Published online Nov 21, 2018. doi: 10.3748/wjg.v24.i43.4939
Peer-review started: September 2, 2018
First decision: October 8, 2018
Revised: October 20, 2018
Accepted: November 13, 2018
Article in press: November 13, 2018
Published online: November 21, 2018
Processing time: 82 Days and 7.9 Hours
To evaluate risk factors for primary sclerosing cholangitis (PSC) recurrence (rPSC) after orthotopic liver transplantation (OLT) in patients with well-preserved colons.
We retrospectively evaluated the medical records of all patients transplanted for PSC in our center between July 1994 and May 2015 and selected 47 with follow-up of at least 60 mo for further analysis based on strict inclusion and exclusion criteria. rPSC was confirmed by magnetic resonance or endoscopic retrograde cholangiopancreatography and liver biopsy. All patients were evaluated by protocolary pre-OLT colonoscopy with randomized mucosal biopsies. Colonoscopy was repeated annually after OLT. Both organ donors and recipients were human leukocyte antigen (HLA) typed by serological and/or DNA methods. All input data were thoroughly analyzed employing relevant statistical methods.
Altogether, 31 men and 16 women with a median (range) age of 36 (15-68) years at the time of OLT and a median follow-up of 122 (60-249) mo were included. rPSC was confirmed in 21/47 (44.7%) of patients, a median 63 (12-180) mo after transplantation. De novo colitis [rPSC in 11/12, P ≤ 0.05, hazard ratio (HR): 4.02, 95% confidence interval (CI): 1.58-10.98] and history of acute cellular rejection (rPSC in 14/25, P ≤ 0.05; HR: 2.66, 95%CI: 1.03-7.86) showed strong positive associations with rPSC. According to the univariate analysis, overlapping features of autoimmune hepatitis (rPSC in 5/5, P ≤ 0.05) and HLA-DRB1*07 in the donor (rPSC in 10/15, P ≤ 0.05) represent other potential risk factors for rPSC, while the HLA-DRB1*04 (rPSC in 0/6, P ≤ 0.05), HLA-DQB1*03 (rPSC in 1/11, P ≤ 0.05), and HLA-DQB1*07 (rPSC in 0/7, P ≤ 0.05) recipient alleles may have protective roles.
De novo colitis and acute cellular rejection are clinical conditions significantly predisposed towards recurrence of PSC after liver transplantation.
Core tip: This study demonstrates that patients with de novo colitis after liver transplantation for primary sclerosing cholangitis (PSC) are significantly predisposed towards primary liver disease recurrence (rPSC). History of acute cellular rejection of the liver graft, overlapping features of autoimmune hepatitis (autoimmune hepatitis /PSC) and certain human leukocyte antigen (HLA)-DQ and HLA-DR alleles in both donors and recipients may also have an impact on rPSC development. These data based on analysis of well-defined cohort with long median follow-up suggest that thorough routine endoscopic, radiological and immunological examination (both pre- and post-transplant) is crucial for identifying high-risk patients.
- Citation: Bajer L, Slavcev A, Macinga P, Sticova E, Brezina J, Roder M, Janousek R, Trunecka P, Spicak J, Drastich P. Risk of recurrence of primary sclerosing cholangitis after liver transplantation is associated with de novo inflammatory bowel disease. World J Gastroenterol 2018; 24(43): 4939-4949
- URL: https://www.wjgnet.com/1007-9327/full/v24/i43/4939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i43.4939
Primary sclerosing cholangitis (PSC), a chronic liver disorder of unknown etiology, is characterized by inflammation, fibrosis, and stenosis of both extra- and/or intrahepatic bile ducts[1,2]. The disease may progress to cirrhosis and eventually to liver failure and death[3]. For those who develop end-stage liver disease, orthotopic liver transplantation (OLT) remains the only effective treatment available[4].
PSC is accompanied by concomitant inflammatory bowel disease (IBD) in up to 80% of patients[5]. IBD in patients with PSC has been proposed to be a phenotype distinct from ulcerative colitis (UC) and Crohn’s disease (CD) and is often referred to as “PSC-IBD”[6,7]. PSC-IBD is usually characterized by a mild course, but often deteriorates after OLT despite long-term immunosuppressive treatment[8].
When compared with other indications for OLT, PSC has satisfactory post-transplantation graft and patient survival, reaching nearly 80% at 5 years after OLT[9-11]. However, a recurrent form of PSC (rPSC) appears in a significant proportion of patients after OLT[12] and may eventually lead to graft loss and liver retransplantation (re-OLT)[13]. The pathogenesis of rPSC is most likely multifactorial, combining both pre- and post-OLT factors with genetic predisposition, and is probably linked with that of the primary disease[14].
rPSC was first described as a unique clinical entity by Lerut et al[15] in 1988. As several other clinical conditions may lead to biliary stricturing post-OLT, thus mimicking rPSC, Graziadei et al[16] proposed a series of diagnostic criteria in 1999.
Risk factors for rPSC have been described previously[14], and include recipient sex[12,17], recipient-donor sex mismatch[18], donor[17] and recipient[19] age, recipient cytomegalovirus (CMV) infection[20], a related living-donor graft[20,21], single and/or multiple episodes of acute cellular rejection (ACR)[17,19,22], corticoresistant ACR[22,23], use of muromonab-CD3 (OKT3)[24], history of cholangiocarcinoma pre-OLT[25], presence of the human leukocyte antigen (HLA)-DRB1*08 gene (particularly in the absence of DQB1*04) in either donor or recipient[22], absence of the HLA-DR52 antigen in the donor[19], UC treatment with corticosteroid continuing for more than 3 mo after OLT[26], and level of liver dysfunction at time of OLT[17,27]. However, the strength of this evidence is not clear, as the previous findings, made mainly from retrospective studies, are inconsistent or even contradictory.
Although the conclusions of previous investigations into the role of IBD in rPSC are inconsistent, a link between the two clinical entities seems probable primarily because of a significant reduction of rPSC in patients without IBD (or with colectomy pre-OLT)[12,26,28,29]. The presence[26,27] or activity of IBD before or after OLT may influence graft survival[17,30,31]. However, not all studies have found a relationship between IBD and rPSC[22,32]. The aim of this study was to assess the risk of rPSC after OLT in a carefully selected cohort of patients with well-preserved colons (no history of colorectal surgery).
In this retrospective study, we analyzed the records of all patients who had received OLT for PSC at the Institute for Clinical and Experimental Medicine, Prague, between July 1994 and May 2015. The most common indication for OLT was recurrent episodes of acute cholangitis and/or liver dysfunction. An electronic database and printed medical records were both evaluated. PSC was diagnosed by pre-OLT cholangiographic examination, then confirmed, and in two cases established, after histological evaluation of liver explants. The stage of liver disease at the time of OLT was determined by the unrevised MELD score (used prior to January 2016). The following data were evaluated in all patients: Sex, age at time of OLT, MELD score, graft configuration, type of anastomosis, cold ischemia time, donor age, presence of donor liver steatosis, donor and recipient AB0 type, donor and recipient CMV status, primary immunosuppressive protocol after OLT, signs of overlap with autoimmune hepatitis (PSC/AIH), presence of cholangiocarcinoma, donor and recipient HLA-DRB1* and -DQB1* types, IBD status, history of pre- or post-OLT colectomy and interim follow-up records after OLT (presence/treatment regimen of ACR, type of primary immunosuppression, dose and duration of corticosteroid administration).
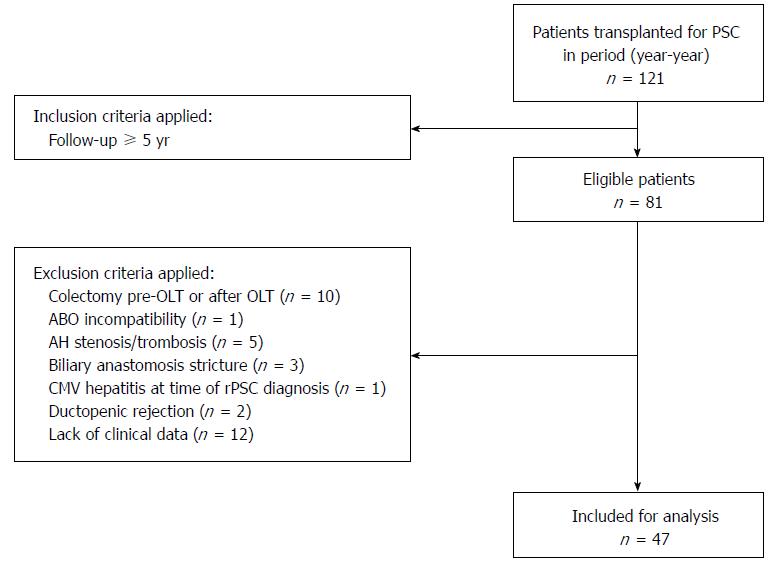
Of 121 patients transplanted for PSC, 47 were included and evaluated (Figure 1). In accordance with the Mayo Clinic Criteria for Diagnosis of rPSC[16], the primary exclusion criteria were presence of hepatic artery thrombosis and/or stenosis, AB0 incompatibility, ductopenic rejection, and presence of biliary strictures within 3 mo after OLT. We excluded one patient with active CMV hepatitis at the time of rPSC diagnosis, patients with a history of anastomotic stricture(s), and patients who had had colon surgery before or within 60 mo after OLT. Twelve patients had insufficient post-OLT data, as their follow-up had been managed at another center. Only patients with complete medical records including annual colonoscopy, regular blood tests, and liver biopsies following the standard protocol were eligible.
The diagnosis of rPSC was based on Mayo Clinic criteria, as proposed by Graziadei et al[16]. These include (1) confirmed diagnosis of PSC before OLT; (2) intra- and/or extrahepatic biliary strictures, beading, and irregularities occurring on cholangiograms > 90 d after OLT; and (3) fibrous cholangitis and/or fibro-obliterative lesions with or without ductopenia, and biliary fibrosis or cirrhosis on liver biopsy. Liver graft biopsies were acquired according to protocol after 1, 2, 3, 5, 7, 10, and 15 years (or as clinically indicated) post-OLT. Liver biopsy specimens were evaluated independently by two experienced pathologists and assessed according to the histological scoring system proposed by Graziadei et al[16]. After re-OLT, rPSC was histologically confirmed in explanted liver grafts. Patients with a Mayo Clinic score of at least 1 (marginal) for rPSC in liver biopsy and those with clinical suspicion (e.g., elevated liver enzymes) were examined cholangiographically to confirm/refute the diagnosis. All cholangiograms were reviewed by an experienced radiologist (RJ) who was blinded to the clinical and histological diagnoses.
All patients were evaluated by pancolonoscopy with randomized mucosal biopsies following the pre-OLT examination protocol. In patients included in the study cohort, colonoscopy was performed annually after OLT. IBD severity was assessed by the Mayo Scoring system[33].
Patients and their organ donors were HLA typed by serological and/or DNA methods. Serological typing of HLA class I antigens was performed by complement-dependent cytotoxicity assay using local or commercial HLA typing trays, following standard procedures (BAG Health Care GmbH, Lich, Germany). DNA typing of HLA class I and class II antigens was performed using PCR-SSP or PCR-SSOP typing trays (Olerup SSP, Stockholm, Sweden; One Lambda Inc, Canoga Park, United States).
All data were recorded and sorted using Microsoft Excel and analyzed using JMP® software (version 10.0.0). Kaplan-Meier analysis was used to estimate graft and patient survival. Student’s t-test or the Mann-Whitney U test (depending on the nature of the data) was used for comparison of continuous variables, which were reported as median (range). Discrete variables were compared using Fisher’s exact test (two-tailed) and expressed as number (n) and percentage (%). Multivariate analysis was performed using the Cox proportional hazards regression model. A P-value ≤ 0.05 was considered as statistically significant. The recurrence (endpoint) time distribution following OLT was estimated by the Kaplan-Meier method, and the respective groups were compared using the log-rank test.
Of the 1139 first liver transplants performed between July 1994 and May 2015, 121 (10.6%) were transplanted for PSC or PSC/AIH (Table 1). All patients received either a whole (n = 116) or reduced (n = 5) liver graft. No patient received a split graft or a graft from a living donor.
| Patients transplanted for PSC (n = 121) | Study cohort (n = 47) | |
| Sex, recipients; male/female | 88/33 (72.7/27.3) | 31/16 (66/34) |
| Median age, recipient, yr (range) | 36 (12-64) | 36 (12-60) |
| Median age, donor, yr (range) | 34 (2-66) | 37 (10-61) |
| Median follow-up, mos (range) | 87 (0-249) | 116 (60-249) |
| MELD score at time of OLT, median (range) | 15 (6-34) | 15 (8-32) |
| Overlap syndrome AIH/PSC pre-OLT | 20 (16.5) | 5 (10.6) |
| Graft configuration: whole/reduced | 116/5 (95.9/4.1) | 47/0 (100/0) |
| Cholangiocarcinoma in liver explant | 8 (6.6) | 0 (0) |
| Cold ischemia median time, min (range) | 300 (125-637) | 319 (175-637) |
| Type of anastomosis: End-to-end/Roux-en-Y | 31/90 (25.6/74.4) | 7/40 (14.9/85.1) |
| Tacrolimus-based primary immunosuppressive regimen | 92 (76.0) | 32 (68.1) |
| Cyclosporine-based primary immunosuppressive regimen | 29 (24.0) | 15 (31.9) |
| IBD pre-OLT | 76 (62.8) | 29 (61.7) |
| UC | 75 (98.7) | 29 (100) |
| CD | 1 (1.3) | 0 (0) |
| Indeterminate colitis | 0 (0) | 0 (0) |
| Mild course of IBD pre-OLT | 62 (81.6) | 29 (100) |
| Active course of IBD pre-OLT | 14 (18.4) | 0 (0) |
| Colectomy pre-OLT | 4 (3.3) | - |
| Liver retransplantation, total | 15 (12.4) | 7 (14.9) |
| Liver retransplantation, for rPSC | 8 (6.6) | 6 (12.8) |
Cholangiocarcinoma was detected in eight liver explants. No other type of malignant tumor, including hepatocellular carcinoma, was found. The primary immunosuppressive protocol was based solely on calcineurin inhibitors. Cyclosporine was routinely used as the primary immunosuppressant until November 2004, when the protocol was universally changed to use tacrolimus. All recipients underwent colonoscopy within 12 mo before OLT. Three patients with UC underwent colectomy, one each for adenocarcinoma, a refractory course of colitis, and toxic megacolon. One patient with CD had a colectomy because of severe bleeding after complicated multiple polypectomy.
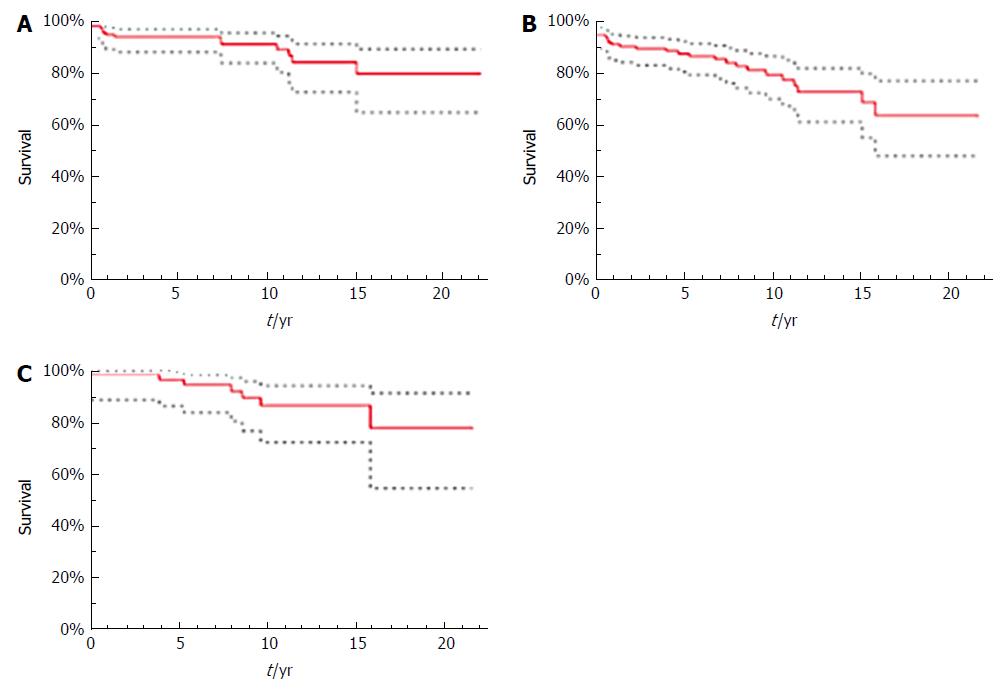
In the total patient population, 1-, 5-, 10-, and 15-year survival was 95.0%, 94.2%, 91.4%, and 84.6%, respectively (Figure 2A). Overall survival in the final study cohort is not shown because no patient died during follow-up. Graft survival in the total population was 91.0% at 1 year, 87.6% at 5, 79.5% at 10, and 72.8% at 15 years (Figure 2B). Of the 15 patients in the total population with re-OLT, eight (53.3%) were indicated because of rPSC. Other indications for re-OLT were primary graft dysfunction, hepatic artery thrombosis and post-hemorrhagic shock. Graft survival in the study cohort was 98.2% at 1 year, 96.2% at 5, 86.2% at 10, and 86.2% at 15 years (Figure 2C). Six of seven patients (85.7%) in the study cohort experienced re-OLT for rPSC, and one (13.3%) had re-OLT for graft failure caused by hepatic artery thrombosis.
Of the 47 recipients included in the study cohort, 31 were men and 16 were women; their median age was 36 (12-60) years at the time of OLT, and median follow-up was 122 (60-249) mo. rPSC was diagnosed in 21 patients (44.7%) during a median of 63 (12-212) mo of follow-up after OLT. Twelve of these patients were men and nine were women, with a median age of 35 (12-68) years at time of OLT (Table 2).
| Total (n = 47) | rPSC (n = 21) | No rPSC (n = 26) | P value | |
| De novo IBD post-OLT, | 12 (25.5) | 11 (91.7) | 1 (8.3) | 0.011 |
| IBD pre-OLT | 29 (61.7) | 8 (27.6) | 21 (72.4) | 0.825 |
| No colitis (pre-OLT or post-OLT) | 6 (12.8) | 2 (33.3) | 4 (66.7) | 0.678 |
| Median age, recipient, yr (range) | 36 (12-60) | 35 (12-60) | 38 (20-60) | 0.337 |
| Median age, donor, yr (range) | 37 (11-61) | 37 (11-60) | 40.5 (13-61) | 0.245 |
| Male; female, recipient | 31; 16 (66.0; 34.0) | 12; 9 (38.7; 56.3) | 19; 7 (61.3; 43.8) | 0.355 |
| Male; female, donor | 24; 23 (51.1; 48.9) | 13; 8 (54.2; 34.8) | 11; 15 (45.8; 65.2) | 0.244 |
| Sex mismatch | 23 (48.9) | 11 (47.8) | 12 (52.2) | 0.773 |
| MELD score at time of OLT (range) | 15 (6-32) | 15 (8-30) | 15 (6-32) | 0.773 |
| History of ACR | 25 (53.2) | 14 (56.0) | 11 (44.0) | 0.114 |
| Multiple episodes of ACR | 12 (25.5) | 7 (58.3) | 5 (41.7) | 0.326 |
| Corticoresistant ACR | 6 (12.8) | 3 (50.0) | 3 (50.0) | 0.665 |
| ACR treated with OKT3 | 4 (8.5) | 3 (75.0) | 1 (25.0) | 0.615 |
| ACR treated with ATG | 2 (4.3) | 0 (0) | 2 (100) | 0.163 |
| CMV IgG positivity, recipient | 27 (57.5) | 12 (44.4) | 15 (55.6) | 1.000 |
| CMV IgG positivity, donor | 36 (76.6) | 18 (50.0) | 18 (50.0) | 0.300 |
| Corticosteroid administration ≥ 3 mo post-OLT | 43 (91.5) | 18 (41.9) | 25 (58.1) | 0.311 |
| Corticosteroid administration ≥ 6 mo post-OLT | 41 (87.2) | 17 (41.5) | 24 (58.5) | 0.386 |
| Corticosteroid administration ≥ 3 mo post-OLT with dose ≥ 10 mg prednisone | 28 (59.6) | 14 (50.0) | 14 (50.0) | 0.551 |
| Corticosteroid administration ≥ 6 mo post-OLT with dose ≥ 10 mg prednisone | 13 (27.7) | 5 (68.5) | 8 (61.5) | 0.747 |
| Overlap with AIH pre-OLT | 5 (10.6) | 5 (100) | 0 (0) | 0.013 |
| Type of anastomosis: End-to-end; Roux-en-Y | 7; 40 (14.9; 85.1) | 4; 17 (57.1; 42.5) | 3; 23 (42.9; 57.5) | 0.684 |
| Liver steatosis, donor | 11 (23.4) | 6 (54.6) | 5 (45.5) | 0.505 |
| Cold ischemia median time, min (range) | 320 (175-637) | 315 (206-618) | 324 (175-637) | 0.627 |
In total, 29 patients (61.7%) were diagnosed with IBD prior to OLT. They all had quiescent pancolitis (Mayo 0-1) with long-term aminosalicylate treatment and ursodeoxycholic acid (8-20 mg/kg/d) used as potential chemopreventive agent against colorectal neoplasia. Eight of these 29 patients (27.6%) were diagnosed with rPSC after 60 (24-212) mo of follow-up after OLT [P = 0.825; odds ratio (OR): 0.76, 95% confidence interval (CI): 0.12-5.01].
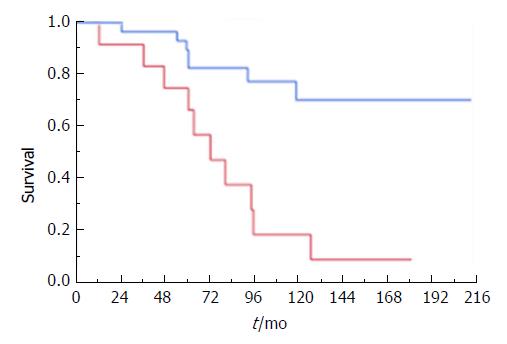
De novo colitis was diagnosed in 12 patients (25.5%) a median of 45 (17-87) mo after OLT. This patient subgroup had a high prevalence of rPSC, confirmed in 11 of the 12 (91.7%) individuals a median of 72 (12-180) mo after OLT. Only two patients were diagnosed with rPSC prior to diagnosis of IBD. Univariate analysis identified de novo IBD as a highly significant risk factor for rPSC (P = 0.011; OR: 22, 95%CI: 1.54-314.31) and, in line with that, recurrence-free survival was substantially (P ≤ 0.001) higher in patients with pre-OLT IBD (median 212 mo, 95%CI: 118-212) compared with de novo IBD patients (median 72 mo, 95%CI: 36-95) (Figure 3). Two of six patients with no evidence of IBD before or after OLT had rPSC (P = 0.678). All patients with de novo IBD were initially treated with mesalazine complementary to their immunosuppressive treatment. None of our patients received biologics after OLT.
All five patients with PSC/AIH confirmed in the liver explant or with evidence pre-OLT had rPSC (P < 0.05), which occurred a median of 72 (61-118) mo after OLT.
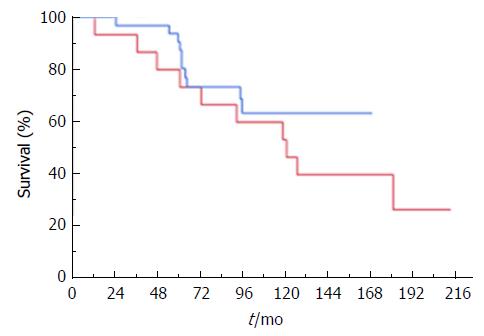
We did not find a difference in time to rPSC between patients using cyclosporine and those using tacrolimus as primary immunosuppressant (P = 0.320; Figure 4).
HLA-DRB1* typing results were available for 46 recipients and 40 donors (Table 3). HLA-DQB1* was typed post hoc (for the purpose of this study) from frozen blood samples, which allowed retrieval of the HLA-DQB1* profile of 35 donors and 41 recipients (Table 3). The presence of a donor HLA-DRB1*07 allele was positively associated with rPSC in 10/15 (66.6%) cases (P = 0.050; OR: 4.25, 95%CI: 1.09-16.61).
| Donor | rPSC | P value | Recipient | rPSC | P value | |
| HLA-DRB1* | ||||||
| 1 | 5 | 2 (40.0) | 1.000 | 9 | 3 (33.3) | 0.711 |
| 3 | 7 | 2 (28.6) | 0.427 | 23 | 11 (47.8) | 0.767 |
| 4 | 9 | 4 (44.4) | 1.000 | 6 | 0 (0) | 0.029 |
| 5 | 1 | 1 (100) | 0.450 | |||
| 6 | 2 | 2 (100) | 0.196 | |||
| 7 | 15 | 10 (66.7) | 0.050 | 2 | 0 (0) | 0.498 |
| 8 | 2 | 1 (50.0) | 1.000 | |||
| 9 | 1 | 0 (0) | 1.000 | 1 | 1 (100) | 0.435 |
| 10 | 1 | 1 (100) | 0.435 | |||
| 11 | 10 | 4 (40.0) | 1.000 | 5 | 1 (20.0) | 0.369 |
| 12 | 2 | 2 (100) | 0.196 | 1 | 1 (100) | 0.435 |
| 13 | 8 | 2 (25.0) | 0.258 | 10 | 5 (50.0) | 0.726 |
| 14 | 4 | 0 (0) | 0.114 | 1 | 1 (100) | 0.435 |
| 15 | 8 | 3 (37.5) | 0.709 | 15 | 5 (33.3) | 0.365 |
| 16 | 1 | 1 (100) | 0.450 | 3 | 2 (66.7) | 0.572 |
| HLA-DQB1* | ||||||
| 2 | 17 | 9 (53.0) | 0.740 | 25 | 10 (40.0) | 1.000 |
| 3 | 20 | 8 (40.0) | 0.740 | 11 | 1 (9.1) | 0.030 |
| 7 | 15 | 6 (40.0) | 0.500 | 7 | 0 (0) | 0.031 |
| 8 | 7 | 5 (71.4) | 0.229 | 4 | 0 (0) | 0.140 |
| 9 | 2 | 1 (50.0) | 1.000 | 1 | 1 (100) | 0.395 |
| 4 | 2 | 1 (50.0) | 1.000 | |||
| 5 | 9 | 4 (44.4) | 1.000 | 10 | 5 (50.0) | 0.481 |
| 6 | 13 | 4 (30.8) | 0.164 | 21 | 9 (42.9) | 0.760 |
None of the six recipients with HLA-DRB1*04 developed rPSC (P = 0.029). Probably because of linkage disequilibrium with the HLA-DRB1*04 allele, the presence of the HLA-DQB1*03 allele in recipients was protective, as only one of 12 patients with this variant had rPSC (P = 0.030; OR: 0.1, 95%CI: 0.01-0.88). These results were duplicated in the HLA-DQB1*07 subgroup: no recipient with this allele (n = 7) had rPSC (P = 0.031). Similarly, no recipient with HLA-DQB1*08 had rPSC. However, this variant did not reach statistical significance (P = 0.140) because of the small number of cases (n = 4). Other HLA-DR or -DQ variants, as well as the number of donor and recipient DR and DQ mismatches, were not associated with rPSC.
Cox proportional hazards regression model for recurrence time confirmed that de novo colitis after OLT is strongly associated with rPSC (P = 0.014), with a HR of 4.02 (95%CI: 1.58-10.98). The model also revealed the occurrence of rPSC to be positively correlated with history of ACR (P = 0.044; HR: 2.66, 95%CI: 1.03-7.86).
In this single-center study of carefully selected patients with long-term follow-up, we confirmed that IBD has an impact on rPSC. To our knowledge, this study is the first to demonstrate that patients with de novo IBD are at significantly higher risk of rPSC than those with IBD pre-OLT. Furthermore, the study confirms the association between ACR and rPSC.
The study represents the longest reported median (122 mo) and maximum (249 mo) follow-up after OLT for PSC.
Study limitations include its retrospective design, but the reliability of the outcome data was increased by care in choosing and adhering to the eligibility criteria, which included evaluation by magnetic resonance or endoscopic retrograde cholangiopancreatography (MRCP/ERCP), biopsy, and annual colonoscopy in all participants. The most obvious limitation of the study is its small sample size, which was a result of meticulous selection of study subjects based on thorough inclusion criteria: only patients with well-preserved colons, proper colonoscopic evaluation before OLT, and follow-up of more than 60 mo were included in the analysis. This approach allowed us to specifically study the influence of IBD on rPSC onset.
In all other aspects, our patient cohort and diagnostics of rPSC were comparable to other published studies. Our study had a single-center design, yet the center is a tertiary care institution that includes more than half of all the transplanted PSC patients in the Czech Republic. This allows the results to be generalized to the broad population, where PSC represents the third leading cause of OLT, accounting for almost 11% of all transplanted patients.
The rate of rPSC occurrence in our study was 44.7%, which is higher than that observed elsewhere; for example, the large study by Ravikumar et al[27] reported only 14.3%. The diagnosis of rPSC in this study was careful, based on MRCP/ERCP and a standard liver biopsy protocol together with clinical signs. That such an approach led to more cases of rPSC being diagnosed suggests that the prevalence of rPSC might be highly underestimated. This is supported by the results of a recent Nordic multicenter study that reported a higher frequency of rPSC in the center that had an established protocol for routine MRCP assessment[29]. The relatively long follow-up in our cohort is another plausible explanation for the observed rPSC rate.
Long-term (15-years) graft survival in all patients transplanted for PSC (84.6%) and in the analyzed cohort (86.2%) did not differ significantly. However, six out of seven retransplants in our study cohort were performed for rPSC, which supports the previous observation that rPSC is associated with high risk of graft failure[13]. Furthermore, de novo IBD was recognized by Kochhar et al[34] as an important risk factor for re-OLT.
In this study, rPSC was diagnosed in the vast majority (91.7%) of patients with de novo colitis. Strikingly, recurrence-free survival was substantially (P ≤ 0.001) lower in patients with de novo IBD compared with those with colitis confirmed pre-OLT. In the study published by Ravikumar et al[27] the frequency of rPSC associated with de novo colitis was 25%, which is less than in our study but almost double the rPSC rate in the entire study group (25% vs 14.3%). Therefore, the ratio of rPSC in the de novo colitis group to rPSC in the entire study group was similar to the ratio in our study (91.7% vs 44.7%).
According to current knowledge on the subject, approximately 14%-30% of patients with PSC may develop de novo IBD during the 10-year period after OLT[35]. This is consistent with our study: De novo colitis was diagnosed in 25.5% of patients after a median of 45 (17-87) mo post-OLT. Apart from in two patients, de novo colitis was diagnosed prior to rPSC, which might be interpreted as study error because evaluations for rPSC and de novo IBD were performed at different times during follow-up. The etiopathogenesis of rPSC is not well understood, but it can be assumed (and presented data support this) that the gut-liver axis plays a crucial role. Pathogenesis of de novo IBD also remains poorly understood. However, Kochhar et al[34] suggested that OLT may reset the “immune thermostat” and trigger the development of bowel disease. Many other factors, including alteration of gut microbiota, gut infections (such as CMV), or use of immunosuppressant agents, may also be involved[36,37]. High incidence of de novo colitis and its tight association with rPSC suggest that regular (maybe even annual) routine colonoscopy after OLT for PSC should be performed in patients with no history of IBD.
The risk of liver graft failure after OLT might be associated with not only the presence but also the activity of IBD[30]. However, available data on the role of IBD activity are not consistent[29,38]. As all patients in our study group experienced quiescent pancolitis before OLT, we were not able to determine whether IBD activity had an impact on increased risk of rPSC.
The role of preventive colectomy has been discussed before, as it may significantly reduce the risk of both colon cancer and rPSC[26,38]. This was further supported by the recent Nordic multicenter study by Lindstrom et al[29], which identified proctocolectomy as a protective factor against rPSC even in the multivariate analysis. However, the association did not appear as strong in the study by Hildebrand et al[17], in which 13.3% of colectomy patients and 19.2% of patients without colectomy had rPSC. Our total population included only three patients who underwent colectomy prior to OLT. As the number was too low to yield any meaningful results, we did not include these patients in the study, preferring to maintain the homogeneity of the study cohort by adding a history of colorectal surgery to the exclusion criteria. Nevertheless, given current knowledge on the topic, proctocolectomy should be considered when evaluating patients with rPSC and concomitant active colitis for re-OLT.
Association between ACR and rPSC has previously been described in several studies[8,19,28]. Multivariate analysis in our cohort identified history of ACR (regardless of number of episodes) as a significant risk factor for rPSC. Neither steroid-resistant ACR nor history of multiple ACR episodes were associated with rPSC. The pathogenetic link between ACR and rPSC remains unresolved. It has been proposed that long-lived memory T-cells may be recruited from the gut due to onset of alloreactive response[39] or that administration of aggressive immunosuppression during rejection treatment might accelerate reactivity against unknown antigens[38]. Given that pathogenesis of rPSC is likely to have similar mechanisms to the primary disease, it is not yet possible to give a credible interpretation to the value of this relationship between ACR and rPSC.
Interestingly, all five patients with overlapping features of AIH developed rPSC and this association was statistically significant in the univariate analysis. This observation is consistent with a previous study that confirmed an increased probability of disease recurrence in PSC patients with overlapping features of AIH when compared with a single autoimmune disease[40]. However, there were too few PSC/AIH patients in our cohort to draw any firm conclusions.
Assuming that genetic predisposition might be involved in rPSC (and PSC) pathogenesis, we performed HLA-DRB1* and HLA-DQB1* patient and donor typing and included the results in the univariate analysis. The presence of a donor HLA-DRB1*07 allele was associated with elevated risk of rPSC. Interestingly, no recipient with the HLA-DRB1*04 allele developed rPSC. Our analysis suggests that the HLA-DQB1*03 allele might also have a protective effect; however, the separate influence of the DRB1*04 and DQB1*03 alleles will have to be determined in future studies. The protective role that the DQB1*08 allele is believed to play remains to be confirmed because of the relatively small number of DQB1*08-positive individuals in our cohort. The presence of HLA-DRB1*08 was associated with increased risk of rPSC by Alexander et al[22], suggesting an immunological mechanism for rPSC, but we could not support that observation. Even though we did not eventually include HLA variants in the multivariate analysis because of our low sample size, these results suggest that routine HLA assessment in OLT is of use.
In summary, all previous studies in the field of rPSC have had a retrospective design and rather inconsistent results, which include the uncertain role of immunosuppressive therapy. Several studies suggested an association of tacrolimus with both rPSC and de novo IBD[29,30,37]; however, the effect of immunosuppression on rPSC is hard to interpret. The cyclosporine-based immunosuppressive protocol used at our institute was changed to a tacrolimus-based regimen during the follow-up period, but patients who were benefiting from cyclosporine were kept on that drug. We did not find a difference in PSC recurrence time distribution between cyclosporine-based and tacrolimus-based immunosuppression (P = 0.320). Furthermore, we were unable to assess the influence of ursodeoxycholic acid as it was given to all patients with PSC, in accordance with our center’s policy.
In conclusion, de novo IBD and history of ACR significantly increase the risk of PSC recurrence after liver transplantation. This association is so close that careful monitoring of patients at risk is critical. Systematic prospective studies are needed to evaluate the role of de novo colitis and the influence of different types of therapy, including biologics, on rPSC rate and risk of graft failure. Proper HLA matching of donors and recipients might be another tool to help avoid development of rPSC, at least in high-risk patients. As rPSC seems likely to be highly underdiagnosed, establishing algorithms for protocolary use of MRCP is justified.
Recurrence of primary sclerosing cholangitis (rPSC) is the most common cause of liver graft failure in patients after liver transplantation (OLT) for PSC. Many previous studies aimed to identify risk factors associated with rPSC. However, results of these studies were often incoherent or even contradictory. This single-center study describes potential risk factors for rPSC in thoroughly selected cohort of patients with longest median and maximum follow-up presented up-to-date.
Identifying relevant risk factors for rPSC is a cornerstone for proper stratification of PSC patients in both pre-OLT and post-OLT arrangement. In the future, this should lead to establishing tailored examination and therapeutic (e.g., immunosuppression regimens) protocols, especially for high-risk individuals. Such measures could reduce morbidity and mortality in patients with this serious clinical condition.
Principal objective of this study was to determine which clinical features are significantly predisposed towards rPSC development. As there was no other study on the topic previously performed in Central Europe, the aim was to assess the outcomes after over twenty years of experience with liver transplantation in our center. Identification of risk factors for rPSC will surely direct future research in the field of recurrent disease pathogenesis.
As this is a study with retrospective design, we analysed all available relevant medical records of patients included in the study while sticking with strict inclusion and exclusion criteria. Standard statistical methods were used to determine significance of obtained results. This included two-tailed Fisher’s exact test, Student’s t-test or the Mann-Whitney U test, analysis by Kaplan-Meier method (with subsequent log-rank test) and Cox proportional hazards regression model.
The most novel finding of this study is represented by very tight association of rPSC and de novo inflammatory bowel disease after OLT. In accordance with several previous studies, we also demonstrated that patients with history of acute cellular rejection after OLT are also at increased risk of rPSC development, similarly as patients with overlapping features of autoimmune hepatitis pre-OLT. Moreover, several human leukocyte antigen (HLA)-DR and HLA-DQ alleles appeared to be promising markers in assessing the risk of rPSC. To confirm these findings, performing of large-scale prospective study would be highly warranted.
Despite the fact, that previous studies described association of IBD and rPSC, this is the first study demonstrating that patients with de novo colitis are at significantly higher risk of rPSC as compared to patients with IBD diagnosed pre-OLT. The work also demonstrates that those who experienced ACR after OLT should be under careful surveillance for signs of rPSC. Moreover, presence of several HLA-DR and HLA-DQ alleles in both donors and recipients had a clear tendency to increase or decrease the risk of PSC. Therefore, routine HLA assessment could lead to more careful donor selection or at least to stratifying the patients for the risk of rPSC development. Routine MRCP should be considered in all those transplanted for PSC as rPSC seems to be highly under-diagnosed. High incidence of de novo colitis raises a question regarding more frequent endoscopic evaluation of patients with no previous history of IBD.
Presented study brings special attention to poorly defined clinical entity of de novo colitis after liver transplantation and its tight relation to PSC recurrence in the liver graft. Future basic and clinical research should define pathogenetic link between both entities and provide diagnostic algorithms and treatment protocols for both these important clinical conditions.
We would like to thank Dr. Vera Lanska for her supervision of the statistical analysis performed in this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Czech Republic
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chu J, Mercado MA, Yuksel I S- Editor: Wang XJ L- Editor: A E- Editor: Bian YN
| 1. | Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 513] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Gow PJ, Chapman RW. Liver transplantation for primary sclerosing cholangitis. Liver. 2000;20:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Levy C, Lindor KD. Primary sclerosing cholangitis: epidemiology, natural history, and prognosis. Semin Liver Dis. 2006;26:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Karlsen TH, Schrumpf E, Boberg KM. Update on primary sclerosing cholangitis. Dig Liver Dis. 2010;42:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Boonstra K, van Erpecum KJ, van Nieuwkerk KM, Drenth JP, Poen AC, Witteman BJ, Tuynman HA, Beuers U, Ponsioen CY. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Moncrief KJ, Savu A, Ma MM, Bain VG, Wong WW, Tandon P. The natural history of inflammatory bowel disease and primary sclerosing cholangitis after liver transplantation--a single-centre experience. Can J Gastroenterol. 2010;24:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Adam R, McMaster P, O’Grady JG, Castaing D, Klempnauer JL, Jamieson N, Neuhaus P, Lerut J, Salizzoni M, Pollard S. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 11. | Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, Poterucha JJ, Rosen CB, Gores GJ, LaRusso NF. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Vera A, Moledina S, Gunson B, Hubscher S, Mirza D, Olliff S, Neuberger J. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (36)] |
| 13. | Rowe IA, Webb K, Gunson BK, Mehta N, Haque S, Neuberger J. The impact of disease recurrence on graft survival following liver transplantation: a single centre experience. Transpl Int. 2008;21:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Lerut J, Demetris AJ, Stieber AC, Marsh JW, Gordon RD, Esquivel CO, Iwatsuki S, Starzl TE. Intrahepatic bile duct strictures after human orthotopic liver transplantation. Recurrence of primary sclerosing cholangitis or unusual presentation of allograft rejection? Transpl Int. 1988;1:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, Hay JE, Gores GJ, Charlton MR, Ludwig J. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Hildebrand T, Pannicke N, Dechene A, Gotthardt DN, Kirchner G, Reiter FP, Sterneck M, Herzer K, Lenzen H, Rupp C. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: A retrospective multicenter analysis. Liver Transpl. 2016;22:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Khettry U, Keaveny A, Goldar-Najafi A, Lewis WD, Pomfret EA, Pomposelli JJ, Jenkins RL, Gordon FD. Liver transplantation for primary sclerosing cholangitis: a long-term clinicopathologic study. Hum Pathol. 2003;34:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Jeyarajah DR, Netto GJ, Lee SP, Testa G, Abbasoglu O, Husberg BS, Levy MF, Goldstein RM, Gonwa TA, Tillery GW. Recurrent primary sclerosing cholangitis after orthotopic liver transplantation: is chronic rejection part of the disease process? Transplantation. 1998;66:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Egawa H, Taira K, Teramukai S, Haga H, Ueda Y, Yonezawa A, Masuda S, Tsuji H, Ashihara E, Takada Y. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation: a single center experience. Dig Dis Sci. 2009;54:1347-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Haga H, Miyagawa-Hayashino A, Taira K, Morioka D, Egawa H, Takada Y, Manabe T, Uemoto S. Histological recurrence of autoimmune liver diseases after living-donor liver transplantation. Hepatol Res. 2007;37 Suppl 3:S463-S469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Alexander J, Lord JD, Yeh MM, Cuevas C, Bakthavatsalam R, Kowdley KV. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Brandsaeter B, Schrumpf E, Bentdal O, Brabrand K, Smith HJ, Abildgaard A, Clausen OP, Bjoro K. Recurrent primary sclerosing cholangitis after liver transplantation: a magnetic resonance cholangiography study with analyses of predictive factors. Liver Transpl. 2005;11:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Kugelmas M, Spiegelman P, Osgood MJ, Young DA, Trotter JF, Steinberg T, Wachs ME, Bak T, Kam I, Everson GT. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003;9:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, Kam I. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008;14:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Cholongitas E, Shusang V, Papatheodoridis GV, Marelli L, Manousou P, Rolando N, Patch D, Rolles K, Davidson B, Burroughs AK. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2008;14:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Ravikumar R, Tsochatzis E, Jose S, Allison M, Athale A, Creamer F, Gunson B, Iyer V, Madanur M, Manas D. Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. J Hepatol. 2015;63:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Lindström L, Jørgensen KK, Boberg KM, Castedal M, Rasmussen A, Rostved AA, Isoniemi H, Bottai M, Bergquist A. Risk factors and prognosis for recurrent primary sclerosing cholangitis after liver transplantation: a Nordic Multicentre Study. Scand J Gastroenterol. 2018;53:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Joshi D, Bjarnason I, Belgaumkar A, O’Grady J, Suddle A, Heneghan MA, Aluvihare V, Rela M, Heaton N, Agarwal K. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 2013;33:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Ueda Y, Kaido T, Okajima H, Hata K, Anazawa T, Yoshizawa A, Yagi S, Taura K, Masui T, Yamashiki N. Long-term Prognosis and Recurrence of Primary Sclerosing Cholangitis After Liver Transplantation: A Single-Center Experience. Transplant Direct. 2017;3:e334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2250] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 34. | Kochhar G, Singh T, Dust H, Lopez R, McCullough AJ, Liu X, Fung J, Shen B. Impact of De Novo and Preexisting Inflammatory Bowel Disease on the Outcome of Orthotopic Liver Transplantation. Inflamm Bowel Dis. 2016;22:1670-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Singh S, Loftus EV Jr, Talwalkar JA. Inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Am J Gastroenterol. 2013;108:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Vindigni SM, Surawicz CM. The gut microbiome: a clinically significant player in transplantation? Expert Rev Clin Immunol. 2015;11:781-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Verdonk RC, Dijkstra G, Haagsma EB, Shostrom VK, Van den Berg AP, Kleibeuker JH, Langnas AN, Sudan DL. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006;6:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Montano-Loza AJ, Bhanji RA, Wasilenko S, Mason AL. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther. 2017;45:485-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, Briskin M, Salmon M, Adams DH. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 40. | Bhanji RA, Mason AL, Girgis S, Montano-Loza AJ. Liver transplantation for overlap syndromes of autoimmune liver diseases. Liver Int. 2013;33:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |