Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.461
Peer-review started: September 12, 2017
First decision: October 18, 2017
Revised: November 22, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: January 28, 2018
Processing time: 136 Days and 11.8 Hours
To study the effect of 18-hydroxy-eicosapentaenoic acid (18-HEPE) and 17-hydroxy-docosahexaenoic acid (17-HDHA) in a murine model of obesity/nonalcoholic fatty liver disease.
C57BL/6 mice were fed with standard chow diet (CD) or high-fat, fructose-enriched diet (HFD) for 16 wk. Then, three groups were treated for 14 d with either, diet switch (HFD for CD), 18-HEPE, or 17-HDHA. Weight and fasting glucose were recorded on a weekly basis. Insulin tolerance test was performed at the end of treatment. Histological analysis (HE and Masson’s trichrome stain) and determination of serum insulin, glucagon, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide, adiponectin and resistin were carried out as well as liver proteins by western blot.
Mice treated with hydroxy-fatty acids 18-HEPE and 17-HDHA displayed no weight loss or improved insulin sensitivity. However, these mice groups showed a significant amelioration on serum GLP-1, adiponectin and resistin levels. Also, a significant reduction on inflammatory infiltrate was observed at both portal and lobular zones. Furthermore, up-regulation of PPARα/γ protein levels was observed in liver tissue and it was associated with decreased levels of NF-κB also determined by western blot analysis. On the other hand, diet switch regimen resulted in a marked improvement in most parameters including: weight loss, increased insulin sensitivity, decreased steatosis, restored levels of insulin, glucagon, leptin, adiponectin and resistin. However, no significant changes were observed regarding inflammatory infiltrate in this last group.
18-HEPE and 17-HDHA differentially exert hepatoprotective effects through up-regulation of nuclear receptors PPARα/γ and amelioration of serum adipokines profile.
Core tip: Our study aimed to prove the efficacy of hydroxy-fatty acids 18-hydroxy-eicosapentaenoic acid and 17-hydroxy-docosahexaenoic acid (17-HDHA) in an obesity/nonalcoholic fatty liver disease (NAFLD) model in mice. We determined the effect of these molecules on histological morphology as well as in protein levels of key nuclear receptors and serum hormones, incretins and adipokines as these parameters are altered in NAFLD. We reported an effect by these hydroxy-fatty acids on the most relevant target proteins involved in this pathological process (PPARα/γ). Also, we demonstrated that diet switch regimen is a selective treatment control as most NAFLD markers and histological alterations were ameliorated by this intervention.
- Citation: Rodriguez-Echevarria R, Macias-Barragan J, Parra-Vargas M, Davila-Rodriguez JR, Amezcua-Galvez E, Armendariz-Borunda J. Diet switch and omega-3 hydroxy-fatty acids display differential hepatoprotective effects in an obesity/nonalcoholic fatty liver disease model in mice. World J Gastroenterol 2018; 24(4): 461-474
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.461
Nonalcoholic fatty liver disease (NAFLD) has become a major chronic liver condition over the last decades[1]. It comprises a wide range of morphological alterations ranging from simple steatosis to an inflammatory state known as nonalcoholic steatohepatitis. Should the inflammation persist throughout the years, it could potentially lead to advanced established fibrosis and ultimately become a form of end-stage liver disease which includes cirrhosis and hepatocellular carcinoma[2]. Notably, NAFLD shows a high growth rate in the Americas, and it is thought to derive mainly from modern lifestyle habits featuring low physical activity and chronic exposure to high-fat, high-fructose diet[3]. Those mentioned factors have dramatically increased the prevalence of obesity and metabolic syndrome along with its comorbidities: dyslipidemia, insulin resistance, and hypertension[4].
Although the typical fat vesicles in the liver can be originated by de novo lipogenesis from an excess of dietary substrates, in the case of NAFLD it is largely the result of a hypertrophied insulin-resistant white adipose tissue. Such event leads to hyperlipidemia in which the released fatty acids reach the liver where they can be esterified and stored within hepatocytes[5]. Remarkably, it has been proposed that NAFLD might be endorsed by a constant vicious cycle operating insulin resistance and progressing fatty liver as both conditions frequently coexist[6]. Furthermore, insulin resistance is primarily triggered by low-grade chronic inflammation. In NASH, just as in many other pathological conditions, persistence of inflammatory cell infiltration (in this case white adipose tissue and liver) is a remarkable feature[7].
Currently, the first line treatment for NAFLD remains weight loss and overall lifestyle modification including physical activity and healthy diet[8]. However, given the complexity of obesity treatment, in many cases it leads to an elevated number of unsuccessful attempts. These facts have prompted a tremendous need for alternative strategies. In this regard, several drugs have been proposed in the clinical scenario over the last years such as pioglitazone, vitamin E, liraglutide, sitagliptine, elafibranor, obeticholic acid, and pentoxifylline just to name a few. Additionally, a large pipeline of preclinical studies are under way[9]. They are generally intended to target major features of NAFLD either separately or combined (lipid accumulation, oxidative stress, inflammation, and fibrosis).
Diet-wise, a low intake of saturated fat and fructose from soft drinks has been part of NAFLD treatment[3,10]. However, much attention has been paid to the anti-inflammatory and lipid-lowering properties of other types of fats such as ω 3 polyunsaturated fatty acids (ω 3 PUFA), which have long been investigated and showed positive impact on cardiovascular and hepatic alterations as well as in overall health[11-13]. Actions exhibited by these fatty acids became a niche in lipid research in the late 1970s after a study conducted in a Greenland Inuit population[14]. Furthermore, there is a wide family of 3 PUFA-derived compounds mainly produced by enzymatic oxidation routes on eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that are thought to exert more potent actions compared to their non-oxidized versions. In this regard, 18-hydroxy-eicosapentaenoic acid (18-HEPE) and 17-hydroxy-docosahexaenoic acid (17-HDHA) have been reported to possess a high affinity for nuclear receptors such as peroxisome proliferator-activated receptors α and γ (PPARα and PPARγ) which in turn orchestrate key processes on lipid metabolism and inflammation[15,16]. In fact, the effects of 18-HEPE and 17-HDHA as well as other hydroxy-fatty acids have been tested on several metabolic and chronic inflammation models at dosages within the nanomolar range and showed protective effects[17-19]. Finally, we conducted this study with the aim to assess the effect of 18-HEPE and 17-HDHA in an obesity/NAFLD model in C57BL/6 mice.
This research protocol was approved by the CUCS Research Committee from Universidad de Guadalajara. Also, it was carried out in accordance with the National Institutes of Health guide for care and use of laboratory animals. five-week old male C57BL/6NHsd mice were purchased from Harlan (Mexico City) and were fed with standard chow for 1 wk to stabilize their metabolism. The animals were group-housed in polycarbonate cages in a moderated environment and temperature at 22 ± 1 °C and a 12 h light/dark cycle. Mice were randomly allocated to cages and fed with either standard chow diet (CD) or high-fat, fructose-enriched diet (HFD) ad libitum for 16 wk to induce obesity and NAFLD. CD group received standard diet Prolab RHM 2500 5P14* (12% of calories from fat) and had free access to pure water, whereas HFD group received Testdiet 58V8 diet (45% of calories from fat) and had free access to high fructose-enriched water at a concentration of 42 g/L (ratios at 55% fructose and 45% sucrose). Treatment of the following began on week 17th and it lasted 14 days: intraperitoneal administration of either 18-HEPE or 17-HDHA every 24 h (Cayman Chemicals; CAS 141110-17-0 and CAS 90780-52-2) in 100 μL 0.9% saline with 2% ethanol as vehicle. Additionally, one HFD-fed group underwent diet switch for chow diet and pure water plus vehicle as a third type of control. At the end of the treatment (18th week) mice were euthanized with tiletamine-zolazepam (15 mg/kg body weight), blood was extracted by cardiac puncture and centrifuged for serum separation, whereas liver tissue was collected and kept at -70 °C for further molecular analysis and fixed in 4% paraformaldehyde for histological analysis.
Animals were weighed on a weekly basis systematically at 9:00-10:00 during the entire protocol. They were five-hour fasted prior blood glucose determination (One Touch Ultra, LifeScan Inc., Wayne, PA, United States). Additionally, to assess insulin sensitivity, all mice underwent an insulin tolerance test (ITT) by the end of the 18th week. Mice were short-fasted for 5 h, basal blood glucose was determined and shortly after this, 100 μl saline solution containing a standardized dose of 0.025 IU of human-recombinant short-acting insulin (Humulin R, Lilly, Indianapolis, IN, United States) was intraperitoneally administered in every animal. Blood glucose measurement was repeated thereafter at 30 min and 60 min. An additional solution of dextrose in sterile water was ready to use in case an animal might be at risk of death by the hypoglycemic effect of short-acting insulin. Once the protocol was finished, all animals were given free access to food and water. No animal losses occurred during the ITT.
Blood was allowed to clot during 20 min at room temperature and then centrifuged at 1500 × g for 10 min in a refrigerated centrifuge. Insulin, glucagon, leptin, ghrelin, glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), adiponectin and resistin were measured in mouse serum by multiplex detection immunoassay (Bio-Plex Pro Diabetes Assay #171F7001M, Bio-Rad Laboratories, Inc., Hercules, CA, United States) according to manufacturer instructions.
Morphological and extracellular matrix deposition assessment was carried out in liver tissue, which was harvested and immersed in a fixation solution (4% paraformaldehyde and 0.1 mol/L PBS at pH 7.4). Afterwards, tissues were embedded in paraffin wax. Serial block (5 μm) sections were subjected to hematoxylin-eosin (HE) and Masson Trichrome staining according to standard procedures. An independent pathologist performed histology grading based on NAS (NASH Activity Score)[20]. All parameters like hepatocyte ballooning, lobular and portal inflammation were scored 0-3. Fibrosis was determined by morphometrical analysis (ImagePro, Rockville, MD, United States).
Liver protein was extracted as follows: total protein was extracted in lysis buffer containing 1 mol/L Tris/HCl pH 7.4, 1% triton X-100, 10% glycerol, 137 mmol/L NaCl, 0.5 mmol/L EDTA, and protease inhibitors (Complete Protease Inhibitor Cocktail, Sigma-Aldrich Corp., Si. Louis, MO, United States; NaF and Sodium Orthovanadate). Homogenate lysates were centrifuged at 12000 g for 30 min at 4 °C. Briefly, aliquots from each sample containing 30 g of total protein quantified by the Bradford protein quantification assay were resuspended in SDS-containing Laemmli sample buffer, heated for 5 min at 95 °C, and separated through 10% SDS-PAGE under reducing conditions (2-mercaptoethanol). Proteins were electro-blotted overnight at 4 °C onto PVDF membranes and the efficiency of the transfer was confirmed by Ponceau staining. Thereafter, membranes were blocked 1 h at room temperature in Tris-buffered saline (20 mmol/L Tris/HCl pH 7.5 and 0.5 mol/L NaCl) containing 0.1% (v/v) Tween 20 (0.1% T-TBS) and 5% (w/v) nonfat dry milk. Blots were washed three times for 5 min each with 0.1% T-TBS and subsequently incubated for 2 h at room temperature with primary mouse/rabbit polyclonal antibodies: anti-PPARα ab8934 (1:1000), anti-LXRα ab3585 (1:300), anti-CPT1A ab128568 (1:1000), anti-ACOX1 ab59964 (1:1000), anti-SREBP1 ab3259 (1:1000), anti-PPARγ ab19481 (1:1000) (Abcam, Cambridge, MA, United States), and anti-NF-κB #8242 (1:1000) (Cell Signaling, Danvers, MA, United States) in 0.05% T-TBS containing 1% BSA. After washing the blots three times for 5 min each with 0.1% T-TBS, the membranes were incubated for 1 h at room temperature with a peroxidase-linked anti-mouse/rabbit antibody (1:16000) in 0.01% T-TBS. To normalize against a loading control, all membranes were stripped and reblotted with anti-β-actin (1:5000). The obtained bands were visualized by chemiluminescence (BM Chemiluminescence Western Blotting substrate POD, Sigma-Aldrich Corp., Si. Louis, MO, United States) kit and quantified using ChemiDoc MP Imaging System with Image Lab software (Bio-Rad Laboratories, Inc, Carlsbad, CA, United States).
Alfa-mouse smooth muscle actin (αSMA) immunohistochemistry
Liver biopsies were subjected to react with anti-mouse smooth muscle actin (α-SMA) antibody, which was obtained from Boehringer (Mannheim, Germany). Briefly, histological-processed liver sections were deparaffinized and endogenous activity of peroxidase was quenched with a solution 0.03% H2O2 in methanol. Tissue was incubated with a 1/100 dilution of a monoclonal goat anti-mouse α-SMA antibody. Anti-goat peroxidase-labeled secondary antibody was revealed with diaminobenzidine and tissue was counterstained with Harris’s hematoxylin
Data are expressed as mean ± standard error of the mean (SEM). Groups were compared using Mann-Whitney U test for quantitative data and Fisher’s exact test for qualitative data. All analyzes were performed using Statistical Program for Social Sciences (SPSS v20.0) for Windows medical pack (Chicago, IL, United States). Statistical significance was determined at P < 0.05.
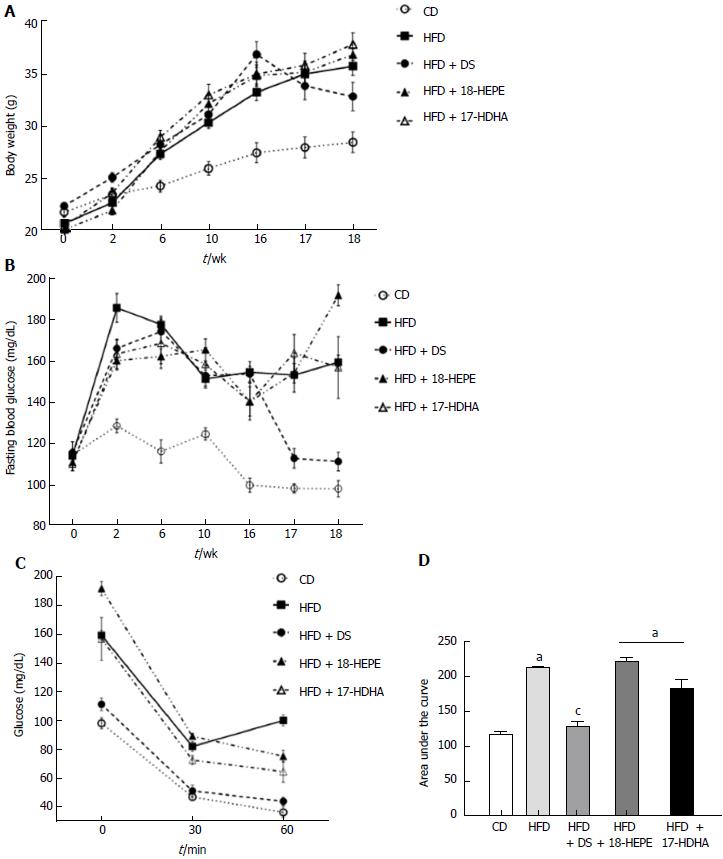
As shown in Figure 1A and B, all HFD-derived groups showed significant increase in weight from the sixth week onwards, whereas fasting glucose alterations appeared sooner at the second week. Diet switch (HFD + DS) reduced body weight and restored fasting glucose levels (32.8 ± 1.3 g and 111.3 ± 1.3 mg/dL respectively) compared to HFD group (35.8 ± 0.9 g and 159.1 ± 3.4 mg/dL). Administration of both 18-HEPE and 17-HDHA showed no differences in weight vs HFD (36.3 ± 0.5 g and 37.3 ± 1.1 g). Regarding fasting glucose, we observed higher levels in the HFD+18-HEPE group (191.5 ± 5.0 mg/dL) while HFD+17-HDHA group showed a similar value to HFD group.
Blood glucose values were plotted (mg/dL) and area under the curve (AUC) was calculated based on these data (Figure 1C and 1D). The lowest AUC value was observed in CD (60.6 ± 19 Arbitrary Units, AU) while the highest values in the graphic are observed in the HFD group (113.8 ± 23 AU), as well as in both HFD + 18-HEPE and HFD + 17-HDHA groups (118.7 ± 37 and 98 ± 29 AU). HFD + DS was the only group that displayed improved insulin sensitivity (68.9 ± 21 AU).
As shown in Table 1, analysis of daily energy intake was divided into two phases: prior and during treatment. First, we observed a significant difference between CD and all HFD-derived groups. While CD showed a mean daily consumption of 11.9 ± 0.3 kcal, HFD, HFD + DS, HFD + 18-HEPE, and HFD + 17-HDHA showed higher energy intake values (13.1.0 ± 0.2, 13.0 ± 0.3, 13.1 ± 0.3, and 13.0 ± 0.2 kcal respectively). Further, during treatment phase, values in CD, HFD, HFD + 18-HEPE, and HFD + 17-HDHA groups remained unaltered. However, we observed a significant decrease in daily energy intake in the HFD + DS group (10.7 ± 0.4).
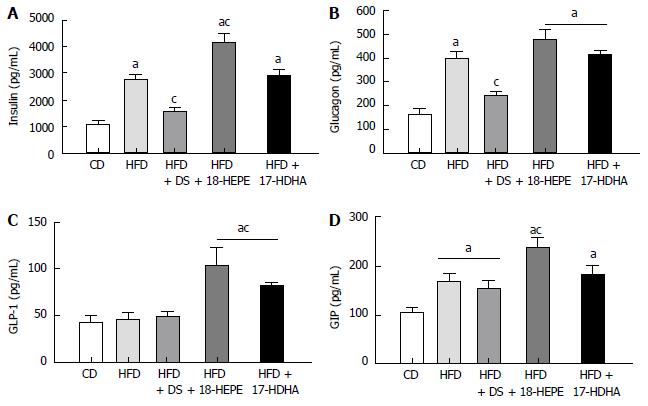
Insulin and glucagon (Figure 2A and B) were significantly increased in HFD, HFD + 18-HEPE and HFD + 17-HDHA groups (insulin: 2736 ± 119, 4138 ± 351, and 2889 ± 1242 pg/mL; glucagon: 397 ± 29, 477 ± 38, and 422 ± 10 pg/mL respectively) compared to CD group (insulin: 1105 ± 142; glucagon: 162 ± 24 pg/mL). In fact, insulin levels in HFD + 18-HEPE group were significantly higher compared to HFD group. On the other hand, HFD + DS group displayed lower levels in both hormones (insulin: 1580 ± 95; glucagon: 239 ± 14 pg/mL). Following with incretins levels, GLP-1 (Figure 2C) remained widely without significant differences among CD, HFD, and HFD+DS groups (42 ± 7, 47 ± 6, and 49 ± 5 pg/mL respectively). Administration of both 18-HEPE and 17-HDHA showed a significant increase in GLP-1 levels (103 ± 18, and 81 ± 4 pg/mL respectively). Finally, GIP levels (Figure 2D) were increased in all HFD-derived groups compared to CD (105.4 ± 8 pg/mL), however HFD + 18-HEPE group showed significantly higher levels compared to HFD group (237 ± 21 vs 169 ± 18 pg/mL). No significant differences were found among the rest of the groups or in comparison to HFD group (HFD + DS 151 ± 18 pg/mL and HFD + 17-HDHA 181 ± 19 pg/mL).
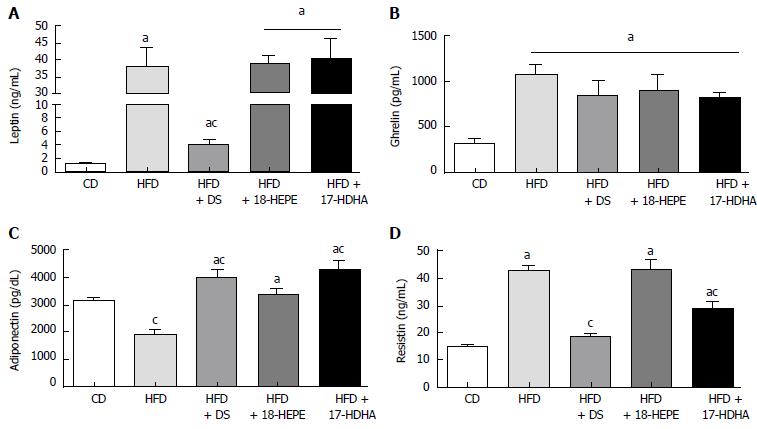
Leptin and ghrelin levels (Figure 3A and B) were significantly increased in HFD, HFD + DS, HFD + 18-HEPE, and HFD + 17-HDHA groups (leptin: 37.8 ± 5.7, 4.1 ± 0.7, 38.4 ± 3.3 and 40.0 ± 6.6 ng/mL; ghrelin: 1070 ± 114, 847 ± 173, 902 ± 176 and 817 ± 68 pg/mL respectively) compared to CD group (leptin: 0.94 ± 0.3 ng/mL; ghrelin: 322 ± 44 pg/mL). However, HFD + DS group showed a significant decrease in leptin levels compared to HFD group. On the other hand, ghrelin remained widely unchanged regardless of treatment. With regards to adipokines, adiponectin (Figure 3C) was significantly reduced in HFD group compared to CD group (1881 ± 213 and 3172 ± 83 pg/mL respectively). Diet switch and administration of both 18-HEPE and 17-HDHA showed a significant increase in adiponectin levels vs HFD group (3998 ± 305, 3367 ± 257, and 4297 ± 333 pg/mL respectively). In contrast, resistin levels (Figure 3D) were significantly increased in HFD group compared to CD (42.8 ± 1.9 and 14.8 ± 1.8 ng/mL respectively). Concerning treated groups, only HFD + DS and HFD + 17-HDHA showed decreased levels of resistin compared to HFD group (18.3 ± 1.6 and 28.8 ± 2.5 ng/mL respectively), while administration of HFD + 18-HEPE (43.4 ± 3.6 ng/mL) showed no effect in resistin levels.
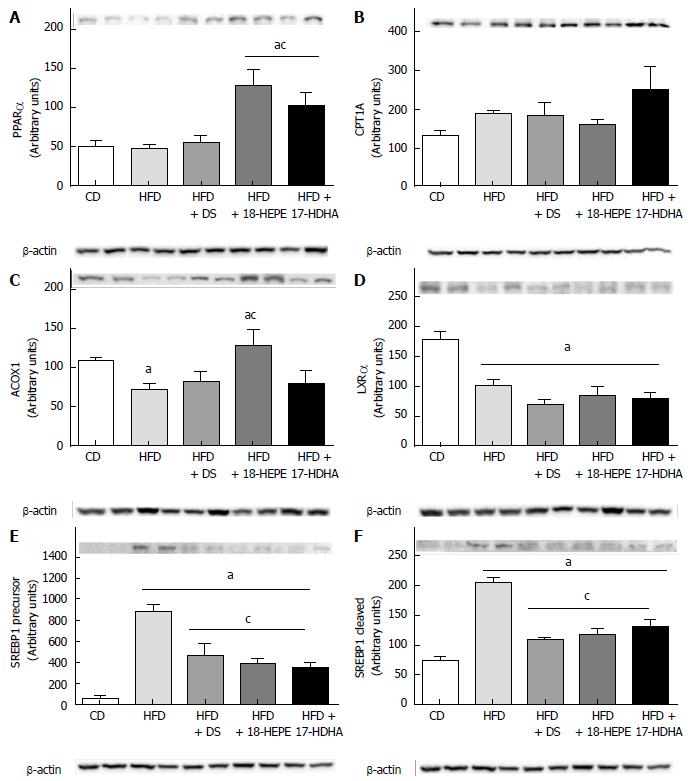
Western blot analysis of lipid oxidation-related proteins comprised PPARα (Figure 4A) and its target genes CPT1A and ACOX1 (Figure 4B and C). Administration of both 18-HEPE and 17-HDHA produced a significant increase in PPARα compared to both CD and HFD groups. While CPT1A showed no statistical significant differences among groups, ACOX1 revealed to be significantly increased only in HFD + 18-HEPE group. On the other hand, analysis of lipid synthesis-related proteins was conducted by quantifying the relative abundance of LXRα (Figure 4D) and its target gene SREBP1 in both, precursor and cleaved forms (Figure 4E and F). In this regard, LXRα was reduced in all HFD-derived groups with or without treatment compared to CD group. Further, SREBP1 in both isoforms was dramatically increased in HFD group vs CD, and, noteworthy, diminished in all treated groups.
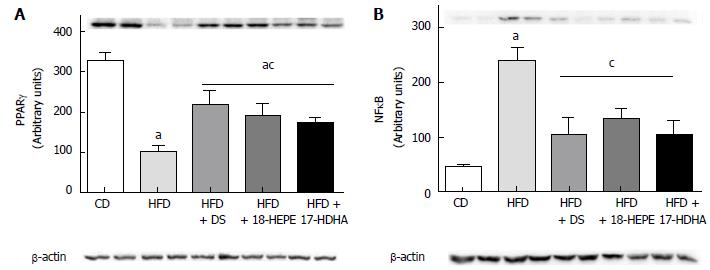
In this regard, two major proteins were analyzed. As shown in Figure 5A, PPARγ showed a dramatic decreased in HFD compared to CD group. Notably, all treated groups produced a significant increase in the relative abundance of PPARγ. In contrast, NF-κB showed a significant increase in HFD compared to CD group. It was also observed that all treatments produced a significant decrease in relative abundance of NF-κB (Figure 5B).
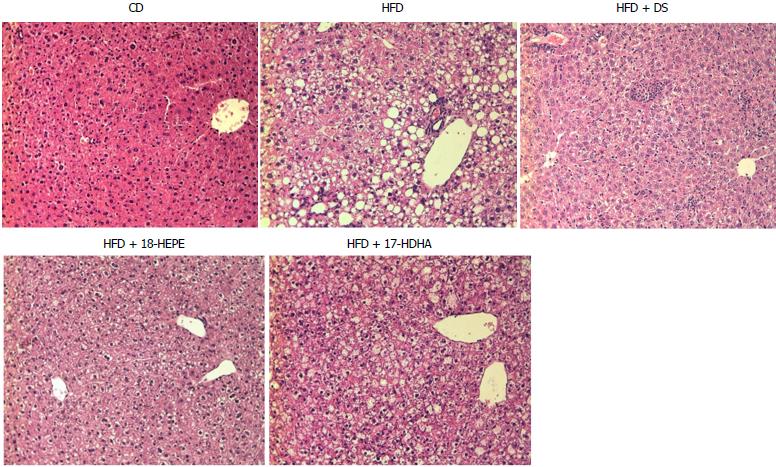
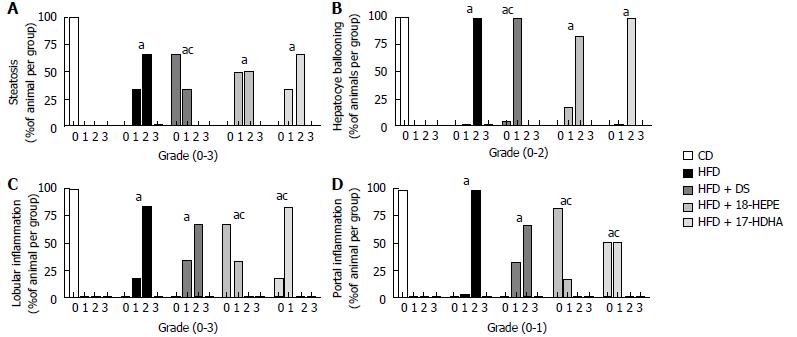
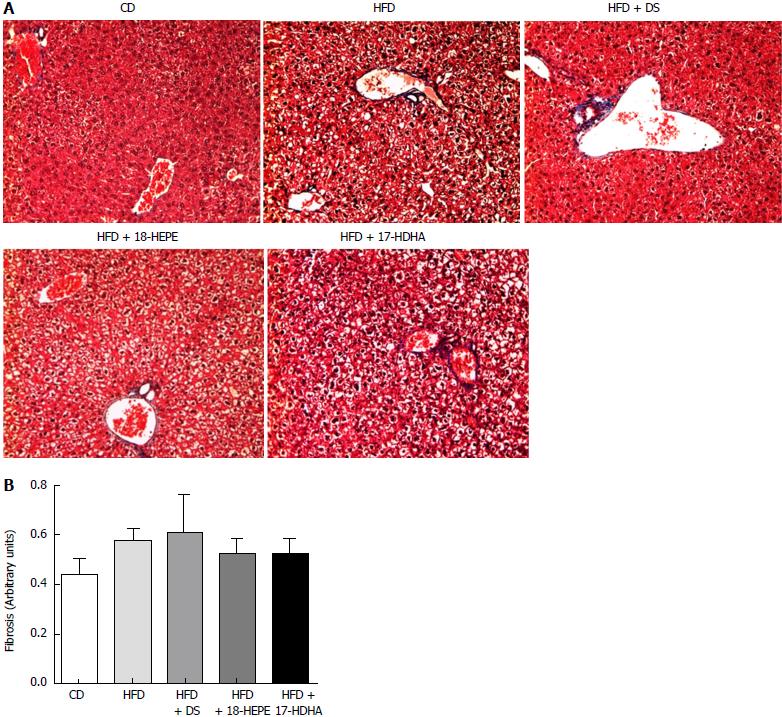
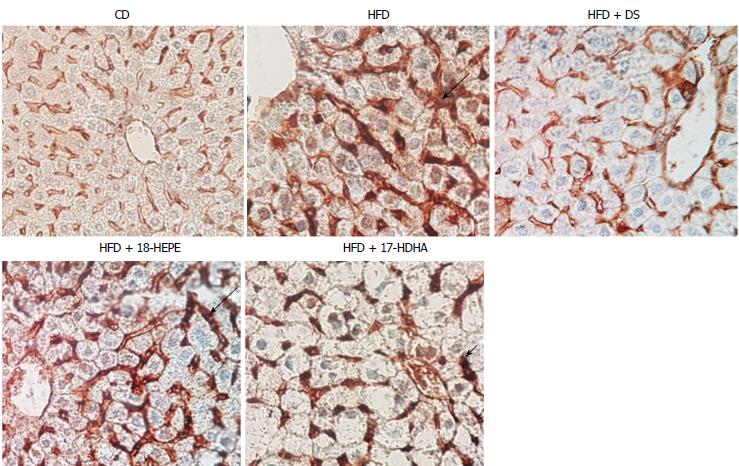
Microscopic liver morphology was conducted following HE standard protocol (Figure 6). HFD group was characterized by steatosis, hepatocyte ballooning and inflammatory infiltrate. The main finding in HFD + DS group was the drastic decrease of number in fat vesicles and ballooning degeneration (Figure 7A and B); however, this group presented a moderate inflammatory-cell aggregates on a great proportion of microphotographs showing no statistical differences vs HFD group. Further, both groups HFD + 18-HEPE and HFD + 17-HDHA showed noticeable changes in steatosis and hepatocyte ballooning compared to HFD group. Besides, both groups displayed scarce presence of inflammatory cells at lobular and portal zones (Figure 7C and D) in different proportions. Analysis of extracellular matrix was also examined, in which we did not observe differences among all groups. (Figure 8A and B). Furthermore, we conducted an immunohistochemistry analysis to determine early development of fibrosis. Therefore, SMA expression was determined. Interestingly enough, HFD group showed an augmented expression of αSMA along the perisinusoidal space compared to CD group. This fact could be representing the prelude of the fibrogenic process. Noteworthy, HFD + DS and HFD + 17-HDHA groups showed a pronounced reduction in αSMA expression, whereas HFD + 18-HEPE group displayed only a modest decrease (Figure 9).
The growing prevalence of obesity and its comorbidities such as NAFLD has urged the need for research on additional alternative therapies. In our study, we demonstrated that administration of 3 hydroxy-fatty acids 18-HEPE and 17-HDHA decreased hepatic inflammation in obese mice. These actions were mainly associated with the up-regulation of PPARα and PPARγ proteins in liver tissue. In addition, evidence for these effects includes the ameliorated production of serum adipokines (i.e., adiponectin and resistin) independently of body weight. Importantly, we compared the effect of these fatty acids to those observed in a group undergoing diet switch (chow) after 16 wk of high-fat, fructose-enriched diet . The importance of approaching obesity and NAFLD with lifestyle modification including diet and exercise is widely accepted and highly recommended[21]. In fact, analysis of on insulin sensitivity in obese mice treated with normocaloric diet for ten wk was examined by Lombardo et al[22], reporting weight loss, lower insulin levels, improved insulin tolerance associated with increased expression of Glut4. Remarkably in our study, mice undergoing diet switch displayed weight loss, restored fasting glucose levels, and insulin sensitivity by the end of treatment period. Alongside with these findings, insulin, glucagon, leptin, adiponectin and resistin showed restoration in this mice group which could be largely explained by weight loss. Plus, liver histology showed decreased steatosis and ballooning, but no relevant changes in inflammatory infiltrate, which has been described in humans when rapid weight loss takes place either by bariatric surgery or low fat diets in rodents[23,24]. It is possible that the virtually unaltered liver inflammation in HFD + DS mice is a product of a sustained release of free fatty acids from visceral fat, which in turn may produce a transient activation of inflammatory cells. Therefore, we hypothesized that a longer period of diet switch regimen might produce amelioration in lobular and portal inflammation. Unlike the study carried out by Kohli et al[25], we found no fibrosis in liver histology after 16 wk of high-fat, fructose-enriched diet . This might be due to the higher fat percentage in the diet they utilized (60% calories). Nevertheless, similarly to what has been reported to occur in NAFLD in humans, we observed higher serum levels of insulin, glucagon, leptin, ghrelin and resistin in our model[26].
Remarkably, administration of both 18-HEPE and 17-HDHA showed significant higher incretin levels. It has been documented that GLP-1 and GIP secretion can be stimulated by -linolenic acid, EPA, DHA and 5-HEPE through GPR120 in the colon[27,28]. However, in the case of GLP-1, it has become a major target in NAFLD treatment whereas GIP seems to be a controversial piece in glucose homeostasis. It has been reported that suppressing GIP in genetically modified mice is rather beneficial under high fat conditions[29]. Furthermore, GPR120 activation by fatty acids might take place either by oral administration or intracolonic delivery[30], but neither of these techniques were conducted in the animals here studied. Notwithstanding, it has been long reported that intraperitoneal injection may lead to inadvertent administration of some material into the gut, abdominal fat and subcutaneous tissues in a relatively frequent occurrence (14%-24% of cases)[31]. Therefore, our results suggest that the increased GLP-1 and GIP levels could be in part due to eventual administration of hydroxy-fatty acids into the gut. However, the increase serum levels we observed on GLP-1 along with the amelioration on adiponectin and resistin was not sufficient to produce any improvement on insulin resistance in these groups.
Analysis of liver tissue showed a marked increase in relative abundance of PPARα and PPARγ in mice treated with 18-HEPE and 17-HDHA. These finding supports the previous reports on ligand activities exerted by these fatty acids over both nuclear receptors[15,16]. However, their activities were seemingly distinct. We found a significant increase in ACOX1 by 18-HEPE administrations and a tendency to enhanced production of CPT1A by 17-HDHA (both PPARα target genes). Restoration or enhancement in the abundance of these enzymes is likely to promote fat oxidation and therefore ameliorate steatosis. In fact, we found a modest improvement in fat accumulation in the mice treated with these fatty acids. However, diet switch displayed a remarkable clearance of fat vesicles even with normal ACOX1 and CPT1A protein levels. These differences may be related to the diminished energy intake observed in these mice group during diet switch, leading to a negative energy balance without the need to increment oxidation enzymes production. More to the point, LXRα is a nuclear receptor known for its s capacity to activate lipogenesis mainly through up-regulation of SREBP1. The latter is a protein that in physiological conditions is stimulated by insulin, but it has been described to be paradoxically activated in NAFLD mainly by endoplasmic reticulum stress[32]. We observed that levels of this protein in both precursor and cleaved forms were significantly blunted by all treatments. This effect plays a key role in inhibiting de novo lipogenesis and thus, steatosis exacerbation. Importantly, the effects observed in SREBP1 were independent of the levels we found in LXRα, as this nuclear receptor showed no significant difference among treated vs non-treated mice. It is important to mention that the role of LXR in obesity is rather controversial. It has been proposed as a pharmacological target for glucose intolerance[33] through agonists and even it has been reported to be important in inhibiting fibrogenesis[34]. Additionally, both nuclear receptors PPARα and PPARγ display anti-inflammatory actions by inhibiting NF-κB. It has been reported that PPARα can increase NF-κB inhibitor alpha (IκBα) expression and thus, prevent p50/p65 NF-κB translocation into the nucleus for DNA binding[35]. On the other hand, PPARγ has been described to mediSate trans repression on inflammatory genes by a SUMOylation dependent pathway also involving p50/p65 NF-κB[36]. These previous data could explain the diminished relative abundance in NF-κB levels observed in liver tissue. Also, lobular and portal inflammation showed marked amelioration in mice groups treated with fatty acids. In our study, hepatoprotective actions elicited by these hydroxy-fatty acids were associated with increased PPARα and PPARγ proteins in liver tissue. In contrast, a phase 2 trial failed to prove histologic amelioration on individuals with non-alcoholic steatohepatitis using ethyl-eicosapentanoic acid[37]. Explanation for the lack of efficacy seems to lie on the administered dose. The dosing for this trial was selected based on existing data for its efficacy for dyslipidemia in Japan. Thus, it is possible that this dose was not sufficient for an American population. It is important to remark that the dosage used in our study was greatly lower (In average: 950 nanograms per day) compared to previous studies in animals with similar objective using EPA and DHA. Just as previous studies have reported, position of the alcohol group in both EPA and DHA is a relevant fact when it comes down to affinity for nuclear receptors[15,16]. Importantly, these hydroxy-fatty acids originated from ω3 PUFAs exert protective actions noticeable at the nanomolar ranges, many of which have been associated with the resolution of unremitting inflammation[17,38-41]. A major proposed mechanism whereby these novel fatty acids exert anti-inflammatory actions is through enzymatic biotransformation into specialized pro-resolving mediators namely lipoxins, resolvins, protectins and maresins. However, since we did not explore this area, further analyses are required to elucidate these possible mechanisms. Finally, we demonstrated in our work the beneficial properties of 18-HEPE and 17-HDHA in an experimental model under high fat conditions as well as a comparative analysis vs dietetic intervention.
In conclusion, We demonstrated that most serum metabolic parameters and histological features in obese mice are reversible by switching diet regimen from high-fat to low-fat for two wk. This finding supports the evidence of diet switch regimen as a valuable reference point for assessing alternative therapies. Finally, administration of 18-HEPE and 17-HDHA exerted hepatoprotective effects in the liver through up-regulation of nuclear receptors PPARα/γ and amelioration of serum adipokines profile.
Nonalcoholic fatty liver disease (NAFLD) is a major chronic liver condition over the last decades. Notably, NAFLD shows a high growth rate worldwide and it is thought to derive mainly from modern lifestyle habits featuring low physical activity and chronic exposure to high-fat, high-fructose diet. Those mentioned factors have dramatically increased the prevalence of obesity and metabolic syndrome along with its comorbidities: dyslipidemia, insulin resistance, and hypertension.
Several drugs have been proposed in the clinical scenario over the last years such as pioglitazone, vitamin E, liraglutide, sitagliptine, elafibranor, obeticholic acid, and pentoxifylline just to name a few. Also, much attention has been paid to the anti-inflammatory and lipid-lowering properties of other types of fats such as ω 3 polyunsaturated fatty acids (ω3 PUFA), which have long been investigated and showed positive impact on cardiovascular and hepatic alterations as well as in overall health.
Metabolic liver disease is currently a major cause of morbidity worldwide. Research on treatment strategies is in fact an interesting area to explore.
To determine the efficacy of hydroxy-fatty acids in experimental NAFLD/obesity as well as comparing the effects with diet switch regimen.
Histological analysis, western bloting analysis and α-mouse smooth muscle actin immunohistochemistry.
Mice treated with hydroxy-fatty acids 18-hydroxy-eicosapentaenoic acid (18-HEPE) and 17-hydroxy-docosahexaenoic acid (17-HDHA) displayed no weight loss or improved insulin sensitivity. However, these mice groups showed a significant amelioration on serum GLP-1, adiponectin and resistin levels. Also, a significant reduction on inflammatory infiltrate was observed at both portal and lobular zones. Furthermore, up-regulation of PPAR α/γ protein levels was observed in liver tissue and it was associated with decreased levels of NF-κB also determined by western blot analysis. On the other hand, diet switch regimen resulted in a marked improvement in most parameters including: weight loss, increased insulin sensitivity, decreased steatosis, restored levels of insulin, glucagon, leptin, adiponectin and resistin. However, no significant changes were observed regarding inflammatory infiltrate in this last group.
Most serum metabolic parameters and histological features in obese mice are reversible by switching diet regimen from high-fat to low-fat for two wk. This finding supports the evidence of diet switch regimen as a valuable reference point for assessing alternative therapies. Finally, administration of 18-HEPE and 17-HDHA exerted hepatoprotective effects in the liver through up-regulation of nuclear receptors PPARα/γ and amelioration of serum adipokines profile.
Just as previous studies have reported, position of the alcohol group in both eicosapentaenoic acid and DHA is a relevant fact when it comes down to affinity for nuclear receptors. Importantly, these hydroxy-fatty acids originated from ω 3 PUFAs exert protective actions noticeable at the nanomolar ranges, many of which have been associated with the resolution of unremitting inflammation. A major proposed mechanism whereby these novel fatty acids exert anti-inflammatory actions is through enzymatic biotransformation into specialized pro-resolving mediators namely lipoxins, resolvins, protectins and maresins. However, since we did not explore this area, further analyses are required to elucidate these possible mechanisms.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inzaugarat E, Sherif Z S- Editor: Chen K L- Editor: A E- Editor: Ma YJ
| 1. | Bellentani S, Bedogni G, Miglioli L, Tiribelli C. The epidemiology of fatty liver. Eur J Gastroenterol Hepatol. 2004;16:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 2. | Wong CR, Nguyen MH, Lim JK. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22:8294-8303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Nseir W, Nassar F, Assy N. Soft drinks consumption and nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:2579-2588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 139] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 4. | Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. 2015;26:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 6. | Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol. 2011;7:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Rius B, López-Vicario C, González-Périz A, Morán-Salvador E, García-Alonso V, Clária J, Titos E. Resolution of inflammation in obesity-induced liver disease. Front Immunol. 2012;3:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 973] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 9. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 10. | Mells JE, Fu PP, Kumar P, Smith T, Karpen SJ, Anania FA. Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J Nutr Biochem. 2015;26:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Yuan F, Wang H, Tian Y, Li Q, He L, Li N, Liu Z. Fish oil alleviated high-fat diet-induced non-alcoholic fatty liver disease via regulating hepatic lipids metabolism and metaflammation: a transcriptomic study. Lipids Health Dis. 2016;15:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Nobili V, Alisi A, Musso G, Scorletti E, Calder PC, Byrne CD. Omega-3 fatty acids: Mechanisms of benefit and therapeutic effects in pediatric and adult NAFLD. Crit Rev Clin Lab Sci. 2016;53:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Jacobson TA. cardiovascular disease. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease 1-3 RESPONSE. Am J Clin Nutr. 2009;87:1981S-1990S. [PubMed] |
| 14. | Dyerberg J, Bang HO. Lipid metabolism, atherogenesis, and haemostasis in Eskimos: the role of the prostaglandin-3 family. Haemostasis. 1979;8:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Yamada H, Oshiro E, Kikuchi S, Hakozaki M, Takahashi H, Kimura K. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J Lipid Res. 2014;55:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, Hochbrugger EE, Fraisl P, Cinti S, Serhan CN. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62:1945-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J Exp Med. 2014;211:1673-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Yamada H, Kikuchi S, Hakozaki M, Motodate K, Nagahora N, Hirose M. 8-Hydroxyeicosapentaenoic Acid Decreases Plasma and Hepatic Triglycerides via Activation of Peroxisome Proliferator-Activated Receptor Alpha in High-Fat Diet-Induced Obese Mice. J Lipids. 2016;2016:7498508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8239] [Article Influence: 412.0] [Reference Citation Analysis (5)] |
| 21. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 22. | Lombardo GE, Arcidiacono B, De Rose RF, Lepore SM, Costa N, Montalcini T, Brunetti A, Russo D, De Sarro G, Celano M. Normocaloric Diet Restores Weight Gain and Insulin Sensitivity in Obese Mice. Front Endocrinol (Lausanne). 2016;7:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 526] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Yamazaki T, Okawa S, Takahashi M. The effects on weight loss and gene expression in adipose and hepatic tissues of very-low carbohydrate and low-fat isoenergetic diets in diet-induced obese mice. Nutr Metab (Lond). 2016;13:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 26. | Petta S, Gastaldelli A, Rebelos E, Bugianesi E, Messa P, Miele L, Svegliati-Baroni G, Valenti L, Bonino F. Pathophysiology of Non Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem. 2015;26:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Yamane S, Harada N, Inagaki N. Mechanisms of fat-induced gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide secretion from K cells. J Diabetes Investig. 2016;7 Suppl 1:20-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Nasteska D, Harada N, Suzuki K, Yamane S, Hamasaki A, Joo E, Iwasaki K, Shibue K, Harada T, Inagaki N. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 2014;63:2332-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Morishita M, Tanaka T, Shida T, Takayama K. Usefulness of colon targeted DHA and EPA as novel diabetes medications that promote intrinsic GLP-1 secretion. J Control Release. 2008;132:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Miner NA, Koehler J, Greenaway L. Intraperitoneal injection of mice. Appl Microbiol. 1969;17:250-251. [PubMed] |
| 32. | Colgan SM, Tang D, Werstuck GH, Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol. 2007;39:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA. 2003;100:5419-5424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 34. | Xing Y, Zhao T, Gao X, Wu Y, DeMatteo RP. Liver X receptor α is essential for the capillarization of liver sinusoidal endothelial cells in liver injury. Sci Rep. 2016;6:21309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 570] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 36. | Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1016] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 37. | Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M; EPE-A Study Group. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 38. | Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 39. | Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 939] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 40. | Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, Sime PJ. Resolvin D1 Reduces Emphysema and Chronic Inflammation. Am J Pathol. 2015;185:3189-3201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671-7676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |