase in the mRNA expression of the antioxidant enzymes SOD and CAT in the HFD groups that drank RW or HRW. However, NAFLD mice already have higher levels of SOD and CAT that are required to help neutralize the enhanced HFD-induced reactive oxygen species (ROS) production[46]. Although many H2 studies have reported increased levels of these antioxidants via activation of the Nrf2 pathway (reviewed here[19]), in some cases, H2 causes a decrease in their levels because it mitigates their need by attenuating the assault[54-58]. Thus, HRW may have attenuated some ROS by mitigating the HFD-induced damage, which reduced the need for higher antioxidant levels.
In the in vitro study, we checked for a residual effect resulting from drinking HRW that could be reflected in hepatocytes overloaded with palmitate in vitro. We compared the function of hepatocytes isolated from mice that were fed regular chow and drank HRW to mice that drank regular water. It has previously been demonstrated that hepatocytes respond to palmitate overload by increasing steatosis and cytokine production[59]. Interestingly, hepatocytes from mice that drank HRW showed less fat accumulation and significantly lower mRNA expression of the cytokines TNF-α and IL-6. Therefore, drinking HRW for four weeks before exposure to palmitate appeared to confer hepatocyte protection to palmitate overload in vitro, which may partly be explained by HRW’s effects on pro-inflammatory cytokine gene expression. This residual effect was sustained for at least 24 h after animals were sacrificed. A similar result was demonstrated in hydrogen preconditioning of ex vivo lung grafts, in which it improved post-transplant graft function[60].
Our study demonstrates that the functional water, HRW, may have the potential for the prevention of NAFLD by attenuating HFD-induced increases in fatty tissues, lipid accumulation in the liver, inflammation, and CD36 expression. However, neither EAW nor L-HRW had beneficial protective effects. This result also confirmed the importance of H2 as the therapeutic agent in these waters. Effectively, all three waters could be considered HRW at different concentrations. EAW still had a negative ORP due to the presence of H2, whereas L-HRW only contained 0.3 mg/L, and H-HRW contained 0.8 mg/L. However, the mice in the H-HRW group drank nearly three times more water than L-HRW or control. Due to the higher volume of consumed water and higher concentration, the actual dose of H2 per day in the H-HRW group would have been significantly more (≈ eight times) than what was ingested in the L-HRW group. It is unknown why mice in the H-HRW group ingested nearly 3 times more water. Perhaps the magnesium/water reaction continued to elevate the pH after our initial measurements, which may then result in an increased thirst sensation. Acidic beverages are generally considered more satisfying to quench thirst than those of higher pH. Additionally, we demonstrated that H2 pretreatment had a residual protective effect by modifying gene expression and conferring future cytoprotection. H2 is already in use without any reports of known side effects. However, more research is needed to determine the benefits and optimal dosing to be recognized as a conventional therapy for the prevention and treatment for NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is a major growing metabolic health condition. Conventional pharmaceuticals and drugs are not effective in preventing or treating this disease condition. There is a deep interest in searching for novel, safe, and effective methods to prevent and treat NAFLD. For many years there have been many claims about healthy functional waters including alkaline ionized water, also known as electrolyzed reduced water. Some of those claims suggest it is beneficial for obesity and metabolic disturbances. However, there is a paucity of scientific data on this electrolyzed water, but significant noise and claims by commercial companies marketing and selling it. There are untenable claims about the benefits of high pH, alkaline water, or the oft claimed, albeit impossible, microclustering/structuring of the water. Strong claims have also been made regarding the water’s negative oxidation-reduction potential. However, electrolysis of water does produce hydrogen gas at the cathode, and H2 gas does produce a negative oxidation-reduction potential (ORP). Interestingly, biomedical investigations of molecular hydrogen have demonstrated that this small molecule does have therapeutic potential. Perhaps electrolyzed alkaline water does have beneficial effects, but they are not due to its high alkaline pH or other impossible properties, but due to the presence of dissolved H2 gas produced during electrolysis. Additionally, molecular hydrogen may be an important molecule to combat against NAFLD.
We first wanted to examine if the claimed functional water, electrolyzed alkaline water, could exert therapeutic effects on a mouse model of NAFLD induced by a high-fat diet. We also wanted to compare these results to water with a similar and a greater concentration of molecular hydrogen. This would allow us to know if electrolyzed water has any beneficial effects on NAFLD, and the importance of molecular hydrogen in that water. Additionally, if molecular hydrogen was shown to be therapeutic as previous studies suggest, then it would add to the body of literature lending support for more research.
To determine the effects of H2 water in preventing NAFLD development under an obesogenic diet. This is the first step to understanding the efficacy of this approach for preventing and treating the disease.
In this research mice under an obesogenic high fat diet were used for developing NAFLD. Control mice ingested regular water while the experimental consumed two types of hydrogen-rich water produced in two different ways: via electrolysis with an alkaline water ionizer or via a chemical reaction between water and metallic magnesium. General parameters as food and water consumption, body weight and composition were measured during the experiment. In the end, livers were sampled for histology and gene expression measuring by means of RT-PCR. In the in vitro experiment hepatocytes obtained from mice drinking either regular water or H2-rich water were exposed to a high-fat environment in order to check the residual protective effects of H2-rich water.
This study demonstrated the positives effects of H2-rich water on NAFLD. Mice fed a high-fat diet that were drinking H2-rich water showed less weight gain, more lean body tissue, less steatosis, and better liver histology when compared to the control group. It was also demonstrated that electrolyzed water with a high pH, -ORP, but a low H2 concentration did not result in any improvement. Hepatocytes derived from mice drinking H2-rich water were more resilient to palmitate overload in vitro when compared to hepatocytes obtained from mice drinking regular water. H2-rich water positively affected the expression of several NAFLD related genes. However, the mechanism of action of H2-rich water needs further investigation.
This study demonstrated that the H2 dissolved in water is the therapeutic agent in functional waters since electrolyzed water with a high pH and a negative ORP did not show any effect on preventing the development of NAFLD. Apparently, H2 works at a molecular level since it changed the expression of specific genes related to the disease. This study also demonstrates a long-term protective effect of H2 in an in vitro experiment. Functional water rich in H2 could be a preventive agent in NAFLD. The Therapeutic aspect of H2 still need to be elucidated and the optimum dosage determined.
H2-rich water is already consumed by humans without any contraindication, which makes it a good candidate for future human clinical studies on NAFLD patients.
We thank Adi Sharabi-Nov for assistance with statistical methods.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Das U, Tarantino G, Xu CF S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9-19. [PubMed] |
| 2. | Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol. 2015;7:846-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (5)] |
| 4. | Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217-9228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (1)] |
| 5. | Xu X, Lu L, Dong Q, Li X, Zhang N, Xin Y, Xuan S. Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis. 2015;14:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-378.e5; quiz e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1625] [Article Influence: 162.5] [Reference Citation Analysis (1)] |
| 7. | Dajani A, AbuHammour A. Treatment of nonalcoholic fatty liver disease: Where do we stand? an overview. Saudi J Gastroenterol. 2016;22:91-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 8. | Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14:20704-20728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 9. | Henry M, Chambron J. Physico-Chemical, Biological and Therapeutic Characteristics of Electrolyzed Reduced Alkaline Water (ERAW). Water. 2013;5:2094-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ignacio RM, Kang TY, Kim CS, Kim SK, Yang YC, Sohn JH, Lee KJ. Anti-obesity effect of alkaline reduced water in high fat-fed obese mice. Biol Pharm Bull. 2013;36:1052-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Shirahata S, Kabayama S, Nakano M, Miura T, Kusumoto K, Gotoh M, Hayashi H, Otsubo K, Morisawa S, Katakura Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem Biophys Res Commun. 1997;234:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Jin D, Ryu SH, Kim HW, Yang EJ, Lim SJ, Ryang YS, Chung CH, Park SK, Lee KJ. Anti-diabetic effect of alkaline-reduced water on OLETF rats. Biosci Biotechnol Biochem. 2006;70:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Tsai CF, Hsu YW, Chen WK, Chang WH, Yen CC, Ho YC, Lu FJ. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2009;47:2031-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Shirahata S, Hamasaki T, Teruya K. Advanced research on the health benefit of reduced water. Trends Food Sci Technol. 2012;23:124-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Hamasaki T, Harada G, Nakamichi N, Kabayama S, Teruya K, Fugetsu B, Gong W, Sakata I, Shirahata S. Electrochemically reduced water exerts superior reactive oxygen species scavenging activity in HT1080 cells than the equivalent level of hydrogen-dissolved water. PLoS One. 2017;12:e0171192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Slezák J, Kura B, Frimmel K, Zálešák M, Ravingerová T, Viczenczová C, Okruhlicová Ľ, Tribulová N. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Physiol Res. 2016;65 Suppl 1:S11-S28. [PubMed] |
| 17. | Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152-154. [PubMed] |
| 18. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1689] [Article Influence: 93.8] [Reference Citation Analysis (1)] |
| 19. | Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Takaki A, Kawai D, Yamamoto K. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2014;15:7352-7379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N, Kitawaki J, Imai S, Nakano K, Ohta M. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Ishibashi T, Sato B, Rikitake M, Seo T, Kurokawa R, Hara Y, Naritomi Y, Hara H, Nagao T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med Gas Res. 2012;2:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Ito M, Ibi T, Sahashi K, Ichihara M, Ito M, Ohno K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med Gas Res. 2011;1:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2012;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Song G, Li M, Sang H, Zhang L, Li X, Yao S, Yu Y, Zong C, Xue Y, Qin S. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res. 2013;54:1884-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Xia C, Liu W, Zeng D, Zhu L, Sun X, Sun X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis B. Clin Transl Sci. 2013;6:372-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Nicolson GL, de Mattos GF, Settineri R, Costa C, Ellithorpe R, Rosenblatt S. Clinical Effects of Hydrogen Administration: From Animal and Human Diseases to Exercise Medicine. Int J Clin Med. 2016;7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Zhai X, Chen X, Lu J, Zhang Y, Sun X, Huang Q, Wang Q. Hydrogen-rich saline improves nonalcoholic fatty liver disease by alleviating oxidative stress and activating hepatic PPARα and PPARγ. Mol Med Rep. 2017;15:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Kawai D, Takaki A, Nakatsuka A, Wada J, Tamaki N, Yasunaka T, Koike K, Tsuzaki R, Matsumoto K, Miyake Y. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology. 2012;56:912-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Iio A, Ito M, Itoh T, Terazawa R, Fujita Y, Nozawa Y, Ohsawa I, Ohno K, Ito M. Molecular hydrogen attenuates fatty acid uptake and lipid accumulation through downregulating CD36 expression in HepG2 cells. Med Gas Res. 2013;3:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Hou C, Wang Y, Zhu E, Yan C, Zhao L, Wang X, Qiu Y, Shen H, Sun X, Feng Z. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem Pharmacol. 2016;103:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes. 2015;64:2847-2858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 449] [Cited by in RCA: 490] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 36. | Seo T, Kurokawa R, Sato B. A convenient method for determining the concentration of hydrogen in water: use of methylene blue with colloidal platinum. Med Gas Res. 2012;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Zhang W, Sargis RM, Volden PA, Carmean CM, Sun XJ, Brady MJ. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS One. 2012;7:e37103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Trump BF. Mouse liver cell culture. II. Primary culture. In Vitro. 1981;17:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 40. | Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 646] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 41. | Jun Y, Teruya K, Katakura Y, Otsubo K, Morisawa S, Shirahata S. Suppression of invasion of cancer cells and angiogenesis by electrolyzed reduced water. In Vitro Cell Dev-An. 2004;40:79A. |
| 42. | Kinjo T, Ye J, Yan H, Hamasaki T, Nakanishi H, Toh K, Nakamichi N, Kabayama S, Teruya K, Shirahata S. Suppressive effects of electrochemically reduced water on matrix metalloproteinase-2 activities and in vitro invasion of human fibrosarcoma HT1080 cells. Cytotechnology. 2012;64:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Kamimura N, Nishimaki K, Ohsawa I, Ohta S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring). 2011;19:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 44. | Liu J, Xu Y, Hu Y, Wang G. The role of fibroblast growth factor 21 in the pathogenesis of non-alcoholic fatty liver disease and implications for therapy. Metabolism. 2015;64:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N, Singh I, Yeldandi AV, Rao MS, Maeda N. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J Biol Chem. 1996;271:24698-24710. [PubMed] |
| 46. | Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351-358. [PubMed] |
| 47. | Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc. 2010;121:206-220. [PubMed] |
| 48. | Wilson CG, Tran JL, Erion DM, Vera NB, Febbraio M, Weiss EJ. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology. 2016;157:570-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 49. | Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009;48:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 533] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 50. | Yoda-Murakami M, Taniguchi M, Takahashi K, Kawamata S, Saito K, Choi-Miura NH, Tomita M. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochem Biophys Res Commun. 2001;285:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 797] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 52. | Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2010;12:365-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Chen B, Liao WQ, Xu N, Xu H, Wen JY, Yu CA, Liu XY, Li CL, Zhao SM, Campbell W. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res. 2009;1273:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Xie K, Yu Y, Huang Y, Zheng L, Li J, Chen H, Han H, Hou L, Gong G, Wang G. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 55. | Wang T, Zhao L, Liu M, Xie F, Ma X, Zhao P, Liu Y, Li J, Wang M, Yang Z. Oral intake of hydrogen-rich water ameliorated chlorpyrifos-induced neurotoxicity in rats. Toxicol Appl Pharmacol. 2014;280:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Gopinath D, Gurupriya VS, Aswathi PB, Anu G. Molecular Hydrogen Therapy: A major milestone in Medicine. World J Pharm Pharma Scien. 2014;3:1201-1205. |
| 57. | Yoon YS, Sajo ME, Ignacio RM, Kim SK, Kim CS, Lee KJ. Positive Effects of hydrogen water on 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Biol Pharm Bull. 2014;37:1480-1485. [PubMed] |
| 58. | Ning Y, Shang Y, Huang H, Zhang J, Dong Y, Xu W, Li Q. Attenuation of cigarette smoke-induced airway mucus production by hydrogen-rich saline in rats. PLoS One. 2013;8:e83429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Noda K, Shigemura N, Tanaka Y, Bhama J, D’Cunha J, Kobayashi H, Luketich JD, Bermudez CA. Hydrogen preconditioning during ex vivo lung perfusion improves the quality of lung grafts in rats. Transplantation. 2014;98:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |