Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.543
Peer-review started: November 11, 2017
First decision: November 30, 2017
Revised: December 11, 2017
Accepted: December 20, 2017
Article in press: December 20, 2017
Published online: January 28, 2018
Processing time: 76 Days and 11.4 Hours
We herein report a case of neuroendocrine carcinoma of the gastric stump found 47 years after Billroth II gastric resection for a benign gastric ulcer. A 74-year-old man was referred to another hospital with melena. Endoscopic examination revealed a localized ulcerative lesion at the gastrojejunal anastomosis. The diagnosis by endoscopic biopsy was neuroendocrine carcinoma. A total gastrectomy of the remnant stomach with D2 lymphadenectomy was performed at our hospital. The lesion invaded the subserosa, and metastasis was found in two of nine the lymph nodes retrieved. The lesion was positive for synaptophysin and chromogranin A, and the Ki-67 labeling index was 60%. The diagnosis of neuroendocrine carcinoma of the gastric stump was confirmed using World Health Organization 2010 criteria. Subsequently, the patient underwent one course of adjuvant chemotherapy with the etoposide plus cisplatin (EP) regimen; however, treatment was discontinued due to grade 3 myelosuppression. The patient showed lymph node metastasis in the region around the gastrojejunal anastomosis in the abdominal cavity 7 mo post-surgery. He then underwent radiotherapy and platinum-based combination chemotherapy; however, the disease progressed and liver recurrence was observed on follow-up computed tomography at 16 mo post-surgery. The patient then received chemotherapy with regimens used for the treatment of small cell lung cancer in first- and second-line settings. The patient died of disease progression 31 months after surgery.
Core tip: The most common form of gastric stump cancer is adenocarcinoma. Various types of malignancies have been reported previously, but the development of neuroendocrine carcinoma from the gastric stump is rare. This case might contribute to improving our understanding of the carcinogenesis, biology, and behavior of gastric neuroendocrine carcinoma and gastric stump cancer.
- Citation: Ma FH, Xue LY, Chen YT, Xie YB, Zhong YX, Xu Q, Tian YT. Neuroendocrine carcinoma of the gastric stump: A case report and literature review. World J Gastroenterol 2018; 24(4): 543-548
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/543.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.543
Gastric stump cancer (GSC) is a well-known long-term complication after distal gastrectomy, and has been reported to account for 1%-8% of all gastric cancers. The most common form of GSC is adenocarcinoma[1], although various types of gastric stump malignancies have been reported[2-6]. Development of neuroendocrine carcinoma (NEC) from the gastric stump is extremely rare. To the best of our knowledge, only a case of NEC in the gastric stump has been reported in the English literature, at the University of Parma, Italy[7]. Herein we report a case of NEC of the gastric stump diagnosed 47 years after distal gastrectomy for a benign gastric ulcer.
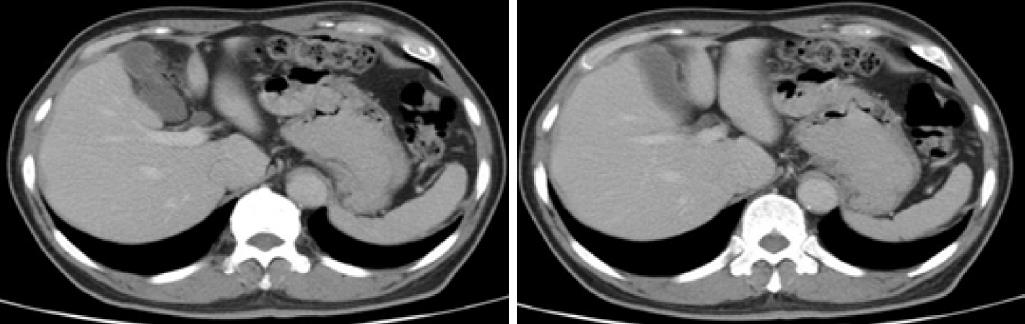
A 74-year-old man consulted a doctor for melena at another hospital. He had undergone a distal gastrectomy with Billroth II reconstruction for a gastric ulcer 47 years ago. He had been having moderate hypertension for 10 years, for which he was taking thiazide daily. A hemorrhage from the upper gastrointestinal tract was suspected. Upper endoscopic examination revealed a localized ulcerative lesion located on the gastrojejunal anastomosis. Contrast-enhanced computed tomography (CT) scans revealed thickening of the stomach wall above the gastrojejunostomy site. There was no evidence of extension of the lesion into the serosa or surrounding soft tissues (Figure 1). An endoscopic biopsy of the tumor was performed. Pathologic examination of the biopsies revealed nests of tumor cells with poor differentiation. The cells showed diffuse positivity for synaptophysin and chromogranin A. Based on biopsy results, the patient was diagnosed with NEC.
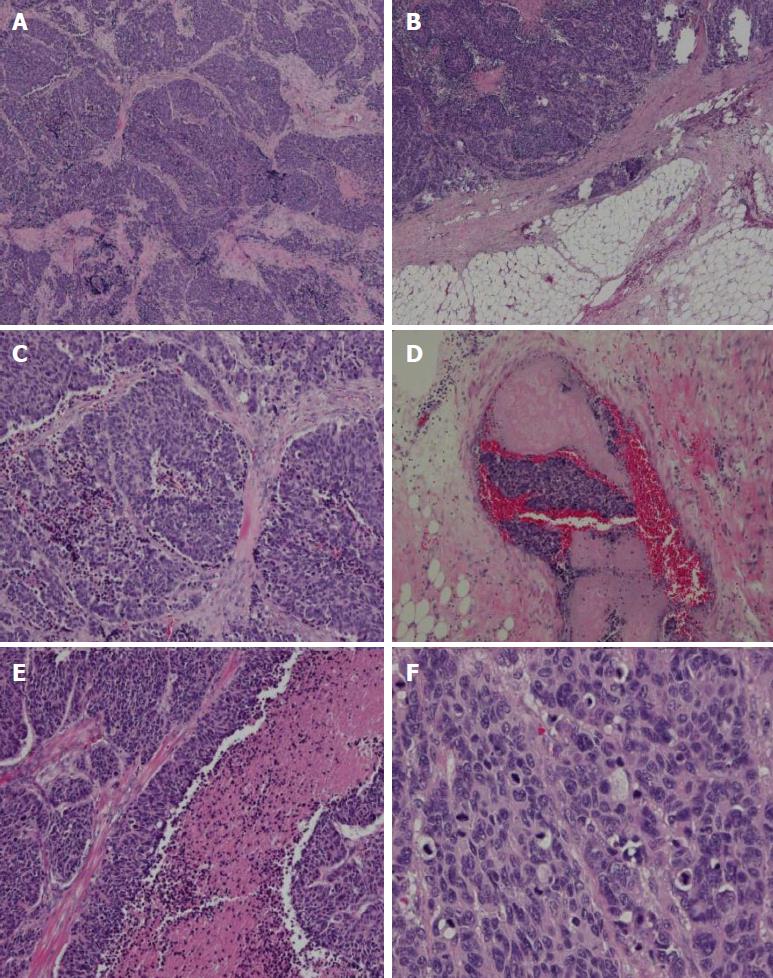
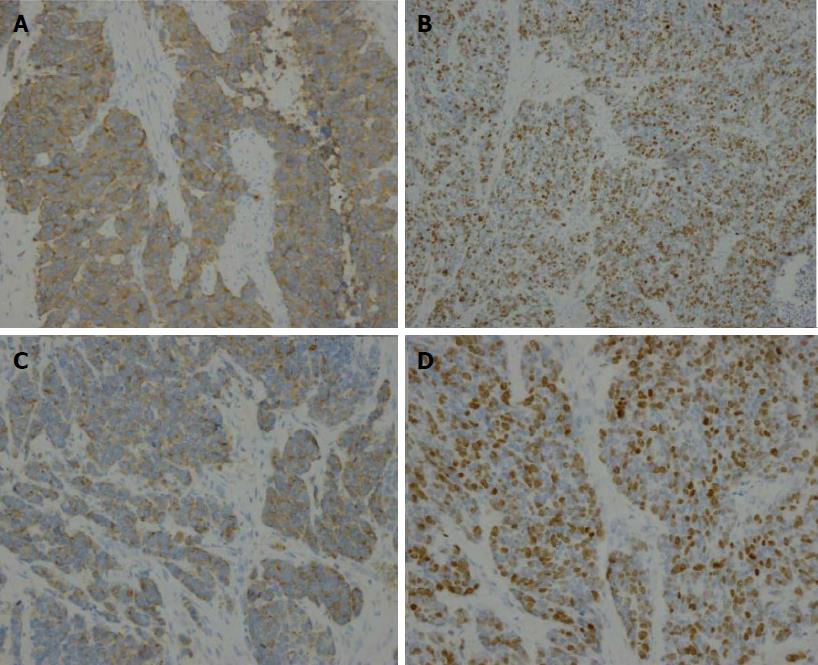
A total gastrectomy of the remnant stomach with D2 lymphadenectomy and Billroth II reconstruction was performed at our hospital. A low-power histological view revealed that tumor cells had invaded entire layers of the stomach wall and showed infiltrative growth from the muscularis propria to the serosa with angiolymphatic invasion and carcinoma cell embolus (Figure 2). The TNM classification was T3N0M0 (stage IIIA). High-power views revealed monotonous large tumor cells with abundant cytoplasm and large irregular nuclei containing prominent nucleoli; mitotic figures were also observed (60 per 10 high-power fields). Immunohistochemical staining revealed that the tumor cells were positive for chromogranin A, CD56, and synaptophysin. The Ki-67 labeling index was 60%. Thus, the diagnosis of gastric stump large-cell NEC was confirmed (Figure 3).
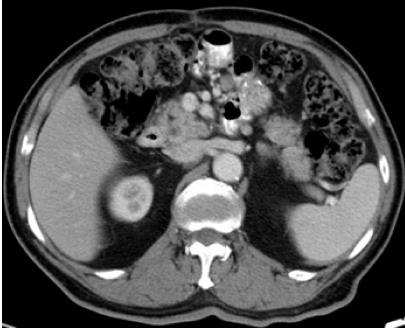
The patient’s postoperative course was favorable, and he was subsequently discharged from the hospital. The patient also commenced a course of adjuvant chemotherapy (EP regimen: 20 mg cisplatin on day 1 and 100 mg etoposide on days 1-4, once a month for one course). However, he experienced grade 3 myelosuppression as a side-effect after this first course of chemotherapy, resulting in treatment suspension due to patient refusal to undergo further treatment. Seven months after the operation, CT scanning revealed lymph node metastasis in the region around the gastrojejunal anastomosis in the abdominal cavity (Figure 4); as a result, the patient received six cycles of chemotherapy (EP regimen: 20 mg cisplatin on days 1-4, and 100 mg etoposide on days 1-3), to which a partial response was achieved. Following this, at 13 mo post-surgery, the patient underwent locoregional radiotherapy, with a total of 60 Gy in 15 fractions. Follow-up CT scanning revealed a recurrence in the liver at 16 mo post-surgery. Two cycles of chemotherapy with the EP regimen were given; however, the patient again experienced grade 3 myelosuppression and disease progression was observed. He then received five cycles of chemotherapy with 240 mg irinotecan on day 1 and 40 mg S-1 on days 1-10, four cycles of chemotherapy with the CAV regimen (0.5 g cyclophosphamide on day 1, 50 mg doxorubicin on days 1-2 and day 21, and 2 mg vincristine on day 1), and two cycles of chemotherapy (200 mg paclitaxel on day 1 and day 14). Despite this treatment, the disease progressed and his performance status deteriorated. He died 31 mo after the operation.
GSC was first reported as a disease entity by Balfour in 1922[8]. It was initially defined as a cancer that arose in the remnant stomach 5 years after gastrectomy for benign diseases such as peptic ulcers[9]. Currently, the concept of GSC has been expanded to include recurrence after gastric cancer resection, which has been reported to account for 1%-7% of all gastric cancers[10]. Gastric NEC (GNEC) is a rare neoplasm known for its aggressive behavior and poor prognosis, accounting for 0.1%-0.6% of all gastric carcinomas[11]. Primary gastric stump NEC is exceptionally rare. A search of the literature revealed documentation of only one such case, described by D’Adda et al[7] who identified a case of metastatic NEC that developed in the gastric stump 25 years after Billroth II gastric resection for a duodenal ulcer in 1991.
The carcinogenesis of GSC is strongly associated with chronic duodenogastric reflux of bile and pancreatic juice, and hypochlorhydria secondary to denervation through vagotomy. It has been generally reported that chronic degenerative changes in the gastric mucosa lead to the development of adenocarcinoma with varying degrees of differentiation[12]. The specific carcinogenetic pathways that lead to GNEC are largely unknown. Whether they are related to the classical mechanisms described for GSC development remains to be better understood.
The World Health Organization 2010 classification defined NEC as a subgroup of neuroendocrine neoplasms (NENs). NENs are divided into neuroendocrine tumors (NET) of grade 1 and grade 2 and NEC grade 3 according to the Ki-67 labeling index[13]. The Japanese classification of gastric carcinoma defined NEC as a special type in its histological classification of gastric tumors, and classified it to be either of the small-cell or the large-cell type. In 1993, Rindi et al[14] proposed a classification system for gastric NETs (GNETs) wherein tumors were divided into three types by their underlying pathophysiology, etiology, and presentation. According to this classification system, type 1 is associated with chronic atrophic gastritis and hypergastrinemia; type 2 is associated with multiple endocrine neoplasia type 1, Zollinger-Ellison syndrome, and hypergastrinemia; and type 3 is sporadic, gastrin-independent, and is believed to be the most biologically aggressive GNET. In the present case, based on histological examination and immunohistochemical staining for neuroendocrine markers, the diagnosis was large-cell NEC and the tumor could be classified as a type 3 GNET.
For patients with GNEC, radical gastrectomy plus regional lymph node dissection should be performed for localized disease; adjuvant chemotherapy should also be provided after surgery[15,16]. Given the rarity of these tumors, there is no standardized chemotherapy for GNEC, and therapy is typically done according to the treatment guidelines for small cell lung cancer. A combination of cisplatin and etoposide (EP regimen) is usually proposed as a first-line therapy for extra-pulmonary high-grade NEC[17]. We chose to treat this patient with chemotherapy regimens used for the treatment of SCLC both in first- and second-line settings. Although the addition of radiotherapy has improved the survival of patients with resectable SCLC, its role in the treatment of GNECs is unclear given the extremely limited information on its usage in this type of cancer.
In conclusion, GNEC is rare and this study presents the exceptionally unusual occurrence of NEC in the gastric stump following Billroth II gastrectomy. This case will contribute to improvements in our understanding of the carcinogenesis, biology, and behavior of GNEC and GSC. This case may also serve as a reminder to gastroenterologists, surgeons, and pathologists who encounter GSC cases in their clinical practice to consider a diagnosis of NEC and undertake the requisite tests for histological and neuroendocrine markers such as chromogranin A and synaptophysin.
The most common form of gastric stump cancer (GSC) is adenocarcinoma. Various types of gastric stump malignancies have been reported previously, but the development of neuroendocrine carcinoma (NEC) from the gastric stump is rare.
Gastric ulcer.
Gastric cancer and lymphoma.
NEC of the gastric stump.
Neoplasm of the gastric stump.
NEC of the gastric stump.
Surgery combined with chemotherapy and radiotherapy.
A case of neuroendocrine carcinoma in the gastric stump has only been reported once in the English literature from the University of Parma, Italy.
Neuroendocrine carcinoma of the gastric stump.
This case will contribute to improvements in our understanding of the carcinogenesis, biology, and behavior of gastric NEC and GSC. This case may also serve as a reminder to gastroenterologists, surgeons, and pathologists who encounter GSC cases in their clinical practice to consider a diagnosis of NEC and undertake the requisite tests for histological and neuroendocrine markers such as chromogranin A and synaptophysin.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fernandez JM, McHenry L, Schmidt J S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Takahashi M, Takeuchi H, Tsuwano S, Nakamura R, Takahashi T, Wada N, Kawakubo H, Saikawa Y, Kitagawa Y. Surgical Resection of Remnant Gastric Cancer Following Distal Gastrectomy: A Retrospective Clinicopathological Study. Ann Surg Oncol. 2016;23:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Greco L, Marino F, Troilo VL, Marzullo A, Gentile A. Gastric stump lymphoma after distal gastrectomy for benign peptic ulcer: Report of a case. Surg Today. 2006;36:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kondo T, Kitazawa R, Kitazawa S. Gastric remnant adenocarcinoma with micropapillary component. Dig Dis Sci. 2008;53:2287-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Chang YS, Kim MS, Kim DH, Park S, You JY, Han JK, Kim SH, Lee HJ. Primary Squamous Cell Carcinoma of the Remnant Stomach after Subtotal Gastrectomy. J Gastric Cancer. 2016;16:120-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cheng CY, Wu IC, Chen YT, Hu HM. A rare hepatoid adenocarcinoma from the gastric remnant. Kaohsiung J Med Sci. 2016;32:482-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Cazzo E, de Saito HP. Mixed adenoneuroendocrine carcinoma of the gastric stump following Billroth II gastrectomy: case report and review of the literature. Sao Paulo Med J. 2016;134:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | D’Adda T, Azzoni C, Franzé A, Bordi C. Malignant enterochromaffinlike cell carcinoid of the gastric stump: an ultrastructural study. Ultrastruct Pathol. 1991;15:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Di Leo A, Pedrazzani C, Bencivenga M, Coniglio A, Rosa F, Morgani P, Marrelli D, Marchet A, Cozzaglio L, Giacopuzzi S. Gastric stump cancer after distal gastrectomy for benign disease: clinicopathological features and surgical outcomes. Ann Surg Oncol. 2014;21:2594-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Huang H, Wang W, Chen Z, Jin JJ, Long ZW, Cai H, Liu XW, Zhou Y, Wang YN. Prognostic factors and survival in patients with gastric stump cancer. World J Gastroenterol. 2015;21:1865-1871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kang SH, Kim KH, Seo SH, An MS, Ha TK, Park HK, Bae KB, Choi CS, Oh SH, Choi YK. Neuroendocrine carcinoma of the stomach: A case report. World J Gastrointest Surg. 2014;6:77-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol. 2014;20:13734-13740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 370] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Zhao P, Shi X, Zhao A, Zhang L, Zhou L. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: a large, retrospective single-centre study. BMC Endocr Disord. 2017;17:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Xie JW, Sun YQ, Feng CY, Zheng CH, Li P, Wang JB, Lin JX, Lu J, Chen QY, Cao LL. Evaluation of clinicopathological factors related to the prognosis of gastric neuroendocrine carcinoma. Eur J Surg Oncol. 2016;42:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Okita NT, Kato K, Takahari D, Hirashima Y, Nakajima TE, Matsubara J, Hamaguchi T, Yamada Y, Shimada Y, Taniguchi H. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |