Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.537
Peer-review started: November 9, 2017
First decision: December 6, 2017
Revised: December 13, 2017
Accepted: December 20, 2017
Article in press: December 20, 2017
Published online: January 28, 2018
Processing time: 77 Days and 19.3 Hours
We report our experience with a synchronous case of gastrointestinal stromal tumor (GIST) and intraductal papillary neoplasm of the bile duct (IPNB) in an elderly woman with neurofibromatosis type 1 (NF-1). A 72-year-old woman presented with a 2-mo history of right upper abdominal pain unrelated to diet and indigestion. Fourteen years earlier, she had been diagnosed with NF-1, which manifested as café au lait spots and multiple nodules on the skin. Computed tomography (CT) revealed a multilocular low-density mass with septation, and mural nodules in the right hepatic lobe, as well as a 1.7-cm-sized well-demarcated enhancing mass in the third portion of the duodenum. The patient subsequently underwent right hepatectomy and duodenal wedge resection. We present here the first report of a case involving a synchronous IPNB and GIST in a patient with NF-1. Our findings demonstrate the possibility of various tumors in NF-1 patients and the importance of diagnosis at an early stage
Core tip: We reported the world wide first case of intraductal papillary adenocarcinoma of bile duct in neurofibromatosis type 1 (NF-1) patient. Because patients with NF-1 have a mutation of the NF-1 gene associated with multiple tumors such as neuroma, and gastrointestinal stromal tumor, consideration for multiple tumors in NF-1 patients would be helpful.
- Citation: Lee JM, Lee JM, Hyun JJ, Choi HS, Kim ES, Keum B, Jeen YT, Chun HJ, Lee HS, Kim CD, Kim DS, Kim JY. Intraductal papillary bile duct adenocarcinoma and gastrointestinal stromal tumor in a case of neurofibromatosis type 1. World J Gastroenterol 2018; 24(4): 537-542
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/537.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.537
Neurofibromatosis type 1 (NF-1) is an autosomal dominant disorder with an incidence of 1 per 3000 births and a prevalence of 1 per 4-5000 individuals[1]. Typically, NF-1 manifests clinically as café-au-lait spots, cutaneous neurofibromas, cognitive impairment, axillary and/or inguinal freckling, Lisch nodules (i.e., pigmented hamartoma of the iris), and bony dysplasia[2]. Clinical manifestations may vary systemically depending on the type of mutation in NF1[1] ; furthermore, patients with NF-1 have an increased incidence of both benign and malignant neoplasms of the nervous system, skin, muscle, and gastrointestinal tract[2]. Approximately 10%-25% of patients with NF-1 are known to develop neoplasms of the gastrointestinal tract[3]. Gastrointestinal stromal tumor (GIST) is the most common gastrointestinal neoplasm in this population, whereas adenocarcinoma occurs at significantly lower frequency relative to other tumor types although the risk remains high[2,3]. To date, intraductal papillary neoplasm of the bile duct (IPNB) has not been reported in a patient with NF-1 patient. Additionally, few reports have described cases of multiple synchronous gastrointestinal tumors in patients with NF-1. In this report, we present a first case of NF-1 patient with coexisting intra-ductal papillary adenocarcinoma of biliary duct and duodenal GIST.
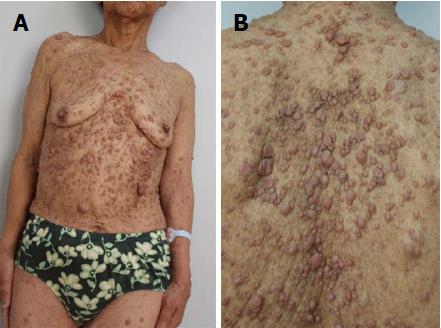
A 72-year-old woman presented with a 2-mo history of right upper abdominal pain unrelated to her diet and indigestion. An examination revealed multiple nodules over her face and body, café au lait spots on the body and limbs, and scoliosis (Figure 1). Fourteen years earlier, she had undergone surgical treatment after receiving a diagnosis of adrenal tumors. Additionally, she was diagnosed with NF-1 after a chromosomal abnormality was detected. Pathology identified the adrenal tumor as a mucinous cystadenoma, but the patient was not subsequently followed up.
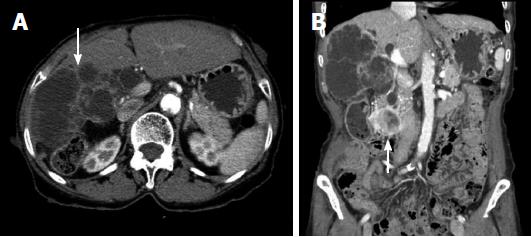
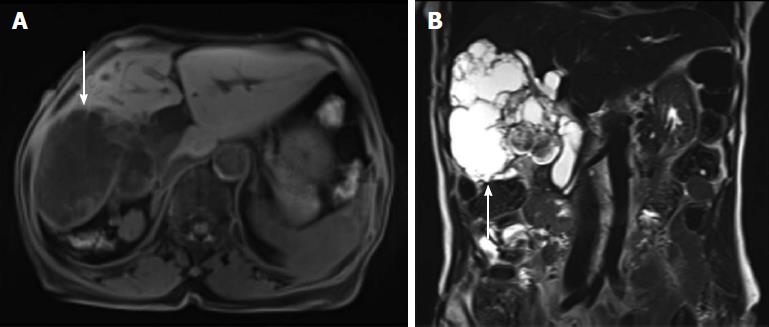
At admission, physical examination revealed a negative Murphy’s sign. The initial laboratory findings were as follows: aspartate transaminase, 19 IU/L; alanine transaminase, 13 IU/L; total bilirubin, 0.43 mg/dL; alkaline phosphatase, 102 IU/L; γ-glutamyltransferase, 70 IU/L; and carcinoembryonic antigen 3.4 ng/mL. However, her cancer antigen (CA) 19-9 level was elevated to 211.3 IU/mL. Abdominal computed tomography (CT) revealed a low-attenuated multilocular mass with septation and a mural nodule with a soft tissue-enhancing lesion in the right hepatic lobe (Figure 2A). CT also indicated diffuse intrahepatic duct (IHD) dilatation, especially in the right hepatic lobe, where a suspicious soft tissue lesion was connected to the IHD. In addition, a 1.7-cm-sized, well-demarcated enhancing mass was observed in the third portion of the duodenum (Figure 2B) Magnetic resonance (MR) cholangiopancreatography showed a lobulated cystic mass in the right hepatic lobe with multifocal intramural enhancing nodules and suspicious communication with the IHD, indicating a malignant transformation (Figure 3). There were no specific findings on brain MR for central nervous system evaluation. We decided to perform a surgical resection histological diagnostic and treatment purposes. Accordingly, the patient underwent right hepatectomy and duodenal wedge resection.
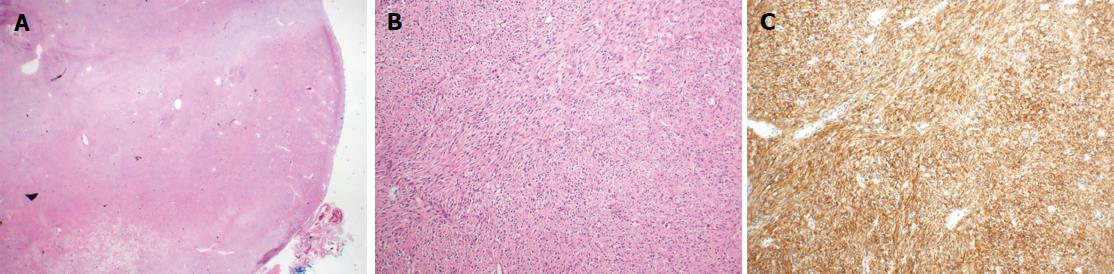
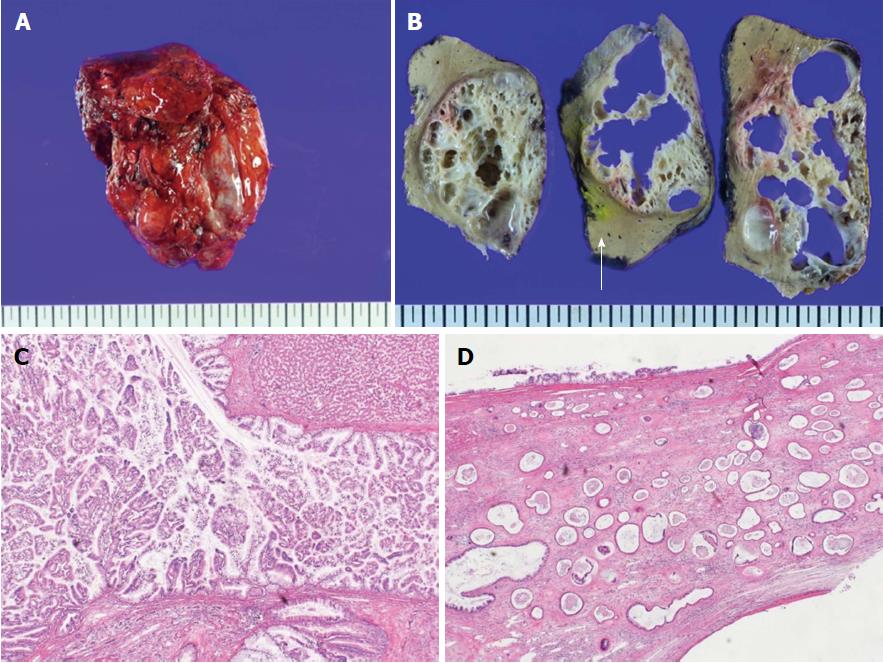
Pathologically, the resected mass from the third portion of the duodenum had a rounded border, contained spindle cells, and was positive for c-kit immunohistochemistry. This led to a diagnosis of a benign GIST (Figure 4). The resected hepatic mass was a multilocular cystic neoplasm with septation and a mural nodule (Figure 5A). This lesion communicated with the segmental bile duct, but was not connected to the main bile duct (Figure 5B). In the dilated bile duct, a normal epithelial lining with an abrupt papillary epithelial portion suggested IPNB (Figure 5C). In a microscopy, multifocal stromal invasion and a tubulopapillary mucin component were visible, suggesting an invasive adenocarcinoma with high-grade dysplasia (Figure 5D). This case described very early stage of IPNB having component of invasive carcinoma arising from most high-grade dysplasia without regional organ involvement, and distant metastasis.
The patient was discharged without any postoperative complications and has remained stable for 8 mo after surgery. During every 3 mo follow-up after discharge, there was no recurrence on follow-up CT scan.
NF-1, which is also known as von Recklinghausen’s disease, is an inherited disorder caused by a mutation of NF1 (chromosome 17q11.2), which acts as a tumor suppressor gene[1]. A previous study reported that intra-abdominal manifestations of NF-1 occurred at frequencies of 5%-25%[4,5]. Intra-abdominal manifestations are known to develop after middle age, particularly following cutaneous manifestations. With age, patients with NF-1 experience increased numbers of both benign and malignant tumors[6]. Neurofibroma is the most common type of benign tumor experienced by NF-1 patients, whereas optic pathway glioma is a common intracranial neoplasm[7].
NF1 encodes a protein called neurofibromin, which regulates RAS activity. RAS activates the stem cell factor/c-KIT signaling pathway and the mitogen-activated protein kinase pathway, leading to cell proliferation[8]. This activation has been linked to a wide spectrum of clinical manifestations of NF-1, including associated tumors[9]. GIST is the most common gastrointestinal manifestation associated with NF-1[9]. Most GISTs in these patients are small and asymptomatic, and follow a benign course. Given the asymptomatic nature of smaller GISTs, early diagnosis is important to reduce the risk of transformation to malignancy. Notably, these GISTs rarely harbor mutations in KIT and PDGFRA (encodes platelet-derived growth factor receptor-alpha)[10,11].
NF-1 is associated with an increased risk of tumors such as neurofibroma and optic pathway ganglioma, but not with gastrointestinal tract adenocarcinoma. As mentioned earlier, IPNB has not previously been reported in patients with NF-1. The term IPNB encompasses both intraductal papillary cholangiocarcinoma and its precursor lesion. IPNB is mainly caused by hepatolithiasis and clonorchiasis, and 40%-80% of these lesions comprise an invasive carcinoma or tubular or mucinous adenocarcinoma[12,13]. The laboratory findings of IPNB mainly involve obstructive jaundice patterns with bilirubin elevation, with elevated CA 19-9 levels in approximately 40% of patients[14].
In the absence of distant metastasis, surgical resection is the first treatment option for IPNB[12,14]. Hepatectomy is performed for IPNB in the IHD, whereas surgical resection is performed for cases involving the common bile duct. In the absence of an increased recurrence risk (i.e., positive lymph node or advanced tumor invasion), subsequent liver transplantation may be an additional treatment option[15]. In the presence of distant metastasis, percutaneous transhepatic biliary drainage, a palliative treatment, helps to improve obstructive jaundice. Patients with IPNB tend to have a better prognosis relative to those with conventional bile duct cholangiocarcinoma[16]. However, prognostic factors include the biology of IPNB and the intraductal growth pattern[12,13,16].
In the present case, a patient with NF-1 presented with various gastrointestinal tumors, including GIST and IPNB, and a benign mucinous cystadenoma of the adrenal gland. As noted earlier, gastrointestinal neoplasms are mostly asymptomatic in patients with NF-1. Kistler et al[2] noted that there are currently no screening guidelines for gastrointestinal tumors in patients with NF-1, regardless of tumor type. Although sufficient clinical evidence of the screening of older NF-1 patients is lacking, it would be advisable to routinely perform endoscopic and CT examinations of older (i.e., beyond middle-aged) patients to screen for gastrointestinal neoplasms, including GIST. This case also demonstrates the possibility of an increased CA 19-9 level with IPNB, and suggests that screening procedures should include CA 19-9 and other potential diagnostic tumor biomarkers may be used as diagnostic biomarker for IPNB in NF-1 patients.
Although there were similar cases of periampullary tumors with GIST in NF-1 patients[17,18] or GIST and somatostatinoma in patients with Von Recklinghausen’s Disease[19], this is the first case in the global literature to demonstrate synchronous IPNB and GIST in a patient with NF-1. According to the references, the prognosis is determined by depth of invasion, component of invasive carcinoma, and grade[12]. We expected a long recurrence-free survival because this case did not have poor prognostic factor. In previous report, the effect of NF-1 on tumor prognosis has not been reported. However, considering the increased risk of developing tumors due to genetic abnormalities, the possibility that NF-1 may be associated with the prognosis of malignant tumors should be considered. Although these tumors are generally very rare, suspicion and early diagnosis are important for asymptomatic patients with NF-1. Further clinical data is needed to establish a guideline for screening gastrointestinal tumors in this patient population.
A 72-year-old Asian woman who was diagnosed with neurofibromatosis type 1 (NF-1) presented with right upper abdominal pain.
The patient had a negative Murphy’s sign, and no evidence of jaundice. There was no palpable mass in right upper quadrant.
Cholecystitis, choledocholithiasis, cholangiocarcinoma, hepatocellular carcinoma
Aspartate transaminase, alanine transaminase, and total bilirubin were within normal range. Alkaline phosphatase, γ-glutamyltransferase was increased slightly.Carcinoembryonic antigen is 3.4 ng/mL, however, cancer antigen 19-9 level was elevated to 211.3 IU/mL.
Computed tomography showed a low-attenuated multilocular mass with septation, and a mural nodule containing a soft tissue-enhancing lesion in the right hepatic lobe. In addition, a 1.7-cm-sized, well-demarcated enhancing mass was observed in the third portion of the duodenum.
Histologic results confirmed the diagnosis of benign gastrointestinal stromal tumor (GIST) in the third portion of the duodenum. In addition, the resected hepatic mass was intraductal papillary neoplasm of bile duct (IPNB).
Surgical resection was curative treatment. Both GIST and IPNB were successfully resected.
NF-1 is associated with an increased risk of tumors. Recently, there were related reports: case of a mixed periampullary adenocarcinoma and a somatostatinoma with a gastrointestinal stromal tumor in NF-1 patients, triad of periampullary carcinoid, duodenal gastrointestinal stromal tumor and plexiform neurofibroma at hepatic hilum in NF-1.
IPNB is a variant of bile duct carcinoma that is characterized by intraductal growth. It have better outcomes compared with common cholangiocarcinoma.
Suspicion and early diagnosis are important for asymptomatic patients with NF-1.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Engin G, Letizia C, Misiakos EP S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Kistler CA, Johnson JM, Winter JM, Baliff JP, Siddiqui AA, Sama AR. A case of periampullary adenocarcinoma in neurofibromatosis type 1. J Gastrointest Oncol. 2014;5:E96-E99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Relles D, Baek J, Witkiewicz A, Yeo CJ. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg. 2010;14:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen’s disease). Histopathology. 1991;19:1-11. [PubMed] |
| 5. | Heuschkel R, Kim S, Korf B, Schneider G, Bousvaros A. Abdominal migraine in children with neurofibromatosis type 1: a case series and review of gastrointestinal involvement in NF1. J Pediatr Gastroenterol Nutr. 2001;33:149-154. [PubMed] |
| 6. | Gutmann DH. Recent insights into neurofibromatosis type 1: clear genetic progress. Arch Neurol. 1998;55:778-780. [PubMed] |
| 7. | Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108:193-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Basile U, Cavallaro G, Polistena A, Giustini S, Orlando G, Cotesta D, Petramala L, Letizia C, Calvieri S, De Toma G. Gastrointestinal and retroperitoneal manifestations of type 1 neurofibromatosis. J Gastrointest Surg. 2010;14:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen’s disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012;5:852-862. [PubMed] |
| 10. | Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Gorgel A, Cetinkaya DD, Salgur F, Demirpence M, Yilmaz H, Karaman EH, Tutuncuoglu P, Oruk G, Bahceci M, Sari AA. Coexistence of gastrointestinal stromal tumors (GISTs) and pheochromocytoma in three cases of neurofibromatosis type 1 (NF1) with a review of the literature. Intern Med. 2014;53:1783-1789. [PubMed] |
| 12. | Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, Zhao HT, Sang XT. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol. 2013;19:8595-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 14. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Vibert E, Dokmak S, Belghiti J. Surgical strategy of biliary papillomatosis in Western countries. J Hepatobiliary Pancreat Sci. 2010;17:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, Fong Y, DeMatteo RP, D’Angelica M, Koea J. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703-712; discussion 712-714. [PubMed] |
| 17. | Tewari N, Rollins K, Gandhi N, Kaye P, Lobo DN. Mixed periampullary adenocarcinoma and somatostatinoma with small bowel gastrointestinal stromal tumour in neurofibromatosis type 1. JOP. 2014;15:600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Yamamoto R, Kato S, Maru T, Ninomiya R, Ozawa F, Beck Y, Abe K, Tamaru J, Nagoshi S, Yakabi K. The Coexistence of Somatostatinoma and Gastrointestinal Stromal Tumor in the Duodenum of a Patient with Von Recklinghausen’s Disease. Intern Med. 2016;55:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Abdessayed N, Gupta R, Mestiri S, Bdioui A, Trimech M, Mokni M. Rare triad of periampullary carcinoid, duodenal gastrointestinal stromal tumor and plexiform neurofibroma at hepatic hilum in neurofibromatosis type 1: a case report. BMC Cancer. 2017;17:579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |