Published online Sep 14, 2018. doi: 10.3748/wjg.v24.i34.3927
Peer-review started: March 17, 2018
First decision: April 11, 2018
Revised: May 25, 2018
Accepted: June 21, 2018
Article in press: June 21, 2018
Published online: September 14, 2018
Processing time: 181 Days and 18.1 Hours
To provide a clear understanding of viral hepatitis epidemiology and their clinical burdens in Somalia.
A systematic review and meta-analysis was conducted as Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A comprehensive literature search of published studies on viral hepatitis was performed from 1977-2016 in PubMed, Google Scholar, Science Direct, World Health Organization African Index Medicus and the Africa Journals Online databases, as well as on the Ministry of Health website. We also captured unpublished articles that were not available on online systems.
Twenty-nine studies from Somalia and Somali immigrants (United Kingdom, United States, Italy, Libya) with a combined sample size for each type of viral hepatitis [hepatitis A virus (HAV): 1564, hepatitis B virus (HBV): 8756, hepatitis C virus (HCV): 6257, hepatitis D virus (HDV): 375 and hepatitis E virus (HEV): 278] were analyzed. The overall pooled prevalence rate of HAV was 90.2% (95%CI: 77.8% to 96%). The HAV prevalence among different age groups was as follows: < 1 year old, 61.54% (95%CI: 40.14% to 79.24%); 1-10 years old, 91.91% (95%CI: 87.76% to 94.73%); 11-19 years old, 96.31% (95%CI: 92.84% to 98.14%); 20-39 years old, 91.3% (95%CI: 83.07% to 95.73%); and > 40 years old, 86.96% (95%CI: 75.68% to 93.47%). The overall pooled prevalence of HBV was 18.9% (95%CI: 14% to 29%). The overall pooled prevalence among subgroups of HBV was 20.5% (95%CI: 5.1% to 55.4%) in pregnant women; 5.7% (95%CI: 2.7% to 11.5%) in children; 39.2% (95%CI: 33.4% to 45.4%) in patients with chronic liver disease, including hepatocellular carcinoma (HCC); 7.7% (95%CI: 4.2% to 13.6%), 12.4% (95%CI: 6.3% to 23.0%) and 11.8% (95%CI: 5.3% to 24.5%) in age groups < 20 years old, 20-39 years old and > 40 years old, respectively. The HBV prevalence among risk groups was 20% (95%CI: 7.19% to 44.64%) in female prostitutes, 21.28% (95%CI: 7.15% to 48.69%) in hospitalized adults, 5.56% (95%CI: 0.99% to 25.62%) in hospitalized children, 60% (95%CI: 31.66% to 82.92%) in patients with acute hepatitis, 33.55% (95%CI: 14.44% to 60.16%) in patients with ancylostomiasis, 12.34% (95%CI: 7.24% to 20.26%) in patients with leprosy and 20.19% (95%CI: 11.28% to 33.49%) in schistosomiasis patients. The overall pooled prevalence of HCV was estimated as 4.84% (95%CI: 3.02% to 7.67%). The prevalence rates among blood donors, risk groups, children and patients chronic liver disease (including HCC) was 0.87% (95%CI: 0.33% to 2.30%), 2.43% (95%CI: 1.21% to 4.8%), 1.37% (95%CI: 0.76% to 2.46%) and 29.82% (95%CI: 15.84% to 48.98%), respectively. The prevalence among genotypes of HCV was 21.9% (95%CI: 15.36% to 30.23%) in genotype 1, 0.87% (95%CI: 0.12% to 5.9%) in genotype 2, 25.21% (95%CI: 18.23% to 33.77%) in genotype 3, 46.24% (95%CI: 37.48% to 55.25%) in genotype 4, 2.52% (95%CI: 0.82% to 7.53%) in genotype 5, and 1.19% (95%CI: 0.07% to 16.38%) in genotype 6. The overall pooled prevalence of HDV was 28.99% (95%CI: 16.38% to 45.96%). The HDV prevalence rate among patients with chronic liver disease, including HCC, was 43.77% (95%CI: 35.09% to 52.84%). The overall pooled prevalence of HEV was 46.86% (95%CI: 5.31% to 93.28%).
Our study demonstrates a high prevalence of all forms of viral hepatitis in Somalia and it also indicates that chronic HBV was the commonest cause of chronic liver disease. This highlights needs for urgent public health interventions and strategic policy directions to controlling the burden of the disease.
Core tip: This is the first article reviewing epidemiology of viral hepatitis in Somalia with systematic review and meta-analysis of the published and unpublished reports from 1977 to 2016 among prevalence of all types’ viral hepatitis in Somalia.
- Citation: Hassan-Kadle MA, Osman MS, Ogurtsov PP. Epidemiology of viral hepatitis in Somalia: Systematic review and meta-analysis study. World J Gastroenterol 2018; 24(34): 3927-3957
- URL: https://www.wjgnet.com/1007-9327/full/v24/i34/3927.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i34.3927
Viral hepatitis is a major public health problem affecting several hundred million people globally. The most common types of viral hepatitis are six distinct types that have been identified as hepatitis A, B, C, D, E, G viruses, and they may present in acute form or chronic form, which causes substantial morbidity and mortality, including chronic hepatitis, cirrhosis and hepatocellular carcinoma. The World Health Organization (WHO) estimates that 257 million people worldwide are infected with hepatitis B virus (HBV), which constitutes 3.5% of the population. Africa has the second-largest number of chronic HBV carriers after the Western Pacific regions. Both regions are considered of high endemicity, and the prevalence rate of each region was 6.1% and 6.2%, respectively. It is estimated that approximately 71 million people were living with hepatitis C virus (HCV) infection, which accounts for 1% of the world’s population, in 2015. The regions with the highest prevalence of HCV were the eastern Mediterranean region (2.3%) and the European region (1.5%), while the prevalence in Africa was 1.0%. Hepatitis D virus (HDV) affects nearly 15 million people, and hepatitis E virus (HEV) annually infects 20 million people, with over 3.3 million symptomatic cases of hepatitis E and 44600 hepatitis E-related deaths being recorded[1] Hepatitis E is one of the leading causes of major outbreaks of acute viral hepatitis worldwide, especially in developing nations[2]. Hepatitis A virus (HAV) infection spans the entire world, with specifically high prevalence rates in older children and adults[3].The number of deaths from viral hepatitis has increased from 1.10 million deaths in 2000 to 1.34 million deaths in 2015 compared to deaths from tuberculosis (from 1.67 to 1.37 million deaths), malaria (from 0.86 to 0.44 million deaths) and human Immunodeficiency virus (from 1.46 to 1.06 million deaths) between 2000 and 2015. Approximately 96% of these deaths resulted from complications of chronic HBV (66%) and HCV (30%), although hepatitis E and HAV infections accounted for 3.3% and 0.8% of these deaths, respectively[1]. HBV (887000 deaths) accounts for more deaths than HCV infection (399000 deaths), while complications of both viruses (HBV and HCV) and cirrhosis (720000 deaths) account for more deaths than hepatocellular carcinoma (470000 deaths)[1].
In Somalia, viral hepatitis, especially HBV, is of significant public health importance. Somalia is an area of the world with a high prevalence HBV infection of > 8. There are several studies of the prevalence of HAV, HBV, HCV, HDV, and HEV in Somalia; however, to the best of our knowledge, there is no meta-analysis to provide an overall estimation of the prevalence of all viral hepatitis infections in this country. A recent report explored the reasons for such a dearth of data[4]. In Somalia, largely due to the unsettling decades-long civil war, medical staffs are underqualified and undertrained, and limited access to modern laboratory facilities poses substantial diagnostic challenges[4]. Somalia is considered to be a country that has no national strategy for the surveillance, prevention and control of viral hepatitis[5]. The provision of high-quality epidemiological data for viral hepatitis in Somalia could help motivate the drafting of action at the policy level. To synthesize such high-quality epidemiological estimates, we decided to undertake this systematic review and meta-analysis of studies that reported the population-level prevalence of each type of viral hepatitis (A, B, C, D and E) in Somalia. We also aimed to understand the burden of viral hepatitis in Somalia, especially HBV and HCV, and to inform public health practitioners, researchers and policy makers.
Somalia is located along the Gulf of Aden and Indian Ocean in the sub-region of East Africa, and it is bordered by Djibouti and the Gulf of Aden to the north, the Indian Ocean to the east, Kenya to the southwest and Ethiopia to the west. Somalia has the longest coastline in Africa. It has a land mass of 637657 km2 and a population of approximately 12 million, 61% of whom live in rural areas. Life expectancy at birth in 2015 was 49 years for males and 54 years for females[6]. The country is divided into eighteen administrative regions and 92 districts. Seventy-one percent of the Somali labor force is in agriculture, and almost everyone living in rural areas is involved in livestock or in farming. The per-capita total health expenditure, as a percentage of gross domestic products, was not mentioned in World Bank estimations. The proportion of Somalia’s population living below the poverty line has not been published to date, but the country was the fifth-poorest country of the world according the World Bank in 2016[7]. According to the United Nation, the Human Development Index of Somalia stood at 0.285, and the country ranked 165th out of 170 countries in 2012[8]. The country entered into a civil war in 1991, and many major structures collapsed, including the health sector and especially the public sectors.
We conducted an electronic literature search in several biomedical databases, including PubMed, Google Scholar, African Journal Online, WHO African Index Medicus and Science Direct, using different combinations of key words. The search encompassed published and unpublished studies from 1977 to 2016 with epidemiological and/or clinical data on the seroprevalence of viral hepatitis in Somalia. The key words used were as follows: [“hepatitis A” AND (seroprevalence OR prevalence) AND “Somalia”], [“hepatitis B” AND (seroprevalence OR prevalence) AND “Somalia”], [“hepatitis C” AND seroprevalence OR prevalence) AND “Somalia”], [“hepatitis D” AND (seroprevalence OR prevalence) AND “Somalia”], [“hepatitis E” AND (seroprevalence OR prevalence) AND “Somalia”]. We also reconducted the search using full written phrases, such as “Viral hepatitis”, “hepatitis A”, “hepatitis B” or “hepatitis B surface antigen”, “hepatitis C”, “hepatitis D”, “hepatitis E”, “epidemiology”, and “Somali immigrants.” We searched unpublished studies from other sources, such as universities and the website of the Ministry of Health (http://www.moh.gov.so/en/) for non-indexed studies or reports on the topic. The statement was reviewed systemically following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[9]. If the full text of studies were not reachable, we contacted the authors by email.
In this systematic review, we considered all studies published in peer-reviewed journals between 1977 and 2016, and unpublished primary data of each type of viral hepatitis in Somalia qualified for inclusion in the review, according to the PRISMA flow diagram[9], which excludes case reports, systematic reviews, case series editorials, letters to the editor, commentaries, magazines, newspaper reports/articles and studies of other Somali ethnicities living in neighboring countries published in peer-reviewed journals. The titles and abstracts were screened for relevance, and full-text papers considered relevant for further screening were obtained wherever possible. The references of all identified full-text articles and reviews of the literature were also checked to identify whether there were any additional articles that were missed during screening. For the study selection of this systematic review, we sub-analyzed all articles according to population sub-groups, such as blood donors, pregnant women, children, and patients with chronic liver disease and in the general population. These subgroups were examined with no age restriction. For other populations, the age restrictions imposed were children, defined as those 12 years of age and below, and adults, defined those 18 years of age and above. The search language was restricted to English. The studies also included Somali people who immigrated to Italy, the United States, United Kingdom and Libya and were screened for hepatitis viruses.
For the data extraction, the two investigators, Hassan-Kadle MA and Mugtaba SO, independently applied the inclusion criteria selected the studies and extracted the data. The extracted data included the following descriptive information: author(s), publication year, country of study (because some studies were conducted among Somali populations outside of Somalia), total sample size, and cases of each type of viral hepatitis in each study.
Calculation of pooled prevalence: The prevalence of viral hepatitis was extracted from every individual observational study by dividing the number of patients who tested positive over the total number included in the study. The prevalence in each study was multiplied by a weight inversely proportional to the total size of the study sample. The pooled prevalence is the sum of these weighted prevalence estimates in each individual study. We used the R software statistical packages “meta” and “metaphor” to calculate the pooled prevalence (Viechtbauer et al[10]; Schwarzer et al[11]). We chose to use a random effects model in the meta-analysis because we expected considerable heterogeneity among studies, due largely to the different settings in which studies were conducted. To compare the results, we included the results for a fixed effects meta-analysis. We attempted a meta-regression model by adjusting for the potential effect of the country where the study was conducted[12].
Testing heterogeneity: Heterogeneity testing was performed using the I2 and the Q statistic methods. We interpreted the I2 statistic results as follows: 0%, no observed heterogeneity; 25%, low; 50%, moderate; and 75%, high heterogeneity. We choose the P value = 0.05 as a cutoff for significant heterogeneity in interpreting the results of the Q statistic.
Publication bias: We used the funnel plot method to visually assess the studies by plotting the sample size against the observed prevalence. We assumed that smaller studies would vary more considerably around the pooled estimate than the larger studies would. Objective measures of the funnel plot symmetry were performed using Duval and Tweedie’s trim and fill procedure (Duval et al[13]) and Egger’s test (Egger et al[14]).
Our search yielded 504 citations, which were retrieved in the literature review. After reviewing the abstracts and titles, we excluded duplicates and irrelevant studies after applying the exclusion criteria. A total of 29 articles were included in this study and were analyzed. The 29 studies were conducted in Somalia and outside of Somalia (among Somali immigrants, Figure 1).
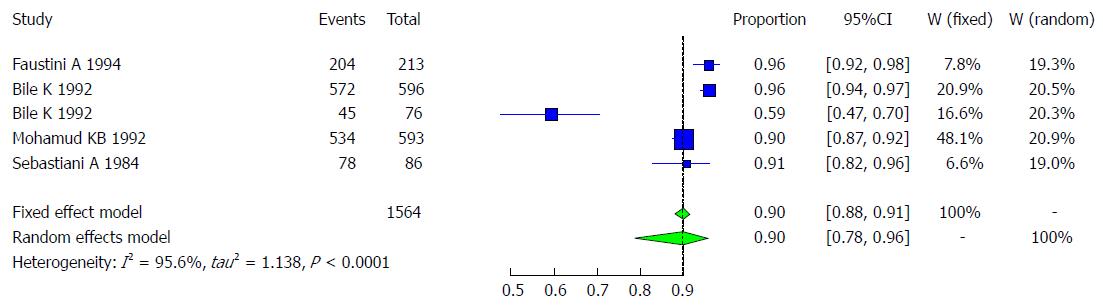
Hepatitis A is a liver disease caused by HAV, and it occurs worldwide. This virus thus creates a public health concern, primarily in developing countries, due to its persistent circulation in the environment. Among the studies presented in Somalia, more than 90% of children had the HAV antibody by the age of 4 years[15-19]. In 1992, Mohamud KB and his colleagues studied a Somali sample of 593 subjects who were healthy rural and urban volunteers and child outpatients ages 0-83 years in three villages in Somalia (Mogadishu area: Buur-Ful village; Jowhar District: Mooda Moode; and Bur-Hakaba District and Bajuni Islands: Kismaio District). This sample showed a very high rate of HAV exposure of approximately 90%[15]. Another study by Bile et al[16] conducted at two institutions for children in Somalia (Shebeli: 596 subjects and Societe Organization Sociale, SOS: 76 subjects) showed a very high rate of HAV in the two samples of 96% and 59%, respectively. One study indicated that HAV in Somalia occurs primarily between 4 mo to 4 years of age, because the child has passive immunity from maternal antibodies during the first 3 mo of life[17]. Sebastiani et al [18] presented a result of 90.6%. Another study conducted in Italy for immigrant communities, which included 213 subjects, mostly originating from Somalia (177 or 83%), Ethiopia (21 or 10%), and Djibouti, Egypt and Saudi Arabia, showed a very high prevalence of HAV of 96%, including children (87.5% of children were under 12 years)[19].These reports of anti-HAV prevalence rates across the 4 studies ranged from 59.2% to 96%[15-19], as shown in Table 1. Four studies met the inclusion criteria for the meta-analysis of the prevalence of HAV infection. The four studies included examined the prevalence of HAV infection in the total of 1564 Somali participants, and the quality of the included studies also varied. We used the extracted data of the 4 studies to quantify the overall pooled prevalence of HAV infection. The pooled effect size for the prevalence of HAV infection among Somali people was 90.2% (95%CI: 77.8% to 96%). The heterogeneity was high (I2 = 95.6%, 95%CI: 92.2% to 97.5%). Another indication of high heterogeneity was the Q-statistic [Q (degrees of freedom = 4) = 90.31, P value < 0.001]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 2.
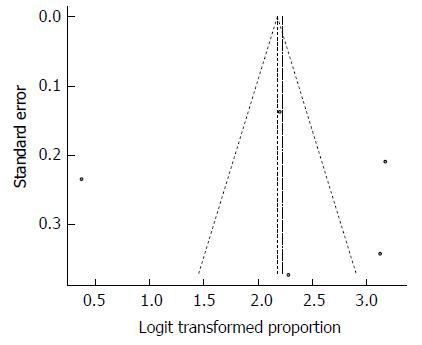
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 3). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 87.6%, which was close to the observed effect size. Notably, we could not perform Egger’s test for the assessment of symmetry of the funnel plot due to the small number of included studies.
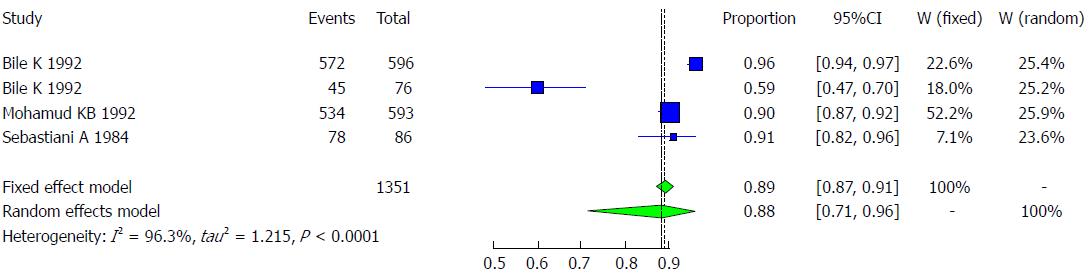
The majority of the studies (n = 3) were conducted in Somalia with a pooled prevalence of HAV of 88.1% (95%CI: 71.1% to 95.7%) and high heterogeneity (I2 = 96.3%). These studies are followed by 1 study conducted in Italy, with a prevalence of HAV of 95.8% (95%CI: 92.1% to 97.8%, Figure 4).
Characteristics of the studies included: All studies met the inclusion criteria for the meta-analysis of the prevalence of HAV infection and examined the prevalence of HAV infection in a total of 1488 Somali participants belonging to 4 major age groups. The quality of the included studies varied (Table 2).
| Age group | Author/Publication year | Total | Cases | Total | Healthy | Serology |
| 0-11 mo | Mohamud et al 1992[15] | 52 | 32 | 52 | 20 | Anti-HAV |
| 1-11 yr | Mohamud et al 1992[15] | 189 | 176 | 189 | 13 | Anti-HAV |
| Sebastiani et al 1984[18] | 35 | 33 | 35 | 2 | Anti-HAV | |
| Faustini et al 1994[19] | 213 | 186 | 213 | 27 | Anti-HAV | |
| Bile et al 1992[16] | 234 | 220 | 234 | 14 | Anti-HAV | |
| Total | 723 | 647 | 723 | 76 | Anti-HAV | |
| 11-19 yr | Mohamud et al 1992[15] | 62 | 58 | 62 | 4 | Anti-HAV |
| Sebastiani et al 1984[18] | 21 | 20 | 21 | 1 | Anti-HAV | |
| Bile et al 1992[16] | 362 | 353 | 362 | 9 | Anti-HAV | |
| Total | 445 | 431 | 445 | 14 | Anti-HAV | |
| 20-39 yr | Mohamud et al 1992[15] | 164 | 153 | 164 | 11 | Anti-HAV |
| Sebastiani et al 1984[18] | 19 | 16 | 19 | 3 | Anti-HAV | |
| Total | 183 | 169 | 183 | 14 | Anti-HAV | |
| 40+ yr | Mohamud et al 1992[15] | 126 | 111 | 126 | 15 | Anti-HAV |
| Sebastiani et al 1984[18] | 11 | 9 | 11 | 2 | Anti-HAV | |
| Total | 137 | 120 | 137 | 17 | Anti-HAV |
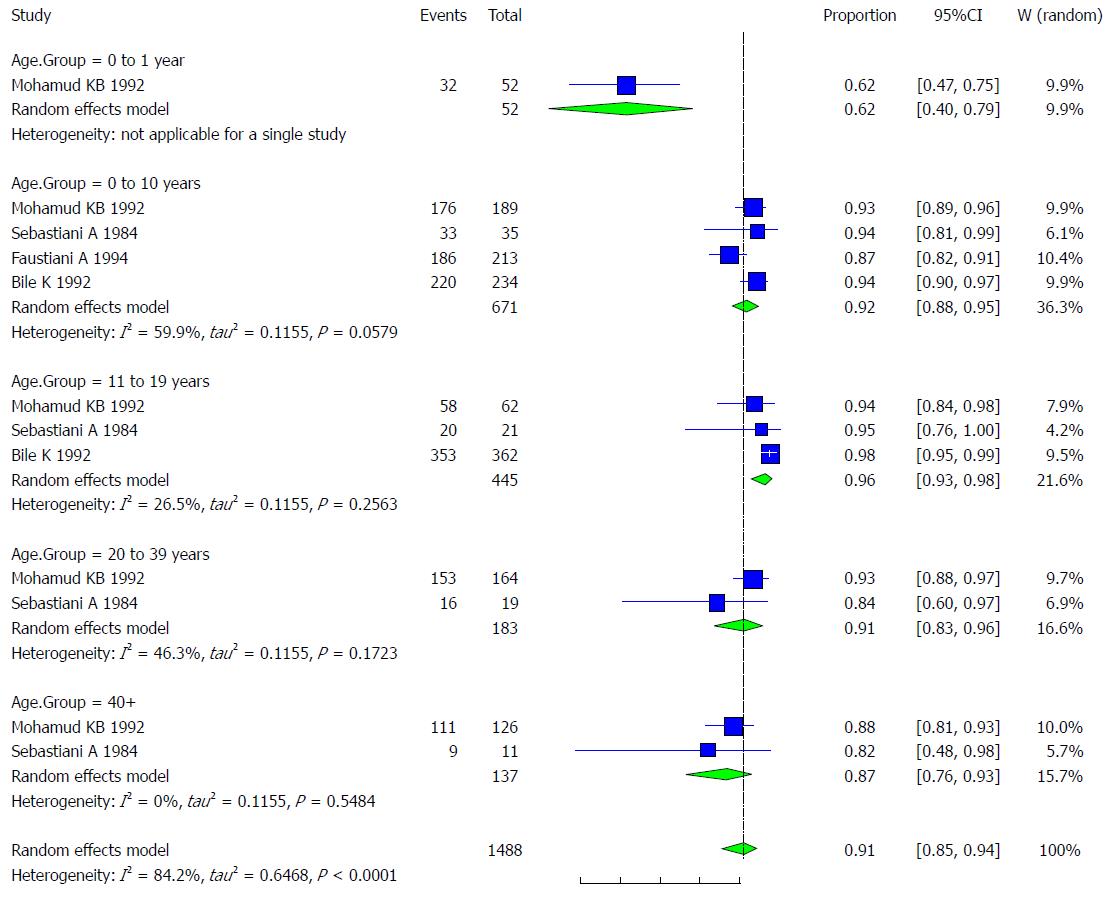
Pooled prevalence of HAV infection per age group: We used the extracted data of the 4 studies to quantify the overall pooled prevalence of HAV infection in each age group.
One study was conducted on patients younger than one year old. The pooled effect size for the prevalence of HAV infection among Somali people aged less than one year was 61.54% (95%CI: 40.14% to 79.24%).
The pooled effect size (out of 4 studies) for the prevalence of HAV infection among Somali people aged 1-10 years old was 91.91% (95%CI: 87.76% to 94.73%). The heterogeneity was moderate (I2 = 59.9%). Another indication of moderate heterogeneity was the Q-statistic [Q (degrees of freedom = 3) = 7.49].
The pooled effect size (out of 3 studies) for the prevalence of HAV infection among Somali people aged 11-19 years old was 96.31% (95%CI: 92.84% to 98.14%). The heterogeneity was low (I2 = 26.5%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 2) = 2.72].
The pooled effect size (out of 2 studies) for the prevalence of HAV infection among Somali people aged 20-39 years old was 91.3% (95%CI: 83.07% to 95.73%). The heterogeneity was moderate (I2 = 46.3%). Another indication of heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 1.86].
The pooled effect size (out of 2 studies) for the prevalence of HAV infection among Somali people aged over 40 years old was 86.96% (95%CI: 75.68% to 93.47%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.36]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CI, as shown in Figure 5.
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 6). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, the Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 88.56%, which was not widely different from the observed effect size.
Somalia is classified among countries as having a high hepatitis B surface antigen (HBsAg) endemicity of more than 8%[20,21]. The first study of HBV in the country was conducted in 1977, in which Delia S and his colleagues were presented with a higher frequency of HBsAg among patients with ancylostomiasis (33.33%) and with urinary schistosomiasis (25.92%) than among leprosy patients (9.67% in the L type and 6.89% in the T type), and the overall prevalence among these patients was shown to be high, with 76.1% (118/155) being observed among patients who were HBsAg positive and 11.11% of the controls. In the leprosy patients with schistosomiasis, the frequency was 40.0%[22]. Another study conducted in 1978 showed that HBsAg was found in 14.8%of these patients (54 cases), while the frequency was 34.0% among controls (47 cases). However, the overall prevalence in this study was 23.7% (77/101)[23].
In 1979, Nuti et al [24] studied 222 Somalian patients with the lepromatous form of leprosy (LL; n = 135 patients) and the tuberculoid form of the disease (TT; n = 87 patients) for HBV markers. The results showed that the proportion of leprosy and tuberculoid patients presenting with HBsAg was 24.4% (54/222) and 11.5% (26/222), respectively, but the overall prevalence of HBsAg-positive patients in this article was 36% (80/222)[24]. Another study published in this year revealed that among patients with acute viral hepatitis who were tested for the presence of HBsAg and the e-antigen and its corresponding antibodies, HBsAg was found in 60% of patients with hepatitis and 34% of controls, and the overall prevalence of HBsAg-positive patients was 48% (49/102)[25]. Nuti M and his colleagues also found that 14% (22/157) of patients were HBsAg-positive for the overall prevalence of their study, but in patients with bladder schistosomiasis and in controls, the prevalence was 19.4% (13/67) and 10% (9/90), respectively[26].
In 1985, a study conducted in three different villages of Somalia found that 12.08% (40/331) of subjects aged 1-83 years were HBsAg-positive, 29.9% were anti-HBs-positive, 43.8% were anti-HBc-positive, 21.4% were anti-HBe-positive, and the overall prevalence in this study was 12% (46/383) of subjects, including those under one year of age. Among the HBsAg-positive subjects, 34.7% were HBeAg-positive and 21.7% had anti-HBcAg-IgM[27]. A survey study in 1987 of HBV epidemiology was carried out among 383 adults from different areas of Somalia and in 135 pregnant women and 428 children from Mogadishu. The study showed a high incidence of HBsAg among nomadic males 20/85; (23%) and a lower incidence among males from agricultural and coastal areas, i.e., 16/93 (17%) and 14/98 (14%), respectively. Meanwhile, the lowest frequency of HBsAg was observed among women from coastal areas (6/72; 8%) and among pregnant women (14/135; 10.4%), none of whom had HBeAg. However, a low number of children were HBsAg-positive, both under 4 years old (3/94; 3%) and 4-13 years of age (5/128; 4%). In the 15-19 age group, 50% of subjects showed seroconversion from HBeAg to anti-HBe. A total of 7 out of 41 HBsAg carriers aged over 20 had HBeAg, while the overall prevalence of 8.2% (78/946) was HBsAg-positive[28].
In 1987, Jama H and his colleagues conducted a study of sexual transmitted diseases among varied population groups, and these diseases were detected in 22.4% (49/218) of subjects in the overall populations, including 37% of pregnant women, 4% of neonates, 22% of educated women and 20% of prostitutes[29]. Another study in the country showed that 50% (52/104) of subjects was HBsAg-positive[30]. In 1989, a total of 1138 subjects with HBsAg were examined from different regions of Somalia; the results showed that 19.3% (220/1138) of subjects were HBsAg-reactive[31]. Bile KM and his colleagues conducted a case-control study that detected that 28.8% (67/232) of the overall prevalence was HBsAg subjects, including cases and control groups[32].
A total of 256 serum samples collected from blood donors (157 subjects) and hospitalized children (42 subjects) and adults (57 subjects) in Mogadishu were examined. The results showed that among 198 samples tested, the HBsAg carrier rate was 19.1% (22/115), 5.6%(2/36) and 21.3% (10/47) among blood donors, hospitalized children and hospitalized adults, respectively, but in the overall prevalence,17.1% (34/198) were positive for HBsAg[33].
The prevalence calculation carried out by Mohamud KB and his colleagues indicated that 10.5% (134/1272) of subjects were positive for HBsAg[15]. Another study published in 1992 showed that 15.9% (95/596) of subjects were HBsAg-positive in one institution at Shabeli residence[16]. In a case-control study published in 1993, it was detected that 13.5% (29/124) of subjects in the overall prevalence of both groups were HBsAg-positive[34]. In 1994, a study conducted in Italy among immigrant communities detected HBsAg among 3.2% (7/213) of subjects[19]. Another study carried out in the United Kingdom among Somali immigrant communities showed that 5.6% (25/439) of subjects were positive for HBsAg[35]. Shire AM and his colleagues conducted a study in the United States and found that 13.6% (151/1109) of their sample were positive for HBsAg[36]. Unpublished studies performed in the country in 2011 and 2012 showed that 40.1% (59/147)[37] and 39.1% (61/156)[38] of subjects, respectively, were HBsAg reactive. A study in an immigrant community mostly originating from Somalia showed that 6.2% (31/500) of subjects had been detected with HBsAg[39].
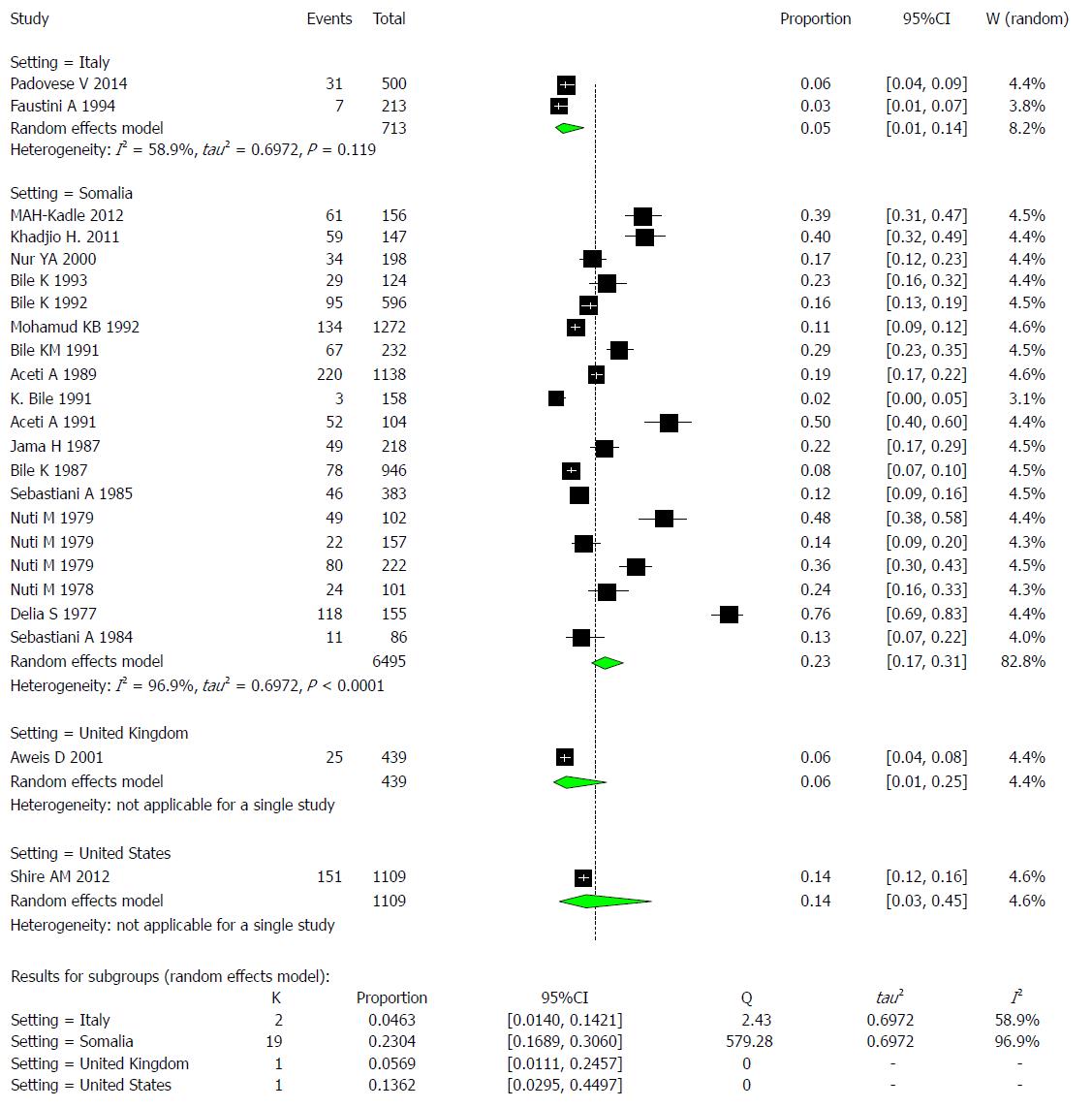
The reported HBsAg prevalence rates across the 23 studies ranged from 1.9% to 76%, as shown in Table 3. Additionally, the percentage of studies that reported prevalence rates that exceeded 8% was 82.6% (19/23), so we define this endemicity level of an area as high. A total of 17.3% (4/23) of studies reported prevalence rates of at least 8%. The 23 studies met the inclusion criteria for the meta-analysis of the prevalence of hepatitis virus infection and examined the prevalence of HBV infection in a total of 8756 Somali participants. The quality of the included studies also varied. We used the extracted data of the 23 studies to quantify the overall pooled prevalence of HBV infection. The pooled effect size for the prevalence of HBV infection among Somali people was 18.9% (95%CI: 14% to 29%). The heterogeneity was high (I2 = 97.6%, 95%CI: 96.2% to 97.6%). Another indication of high heterogeneity was the Q-statistic [Q (degrees of freedom = 22) = 726.17, P value < 0.001]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CI, as shown in Figure 7.
| No. | Author | Publication year | Total | HBsAg | Healthy | Setting | Population |
| 1 | Padovese et al[39] | 2014 | 500 | 31 (6.2) | 469 | Italy | Immigrants |
| 2 | Kadle et al[38] | 2012 | 156 | 61 (39.1) | 95 | Somalia | Local |
| 3 | Shire et al[36] | 2012 | 1109 | 151 (13.6) | 958 | United States | Immigrants |
| 4 | Khadjio et al[37] | 2011 | 147 | 59 (40.1) | 88 | Somalia | Local |
| 5 | Aweis et al[35] | 2001 | 439 | 25 (5.6) | 414 | United Kingdom | Immigrants |
| 6 | Nur et al[33] | 2000 | 198 | 34 (17.1) | 164 | Somalia | Local |
| 7 | Faustini et al[19] | 1994 | 213 | 7 (3.2) | 206 | Italy | Immigrants |
| 8 | Bile et al[34] | 1993 | 124 | 29 (13.5) | 95 | Somalia | Local |
| 9 | Bile et al[16] | 1992 | 596 | 95 (15.9) | 501 | Somalia | Local |
| 10 | Mohamud et al[15] | 1992 | 1272 | 134 (10.5) | 1138 | Somalia | Local |
| 11 | Bile et al[32] | 1991 | 232 | 67 (28.8) | 165 | Somalia | Local |
| 12 | Aceti et al[31] | 1989 | 1138 | 220 (19.3) | 918 | Somalia | Local |
| 13 | Bile et al[40] | 1991 | 158 | 3 (1.9) | 155 | Somalia | Local |
| 14 | Aceti et al[30] | 1991 | 104 | 52 (50) | 52 | Somalia | Local |
| 15 | Jama et al[29] | 1987 | 218 | 49 (22.4) | 169 | Somalia | Local |
| 16 | Bile et al[28] | 1987 | 946 | 78 (8.2) | 868 | Somalia | Local |
| 17 | Sebastiani et al[27] | 1985 | 383 | 46 (12) | 337 | Somalia | Local |
| 18 | Nuti et al[26] | 1979 | 102 | 49 (48) | 58 | Somalia | Local |
| 19 | Nuti et al[25] | 1979 | 157 | 22 (14) | 135 | Somalia | Local |
| 20 | Nuti et al[24] | 1978 | 101 | 24 (23.7) | 77 | Somalia | Local |
| 21 | Nuti et al[23] | 1979 | 222 | 80 (36) | 142 | Somalia | Local |
| 22 | Delia et al[22] | 1977 | 155 | 118 (76.1) | 37 | Somalia | Local |
| 23 | Sebastiani et al[18] | 1984 | 86 | 11 (12.7) | 75 | Somalia | Local |
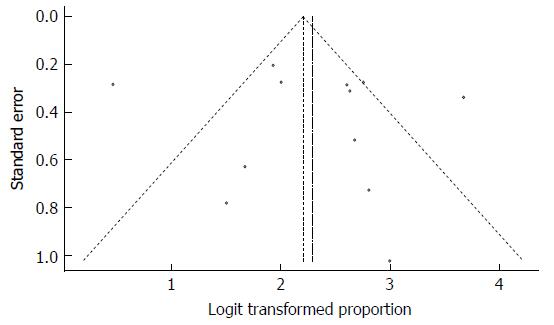
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 8). Notably, the total number of studies was reasonable, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 18.9%, which was notably similar to the observed effect size. Furthermore, Egger’s test for assessment of symmetry of the funnel plot was not statistically significant (t = 0.6158, degrees of freedom = 21, P-value = 0.5447), providing further support for the absence of publication bias.
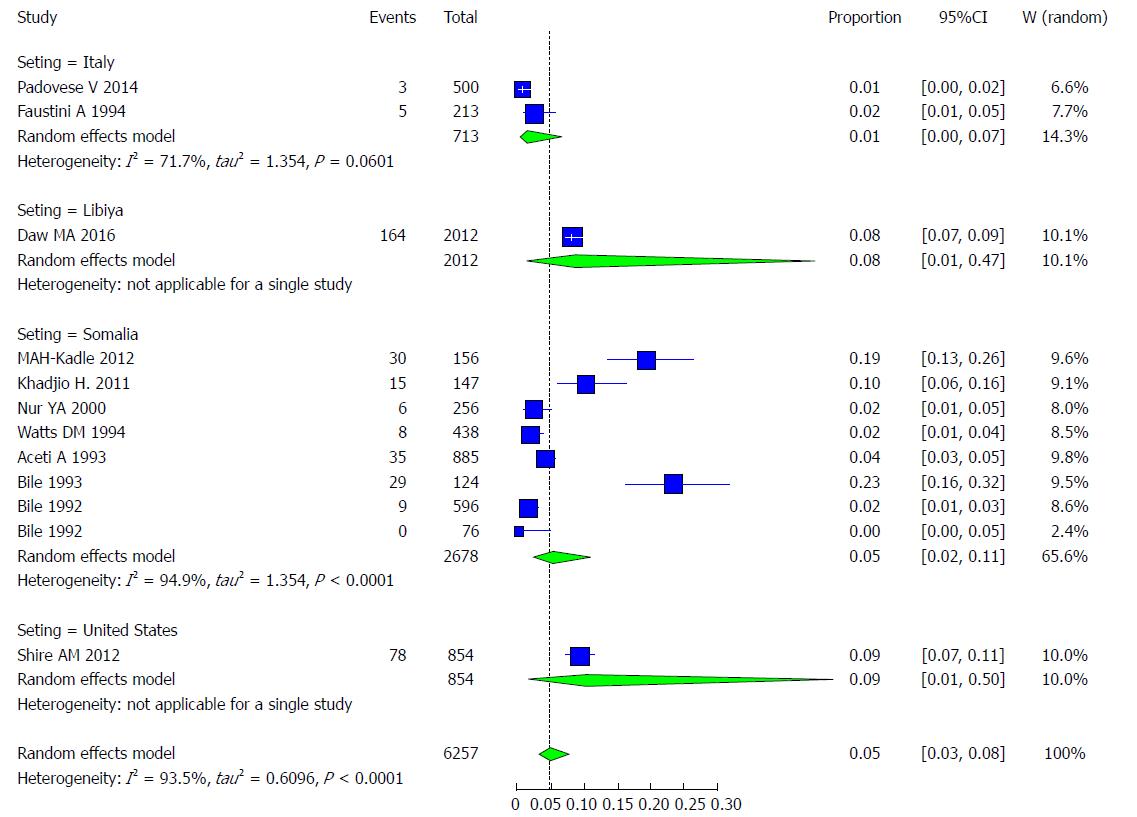
The majority of the studies (n = 19) were conducted in Somalia, with a pooled prevalence of HBV of 23% (95%CI: 16.9% to 30.6%) and high heterogeneity (I2 = 96.9%). These findings are followed by 2 studies conducted in Italy, with a pooled prevalence of HBV of 4.6% (95%CI: 1.4% to 14.2%) and moderate heterogeneity (I2 = 58.9%). One study was conducted in the United Kingdom, with a prevalence of HBV of 5.7% (95%CI: 1.1% to24.6%), and another study was conducted in the United States, with a prevalence of HBV of 13.6% (95%CI: 3.0% to 45.0%) (Figure 9).
Examining the effect of setting, significant variability could be explained by meta-regressing the meta-analysis over the four settings: Italy, Somalia, the United Kingdom, and the United States. Tests of moderators indicated the following: coefficient(s) 2, 3, 4: QM (df = 3) = 10.5687, P-value = 0.0143. For the prevalence results in Somalia compared to those of Italy, the P value was 0.0056, and the estimate was 1.8193. For the United Kingdom and the United States, the variability was not significantly different from that of Italy (P value=0.8373 and 0.2608, respectively).
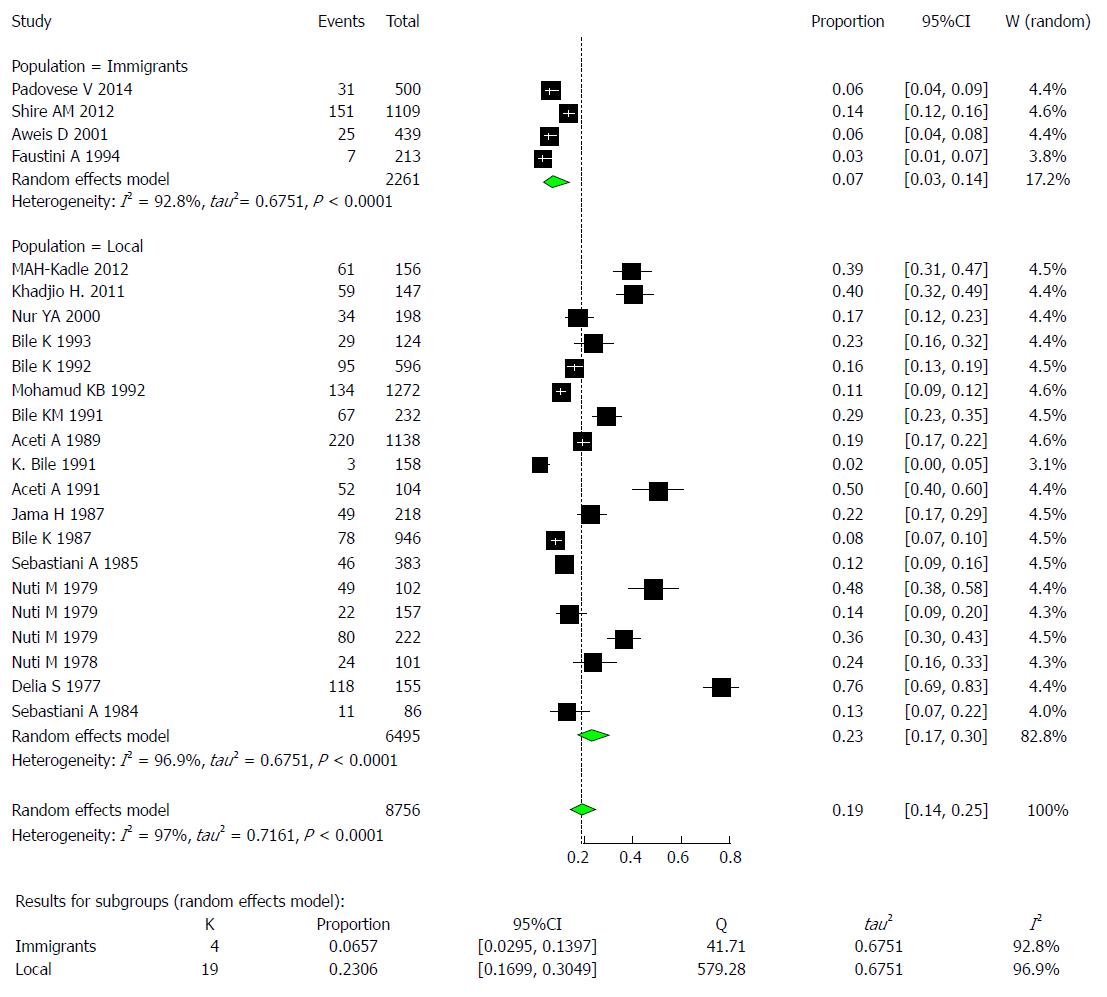
The majority of the studies (n = 19) were conducted on the local population in Somalia with a pooled prevalence of HBV of 23% (95%CI: 16.9% to 30.6%) and high heterogeneity (I2 = 96.9%). The remaining 4 studies were conducted on immigrants with a pooled prevalence of HBV of 23.1% (95%CI: 17.0% to 30.5%) and high heterogeneity (I2 = 92.8%) (Figure 10).
Examining the effect of population, significant variability could be explained by meta-regressing the meta-analysis over the two population types: immigrants and locals. Tests of moderators indicated the following: coefficient 2: QM (df = 1) = 9.5448, P-val = 0.0020. For the prevalence results of the local Somali population compared to the immigrant population, the P value was 0.002, and the estimate was 1.4498.
A total of 2 studies presented HBV prevalence rates among pregnant women, and these studies showed that 10.4% (14/135) of subjects were HBsAg-positive[28], while the other study presented 37% (19/52) of subjects as positive for HBsAg[29], as shown in Table 4. The two studies met the inclusion criteria for the meta-analysis of the prevalence of HBV infection during pregnancy. The pooled effect size for the prevalence of HBV infection during pregnancy among Somali people was 20.5% (95%CI: 5.1% to 55.4%). The heterogeneity was high (I2 = 93.7%). See Figure 11.
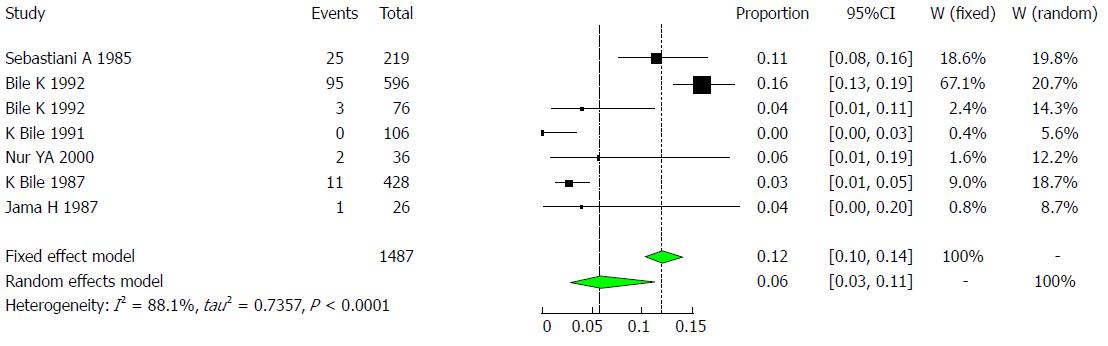
A total of six studies showed HBV prevalence rates between 0% to 16% among children[16,27-30,40]. See Table 5. Six studies met the inclusion criteria for the meta-analysis of the prevalence of HBV infection among children. The pooled effect size for the prevalence of HBV infection in Somali children was 5.7% (95%CI: 2.7% to 11.5%). The heterogeneity was high (I2 = 88.1%, 95%CI: 77.8% to 93.6%, Figure 12).
| Author | Year | Total | Cases | HBsAg | Healthy | Setting | Population |
| Sebastiani et al[27] | 1985 | 219 | 25 | 11% | 194 | Jowhar, Bur-Hakaba, Kismaio and Afgoy | Children living in four villages |
| Bile et al[16] | 1992 | 596 | 95 | 16% | 501 | Mogadishu area | Children in government-operated residence for abandoned children in Shebeli |
| Bile et al[16] | 1992 | 76 | 3 | 4% | 73 | Mogadishu | Children in government-operated residences for abandoned children in SOS institution |
| Bile et al[40] | 1991 | 106 | 0 | 0% | 106 | Lower Shabelle | Children living in Mukay Dumis |
| Nur et al[30] | 2000 | 36 | 2 | 6% | 34 | Mogadishu | Hospitalized children |
| Bile et al[28] | 1987 | 428 | 11 | 3% | 417 | Mogadishu | Children living in Mogadishu |
| Jama et al[29] | 1987 | 26 | 1 | 4% | 25 | Mogadishu | Newborn babies |
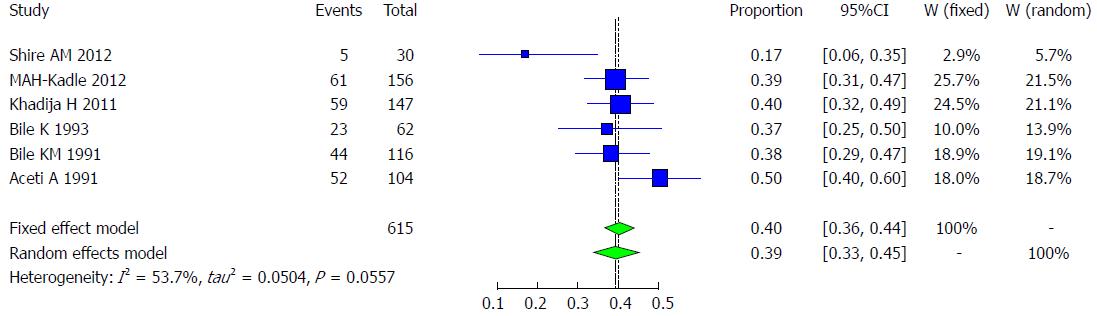
Six studies met the inclusion criteria for the meta-analysis of the prevalence of HBV infection among patients with chronic liver disease, including patients with hepatocellular carcinoma, in Somali people. Their prevalence ranges from 17.9% to 50%[30,32,34,36-38] (Table 6). The pooled effect size for the prevalence of hepatitis B among chronic liver disease patients in Somali people was 39.2% (95%CI: 33.4% to 45.4%). The heterogeneity was moderate (I2 = 53.7%, 95%CI: 0% to 81.5%, Figure 13).
| Author | Year | Total | HBsAg | Healthy | Setting | Population |
| Shire et al[36] | 2012 | 30 | 5 (17.9) | 25 | United States | Immigrants |
| Kadle et al[38] | 2012 | 156 | 61 (39.1) | 95 | Somalia | Local |
| Khadija et al[37] | 2011 | 147 | 59 (40.1) | 88 | Somalia | Local |
| Bile et al[34] | 1993 | 62 | 23 (37.1) | 39 | Somalia | Local |
| Bile et al[32] | 1991 | 116 | 44 (37.9) | 72 | Somalia | Local |
| Aceti et al[30] | 1991 | 104 | 52 (50) | 52 | Somalia | Local |
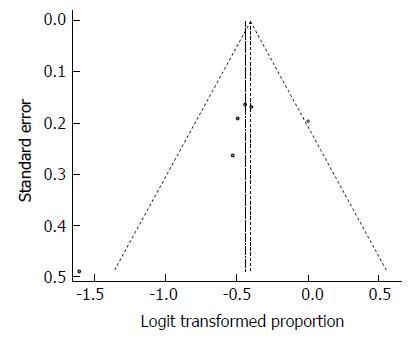
Despite the moderate heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 14). Moreover, the Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 41.5%, which was close to the observed effect size (i.e., 39.2%). However, the heterogeneity, expectedly, was statistically significant (Q = 18.28, df = 7, P = 0.0108).
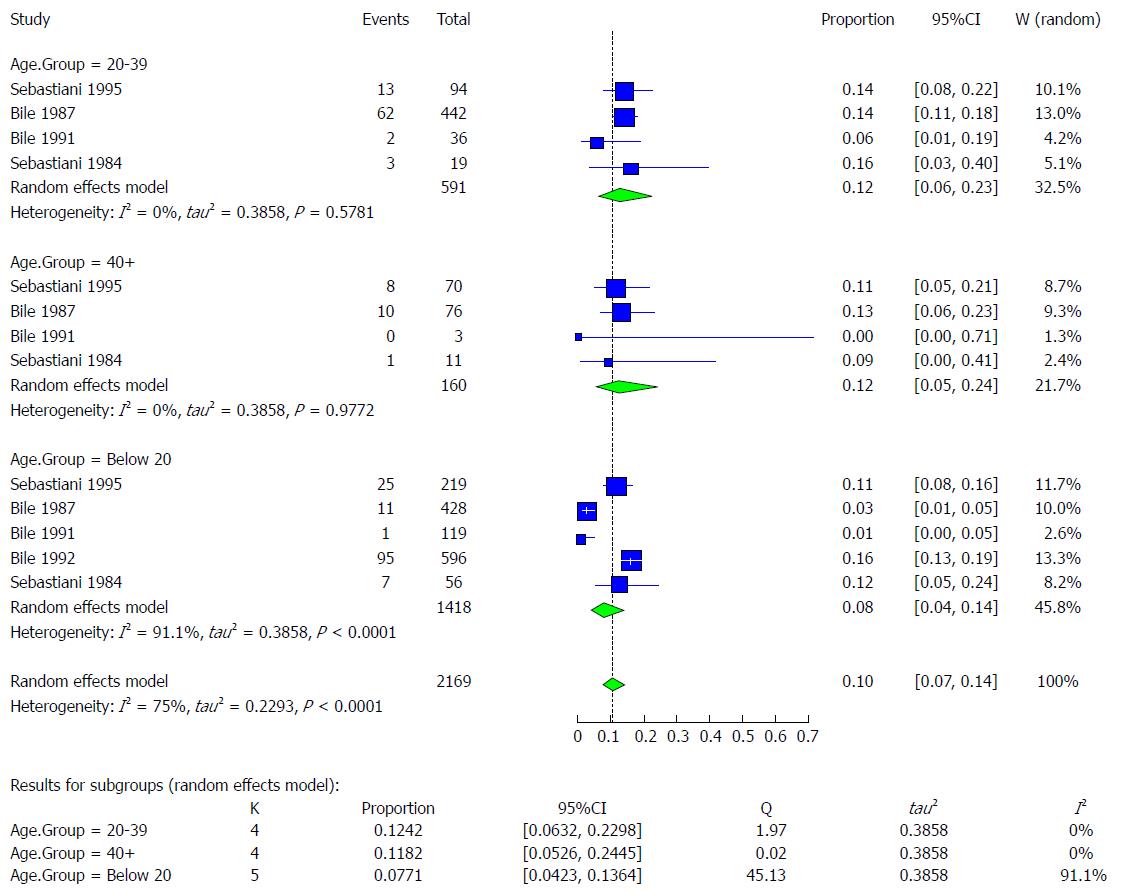
The available studies (n = 5) contained data on the prevalence of HBV infection among three age groups (below 20 years, 20-40 years, and above 40 years) that were available for extraction. The reported prevalence rate of these studies according to age group was as follows: below 20 (1% to 16%)[16,18,28,40]; 20-39 (6% to 16%)[18,28,40]; and above 40 (0% to 13%)[18,28,40] (Table 7). The HBV infection pooled effect was highest among the 20-39 age group at 12.4% (95%CI: 6.3% to 23.0%) followed by the above-40 age group at 11.8% (95%CI: 5.3% to 24.5%). The lowest pooled prevalence was in the below-20 age group at 7.7% (95%CI: 4.2% to 13.6%). The heterogeneity was very low for the 20-39 and the above-40 age groups (I2 = 0%) but was very high for the below-20 age group (I2 = 91.1%) (Figure 15).
| Age group | Author | Publication year | Total | HBsAg | Healthy |
| 0-20 | Sebastiani et al[40] | 1985 | 219 | 25 (11) | 194 |
| Bile et al[28] | 1987 | 428 | 11 (6) | 417 | |
| Bile et al[40] | 1991 | 119 | 1 (1) | 118 | |
| Bile et al[16] | 1992 | 596 | 95 (16) | 501 | |
| Sebastiani et al[18] | 1984 | 56 | 7 (13) | 49 | |
| Total | 1418 | 139 (9.8) | 1279 | ||
| 20-39 | Sebastiani et al[40] | 1985 | 94 | 13 (14) | 81 |
| Bile et al[28] | 1987 | 442 | 62 (14) | 380 | |
| Bile et al[40] | 1991 | 36 | 2 (6) | 34 | |
| Sebastiani et al[18] | 1984 | 19 | 3 (16) | 16 | |
| Total | 591 | 80 (14) | 511 | ||
| 40+ | Sebastiani et al[40] | 1985 | 70 | 8 (11) | 62 |
| Bile et al[28] | 1987 | 76 | 10 (13) | 66 | |
| Bile et al[40] | 1991 | 3 | 0 (0) | 3 | |
| Sebastiani et al[18] | 1984 | 11 | 1 (9.1) | 10 | |
| Total | 160 | 19 (11.8) | 141 |
The funnel plot indicated little evidence to support publication bias. Furthermore, Egger’s test for the assessment of symmetry of the funnel plot was not statistically significant (t = -1.8748, degrees of freedom = 11, P-value = 0.0876), providing some support, at the 5% significance level, for the absence of publication bias (Figure 16).
Characteristics of the studies included: Seven studies met the inclusion criteria for the meta-analysis of the prevalence of HBV infection and their risk groups, as shown in Table 8. The 7 included studies examined the prevalence of HBV infection in a total of 1488 Somali participants belonging to 7 major risk groups. The quality of the included studies also varied.
| Author | Year | Total | Cases | Healthy | Population |
| Nuti et al[24] | 1979 | 135 | 33 | 102 | Patients with leprosy |
| Nuti et al[24] | 1979 | 87 | 10 | 77 | Patients with leprosy |
| Nuti et al[23] | 1978 | 54 | 8 | 46 | Patients with schistosomiasis |
| Nuti et al[26] | 1979 | 67 | 13 | 54 | Patients with schistosomiasis |
| Delia et al[22] | 1977 | 155 | 52 | 103 | Patients with ancylostomiasis |
| Delia et al[22] | 1977 | 155 | 40 | 115 | Patients with schistosomiasis |
| Delia et al[22] | 1977 | 155 | 15 | 140 | Patients with leprosy |
| Delia et al[22] | 1977 | 155 | 11 | 144 | Patients with leprosy |
| Nuti et al[25] | 1979 | 55 | 33 | 22 | Patients with acute viral hepatitis |
| Jama et al[29] | 1987 | 85 | 17 | 68 | Female Prostitutes |
| Nur et al[33] | 2000 | 36 | 2 | 34 | Hospitalized children |
| Nur et al[33] | 2000 | 47 | 10 | 37 | Hospitalized adult |
Pooled prevalence of HBV infection of Risk groups: We used the extracted data of the 12 studies to quantify the overall pooled prevalence of HAV infection in each group.
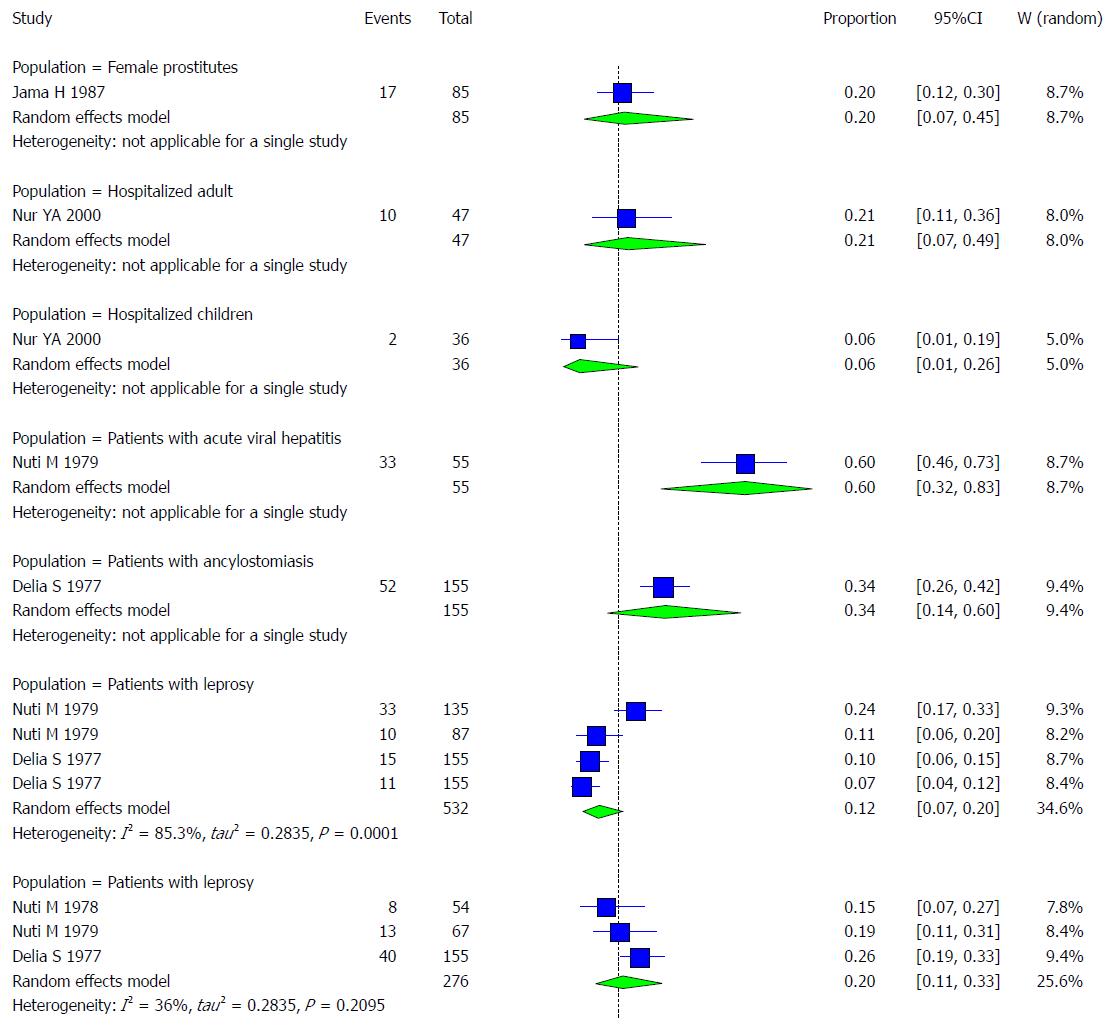
One study was conducted on female prostitutes. The pooled effect size for the prevalence of HBV infection among Somali prostitutes was 20% (95%CI: 7.19% to 44.64%).
One study was conducted on hospitalized adults. The pooled effect size for the prevalence of HBV infection among Somali hospitalized adults was 21.28% (95%CI: 7.15% to 48.69%).
One study was conducted on hospitalized children. The pooled effect size for the prevalence of HBV infection among Somali hospitalized children was 5.56% (95%CI: 0.99% to 25.62%).
One study was conducted on patients with acute hepatitis. The pooled effect size for the prevalence of HBV infection among Somali acute hepatitis patients was 60% (95%CI: 31.66% to 82.92%).
One study was conducted on patients with ancylostomiasis. The pooled effect size for the prevalence of HBV infection among Somali ancylostomiasis patients was 33.55% (95%CI: 14.44% to 60.16%).
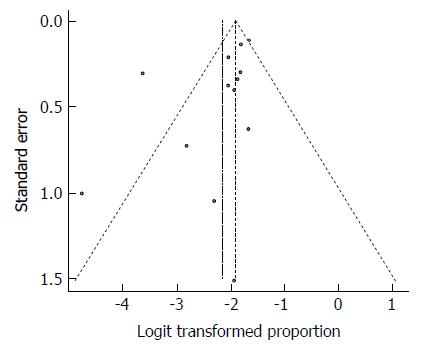
Four studies were conducted on leprosy patients. The pooled effect size for the prevalence of HBV infection among Somali leprosy patients was 12.34% (95%CI: 7.24% to 20.26%). The heterogeneity was high (I2 = 85.3%). Another indication of high heterogeneity was the Q-statistic [Q (degrees of freedom = 3) = 20.38].
Three studies were conducted on schistosomiasis patients. The pooled effect size for the prevalence of HBV infection among Somali schistosomiasis patients was 20.19% (95%CI: 11.28% to 33.49%). The heterogeneity was low (I2 = 36%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 2) = 3.13].
The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 17.
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 18). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 28.5% which was not widely different from the observed effect size.
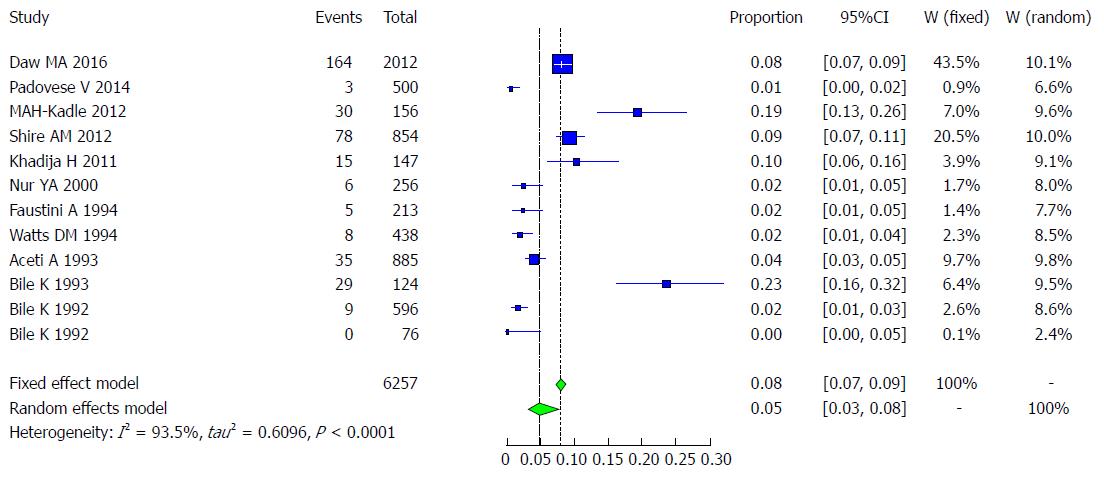
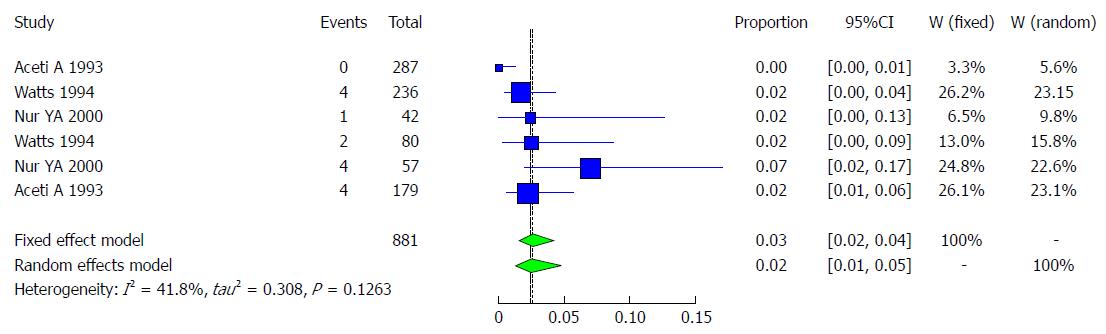
There are few studies on HCV infection in Somalia. Watts DM and his colleagues studied 438 subjects, including female prostitutes (1.7% or 4/236), patients from a sexually transmitted disease clinic (2% or 2/80), male soldiers (1.3% or 1/79), and tuberculosis patients (2.3% or 1/43), while the overall prevalence showed that 1.8% (8/438) were anti-HCV positive[41].Another study investigated children from two institutions for children in Somalia, showing that 1.5% of children were positive for anti-HCV in one residence of 596 children, including boys (1.9% or 6/309) and girls (1% or 3/287). However, in corresponding individuals, 76 children (boys and girls) showed a result of 0%[16]. A study conducted among blood donors revealed that (0.6% or 1/157) were anti-HCV-positive, while the overall presence of anti-HCV in this study was (2.4% or 6/256)[33]. A case-control study of HCV in chronic liver disease patients showed an estimate of 4% (35/885) in overall subjects[42]. Bile K and his colleagues found that 23.3% (29/124) of subjects had antibodies for HCV in the overall prevalence of cases and controls[34]. In 2014, a study conducted in Malta among asylum seekers showed that 0.6 %(3/500) of subjects were anti-HCV-positive[39]. Another study in Italy among immigrant communities that mostly came from Somalia indicated that 2.3% (5/213) had HCV-positive antibodies[19], and a study conducted in immigrant Somali communities in the United States showed that 9.1% (78/854) of subjects were anti-HCV-positive[36] (Table 9). Among population groups at risk for HCV in Somalia, studies have shown a result of 0% to 7%[33,41,42], shown in Table 10. Eleven studies met the inclusion criteria for the meta-analysis of the prevalence of HCV infection (Table 9).
| Year | Total | Cases | Total | Healthy | Setting | Population | |
| Daw et al[43] | 2016 | 2012 | 164 | 2012 | 1848 | Libiya | Immigrants |
| Padovese et al[39] | 2014 | 500 | 3 | 500 | 497 | Italy | Immigrants |
| Kadle et al[38] | 2012 | 156 | 30 | 156 | 126 | Somalia | Local |
| Shire et al[36] | 2012 | 854 | 78 | 854 | 776 | United States | Immigrants |
| Khadjio et al[37] | 2011 | 147 | 15 | 147 | 132 | Somalia | Local |
| Nur et al[33] | 2000 | 256 | 6 | 256 | 250 | Somalia | Local |
| Faustini et al[19] | 1994 | 213 | 5 | 213 | 208 | Italy | Immigrants |
| Watts et al[41] | 1994 | 438 | 8 | 430 | 90 | Somalia | Local |
| Aceti et al[42] | 1993 | 885 | 35 | 885 | 850 | Somalia | Local |
| Bile et al[34] | 1993 | 124 | 29 | 124 | 95 | Somalia | Local |
| Bile et al[16] | 1992 | 596 | 9 | 596 | 587 | Somalia | Local |
| Bile et al[16] | 1992 | 76 | 0 | 76 | 76 | Somalia | Local |
| Author | Year | Total | Cases | Total | Healthy | Setting | Population |
| Aceti et al[42] | 1993 | 287 | 0 | 287 | 278 | Mogadishu | Hospitalized children with diseases other than hepatitis |
| Watts et al[41] | 1994 | 236 | 4 | 236 | 232 | Mogadishu, Marka, Kismayo | Female prostitutes |
| Nur et al[33] | 2000 | 42 | 1 | 42 | 41 | Mogadishu | Hospitalized children with measles, tuberculosis, anemia and other febrile illnesses |
| Watts et al[41] | 1994 | 80 | 2 | 80 | 78 | Mogadishu, Marka, Kismayo | Sexually transmitted disease patients |
| Nur et al[33] | 2000 | 57 | 4 | 57 | 53 | Mogadishu | Hospitalized adults with tuberculosis, malaria, acute respiratory infections, and unknown diagnosis (no clinically evident case of hepatitis) |
| Aceti et al[42] | 1993 | 179 | 4 | 179 | 175 | Mogadishu | Mixed populations (98 prisoners and 81 psychiatric patients) |
The 11 included studies examined the prevalence of HCV infection in a total of 6257 Somali participants. The quality of the included studies also varied.
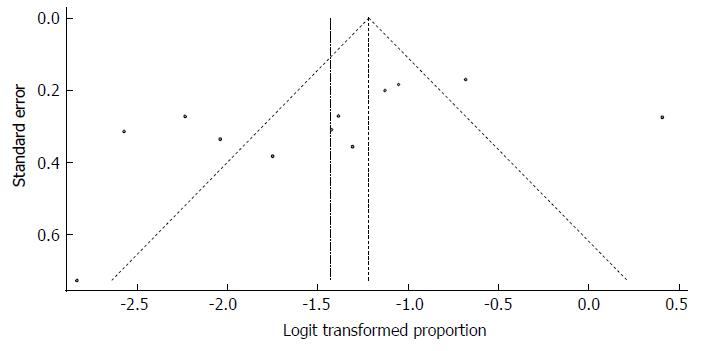
We used the extracted data of the 12 studies to quantify the overall pooled prevalence of HCV infection. The pooled effect size for the prevalence of HCV infection among Somali people was 4.84% (95%CI: 3.02% to 7.67%). The heterogeneity was high (I2 = 93.5%, 95%CI: 90.4% to 95.6%). Another indication of high heterogeneity was the Q-statistic [Q (degrees of freedom = 10) = 168.5, P value < 0.001]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CI, as shown in Figure 19.
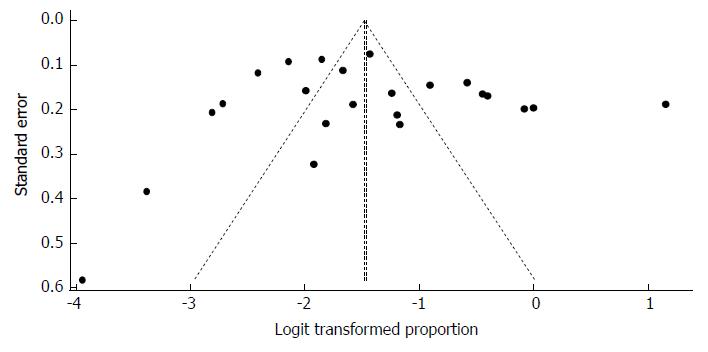
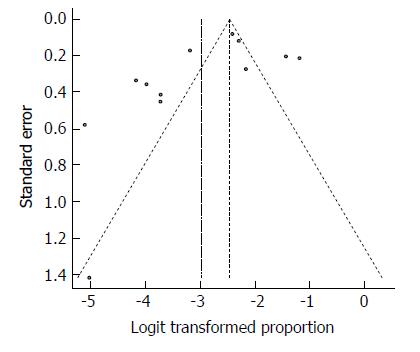
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 20). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 8.14%, which was not widely different from the observed effect size. Furthermore, Egger’s test for the assessment of symmetry of the funnel plot was not statistically significant (t = -1.6334, degrees of freedom = 10, P-value = 0.1334), providing further support for the absence of publication bias.
The majority of the studies (n = 8) were conducted in Somalia, with a pooled prevalence of HCV of 5.02% (95%CI: 2.18% to 11.13%) and high heterogeneity (I2 = 94.9%). These findings are followed by 2 studies conducted in Italy, with a pooled prevalence of HCV of 1.22% (95%CI: 0.21% to 6.74%) and moderate heterogeneity (I2 = 71.7%). There was 1 study from the United States with a prevalence of HCV of 9.13% (95%CI: 1.01% to 49.87%). There was 1 study from Libya with a prevalence of HCV of 8.15% (95%CI: 0.89% to 46.60%) (Figure 21).
Examining the effect of setting, insignificant variability could be explained by meta-regressing the meta-analysis over the three settings: Italy, Somalia, and the United States. Tests of moderators indicated the following: coefficient(s) 2,3: QM (df = 2) = 2.6557, P = 0.2651. For the prevalence results in Somalia compared to Italy, the P value was 0.1476, and the estimate was 1.4512. For the United States, the variability was not significantly different from that of Italy (P value=0.1560 and estimate = 2.0938).
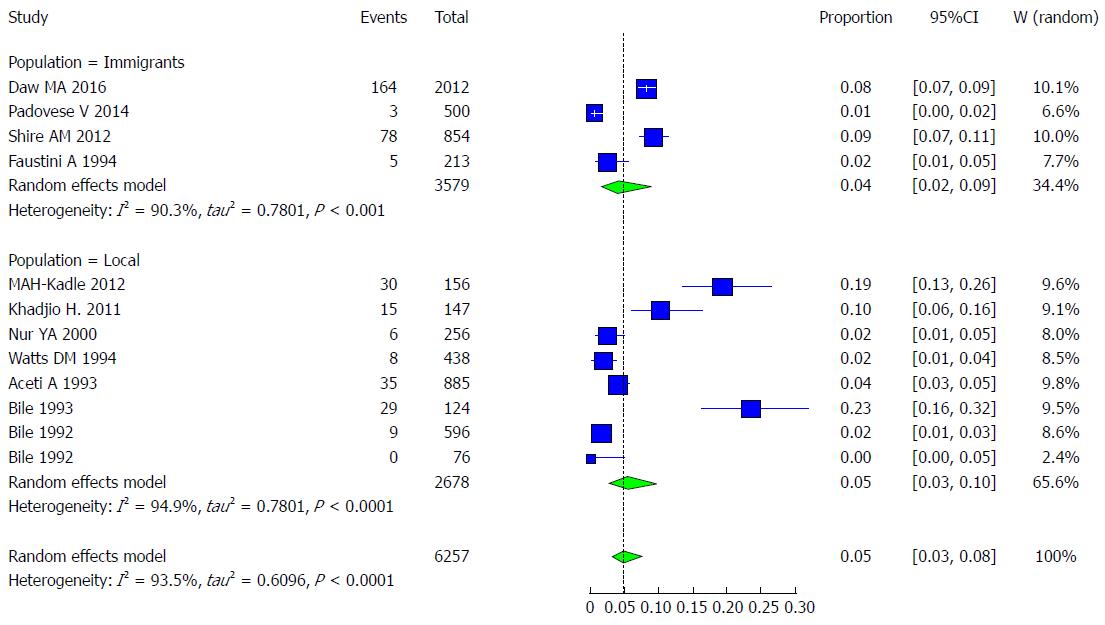
The majority of the studies (n = 8) were conducted in Somalia among non-immigrant populations, with a pooled prevalence of HCV of 4.99% (95%CI: 2.11% to 11.36%) and high heterogeneity (I2 = 94.9%). The remaining 4 studies were conducted in Italy, Libya and the United States among refugees, showing a pooled prevalence of HCV of 3.81% (95%CI: 1.54% to 9.13%) and high heterogeneity (I2 = 90.3%) (Figure 22).
When examining the effect of refugee status type, insignificant variability could be explained by meta-regressing the meta-analysis over the two statuses: immigrants vs the local population. Tests of moderators indicated the following: coefficient(s) 2: QM (df = 1) = 0.3335, P = 0.5636. For the prevalence in the local population compared to that in immigrants, the P value was 0.5636, and the estimate was 0.3383.
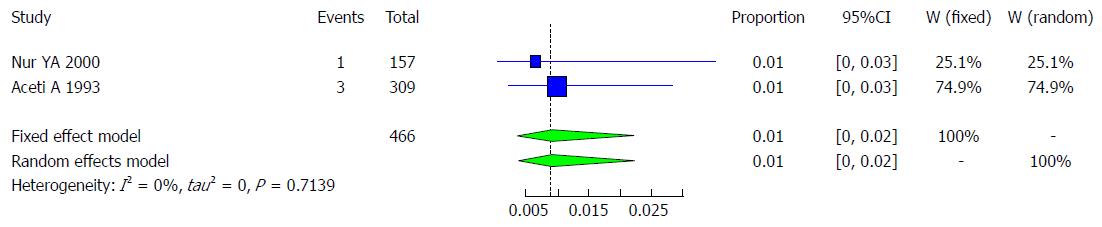
Characteristics of the studies included: There are 2 studies that met the inclusion criteria for the meta-analysis of the prevalence of HCV infection among blood donors (Table 11). The included 2 studies examined the prevalence of HCV infection in a total of 466 Somali blood donors. The quality of the included studies also varied.
We used the extracted data of the 2 studies to quantify the overall pooled prevalence of HCV infection in Somali blood donors. The pooled effect size for the prevalence of HCV infection among Somali blood donors was 0.87% (95%CI: 0.33% to 2.30%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.13, P = 0.7139]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 23.
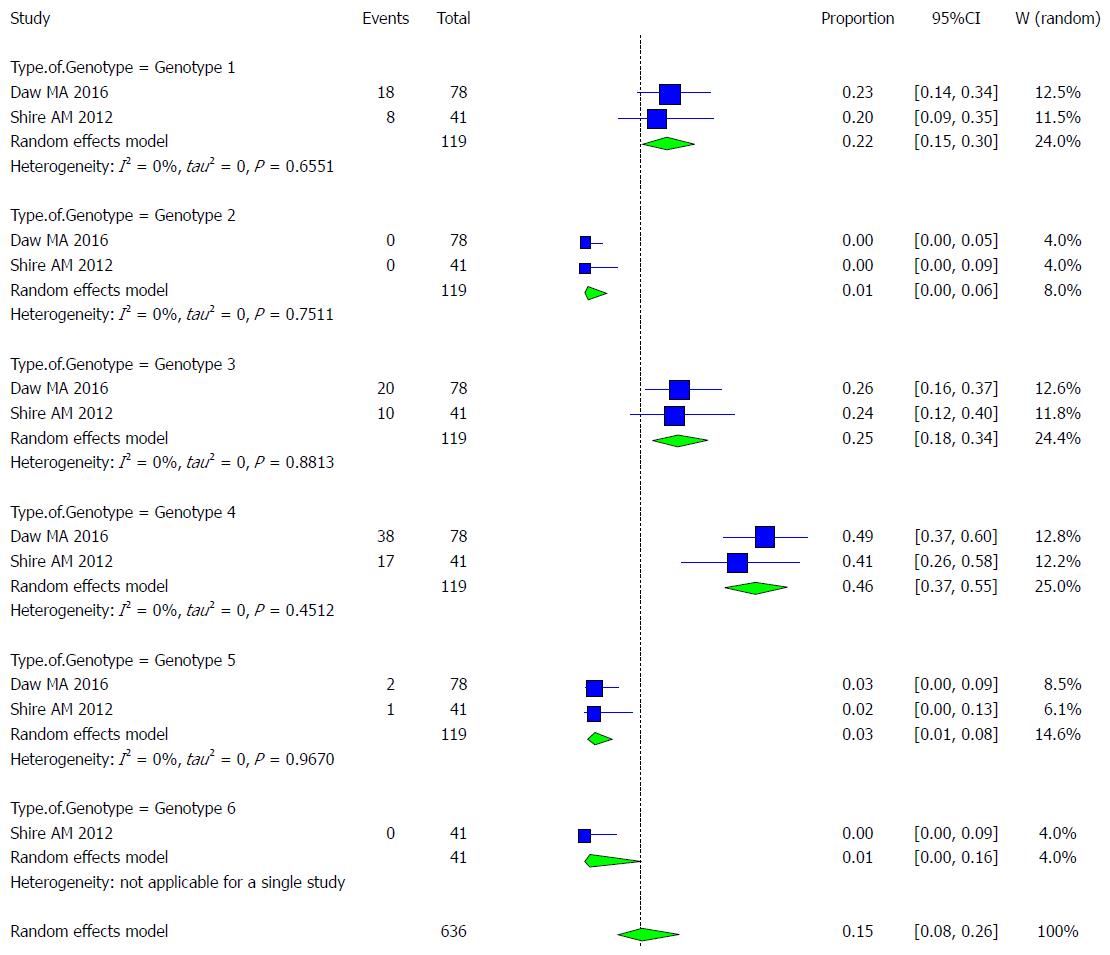
Characteristics of the studies included: Two studies met the inclusion criteria for the meta-analysis of the prevalence of HCV infection among different genotypes (Table 12)[36,43].
| Type of genotype | Author/Publication Year | Total | Cases | Healthy | Serology |
| Genotype 1 | Daw et al[43] /2016 | 78 | 18 | 60 | Anti-HCV |
| Genotype 1 | Shire et al[36] / 2012 | 41 | 8 | 33 | Anti-HCV |
| Genotype 2 | Daw et al[43] /2016 | 78 | 0 | 78 | Anti-HCV |
| Genotype 2 | Shire et al[36] / 2012 | 41 | 0 | 41 | Anti-HCV |
| Genotype 3 | Daw et al[43] /2016 | 78 | 20 | 58 | Anti-HCV |
| Genotype 3 | Shire et al[36] / 2012 | 41 | 10 | 31 | Anti-HCV |
| Genotype 4 | Daw et al[43] /2016 | 78 | 38 | 40 | Anti-HCV |
| Genotype 4 | Shire et al[36] / 2012 | 41 | 17 | 24 | Anti-HCV |
| Genotype 5 | Daw et al[43] /2016 | 78 | 2 | 76 | Anti-HCV |
| Genotype 5 | Shire et al[36] / 2012 | 41 | 1 | 10 | Anti-HCV |
| Genotype 6 | Shire et al[36] / 2012 | 41 | 0 | 40 | Anti-HCV |
Pooled prevalence of HCV infection per genotype group: We used the extracted data of the 12 studies to quantify the overall pooled prevalence of HCV infection in each genotype group.
The pooled effect size (out of 2 studies) for the prevalence of HCV infection among Somali people of genotype 1 was 21.9% (95%CI: 15.36% to 30.23%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.20].
The pooled effect size (out of 2 studies) for the prevalence of HCV infection among Somali people of genotype 2 was 0.87% (95%CI: 0.12% to 5.9%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.10].
The pooled effect size (out of 2 studies) for the prevalence of HCV infection among Somali people of genotype 3 was 25.21% (95%CI: 18.23% to 33.77%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.02].
The pooled effect size (out of 2 studies) for the prevalence of HCV infection among Somali people of genotype 4 was 46.24% (95%CI: 37.48% to 55.25%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.57].
The pooled effect size (out of 2 studies) for the prevalence of HCV infection among Somali people of genotype 5 was 2.52% (95%CI: 0.82% to 7.53%). The heterogeneity was low (I2 = 0%). Another indication of low heterogeneity was the Q-statistic [Q (degrees of freedom = 1) = 0.00].
The pooled effect size (out of 1 studies) for the prevalence of HCV infection among Somali people of genotype 6 was 1.19% (95%CI: 0.07% to 16.38%).
The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 24.
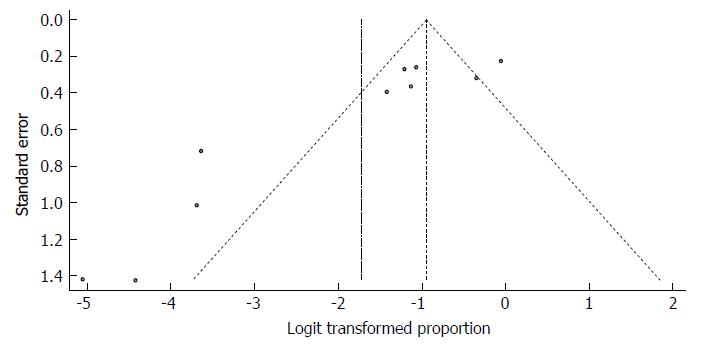
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 25). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 25.84%, which was not widely different from the observed effect size.
Characteristics of the studies included: Three studies met the inclusion criteria for the meta-analysis of prevalence of HCV infection (Table 10). The 3 included studies examined the prevalence of HCV infection in the total of 881 Somali participants. The quality of the included studies also varied.
Pooled prevalence of HCV infection in risk groups: We used the extracted data of the 6 studies to quantify the overall pooled prevalence of HCV infection in Somali risk groups. The pooled effect size for the prevalence of HCV infection among Somali risk groups was 2.43% (95%CI: 1.21% to 4.8%). The heterogeneity was low (I2 = 41.8%, 95%CI: 0% to 77%). Another indicator of low heterogeneity was the Q-statistic [Q (degrees of freedom = 5) = 8.6, P value = 0.1263]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 26.
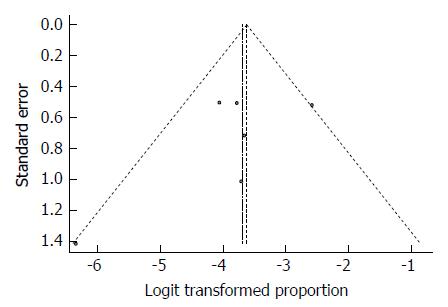
As expected from the low heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 27). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 3.36%, which was close to the observed effect size.
Characteristics of the studies included: Three studies met the inclusion criteria for the meta-analysis of the prevalence of HCV infection (Table 13). The 3 included studies examined the prevalence of HCV infection in a total of 1001 Somali participants. The quality of the included studies also varied.
| Author | Year | Total | Cases | Total | Healthy | Setting | Population |
| Bile et al[16] | 1992 | 596 | 9 | 596 | 587 | Mogadishu area | Children in government-operated residences for abandoned children in Shebeli |
| Bile et al[16] | 1992 | 76 | 0 | 76 | 76 | Mogadishu area | Children in government-operated residence for abandoned children in SOS institution |
| Nur et al[33] | 2000 | 42 | 1 | 42 | 41 | Mogadishu | Hospitalized children |
| Aceti et al[42] | 1993 | 287 | 0 | 287 | 278 | Mogadishu | Hospitalized children with diseases other than hepatitis |
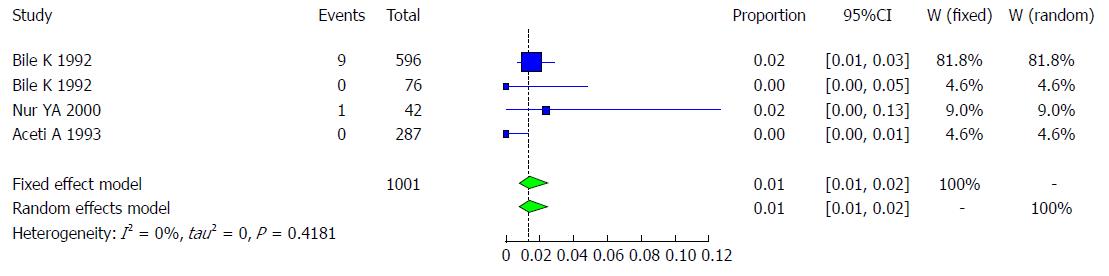
Pooled prevalence of HCV infection in children: We used the extracted data of the 4 studies to quantify the overall pooled prevalence of HCV infection in Somali children. The pooled effect size for the prevalence of HCV infection among Somali children was 1.37% (95%CI: 0.76% to 2.46%). The heterogeneity was low (I2 = 0%, 95%CI: 0% to 83.8%). Another indicator of low heterogeneity was the Q-statistic [Q (degrees of freedom = 3) = 2.83, P value = 0.4181]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 28.
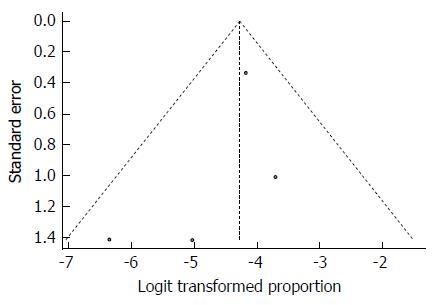
As expected from the low heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 29). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 1.63%, which was close to the observed effect size.
Characteristics of the studies included: Five studies met the inclusion criteria for the meta-analysis of the prevalence of HCV infection (Table 14). The 5 included studies examined the prevalence of HCV infection in a total of 505 Somali participants. The quality of the included studies also varied.
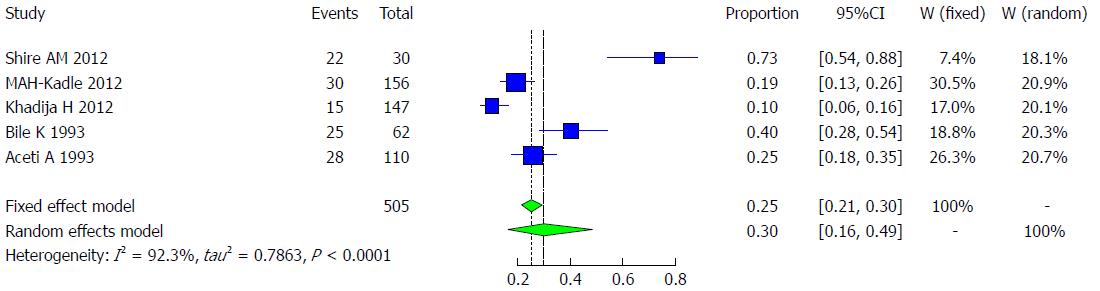
Pooled prevalence of HCV infection in chronic liver disease groups: We used the extracted data of the 6 studies to quantify the overall pooled prevalence of HCV infection in Somali chronic liver disease groups. The pooled effect size for the prevalence of HCV infection among Somali chronic liver disease groups was 29.82% (95%CI: 15.84% to 48.98%). The heterogeneity was high (I2 = 92.3%, 95%CI: 85% to 96%). Another indicator of high heterogeneity was the Q-statistic [Q (degrees of freedom = 4) = 51.92, P value < 0.0001]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 30.
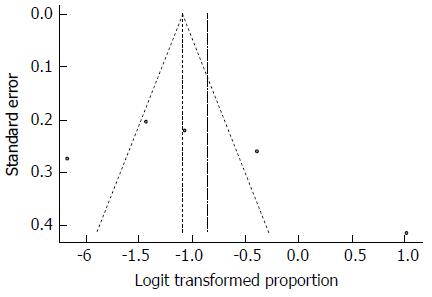
Despite the high heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 31). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 22.2%, which was close to the observed effect size.
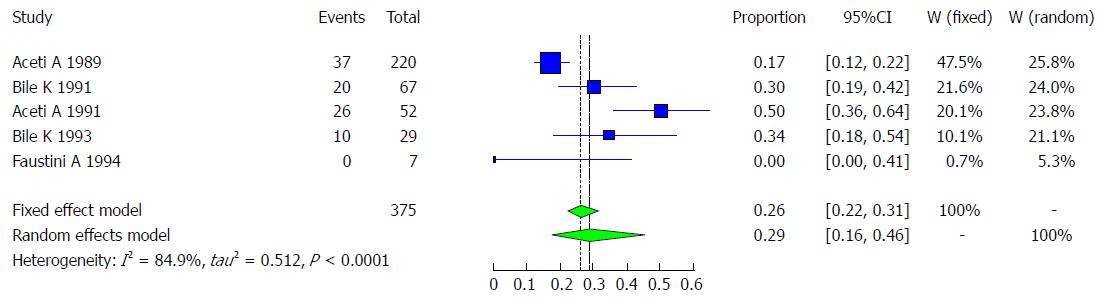
Hepatitis D, or delta hepatitis, is caused by the HDV, a unique RNA pathogen. It requires the HBV for its own replication and to infect as co-infection. HDV is transmitted by percutaneous or sexual contact with infected blood or blood products. The main vulnerable group consists of patients with chronic HBsAg infection who become super-infected with the virus. It is estimated that 5% of HBV-infected persons are also co-infected with HDV[1]. Aceti A and his colleagues conducted a study in 1989 showing that among 220 asymptomatic HBsAg-positive carriers, 16.80% (37) had the antibody for HDV[31]. In a study of the prevalence of HDV infection in patients with chronic liver disease in Somalia, 52 of the 104 patients studied were positive for HBsAg, and 26 (50%) had anti-delta antibodies[30]. In a 1993 study by Khalif Bile and his colleagues with 62 Somali patients with chronic liver disease, including hepatocellular carcinoma, 37.1% (23) of cases with chronic liver disease were positive for HBsAg, and 34.6% (8) of chronic liver disease patients had anti-HDV. Meanwhile, in the control group, 14.3% of those who were positive for HBsAg had anti-HDV[32]. Another study conducted among 67 HBsAg-positive patients showed that 30% (20) of these patients were anti-HDV-positive. Additionally, this study showed that 38.6% (17) of the 44 cases of chronic liver disease patients were HBsAg-positive[32]. One study examining the prevalence of HDV showed that 34.4% (10/29) of those who were HBsAg-positive and 39.1% (9/23) of chronic liver disease patients who were HBsAg-positive had antibodies for HDV[34]. Additionally, 0% of community immigrants from Somalia and neighboring countries who were positive for HBsAg were shown to have anti-HDV[19]. Five studies met the inclusion criteria for the meta-analysis of the prevalence of HDV infection, and the five included studies examined the prevalence of HDV infection in a total of 375 Somali participants. The quality of the included studies also varied (Table 15).
We used the extracted data of the 5 studies to quantify the overall pooled prevalence of HDV infection. The pooled effect size for the prevalence of HDV infection among Somali people was 28.99% (95%CI: 16.38% to 45.96%). The heterogeneity was high (I2 = 84.9%, 95%CI: 66% to 93%). Another indicator of high heterogeneity was the Q-statistic [Q (degrees of freedom = 4) = 26.4, P value < 0.001]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 32.
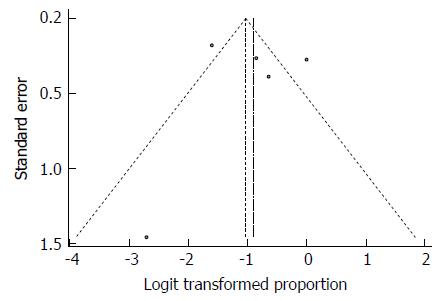
Despite the significant heterogeneity, the funnel plot displayed a symmetric spread of studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 33). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 28.99%, which was identical to the observed effect size.
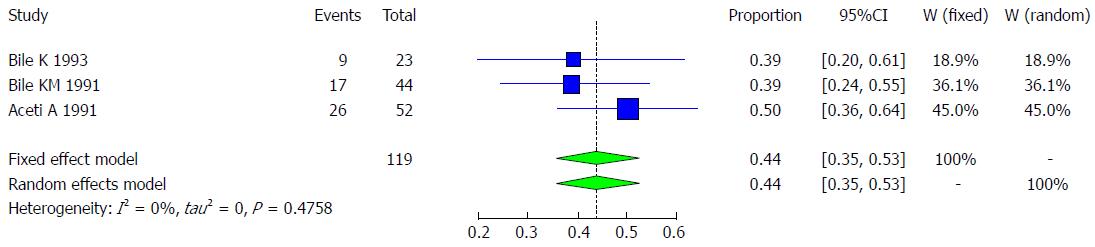
Three studies met the inclusion criteria for the meta-analysis of prevalence of chronic liver disease and HDV infection. These three included studies had a total of 119 Somali participants, and the quality of the included studies varied (Table 16).
We used the extracted data of the 3 studies to quantify the overall pooled prevalence of chronic liver disease and HDV infection. The pooled effect size for the prevalence of chronic liver disease and HDV infection among Somali people was 43.77% (95%CI: 35.09% to 52.84%). The heterogeneity was low but had a wide confidence interval (I2 = 0%, 95%CI: 0% to 86%). Another indicator of low heterogeneity was the Q-statistic [Q (degrees of freedom = 2) = 1.49, P value = 0.4758]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 34.
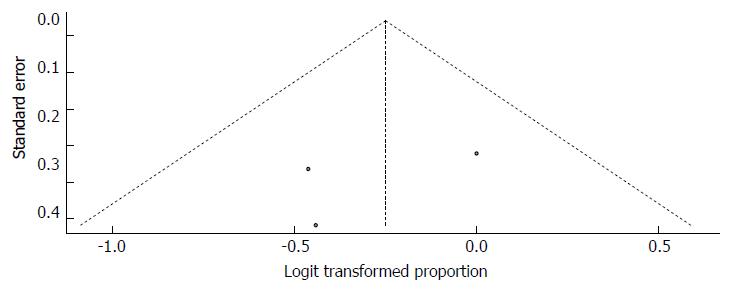
The funnel plot displayed a symmetric spread of the three studies in terms of relative weight and effect size, thereby indicating little evidence of publication bias (Figure 35). Notably, the total number of studies was small, and the individual studies were of variable sample size.
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did not support the possibility of missing studies from the analysis. The effect size imputed was 43.77%, which was identical to the observed effect size.
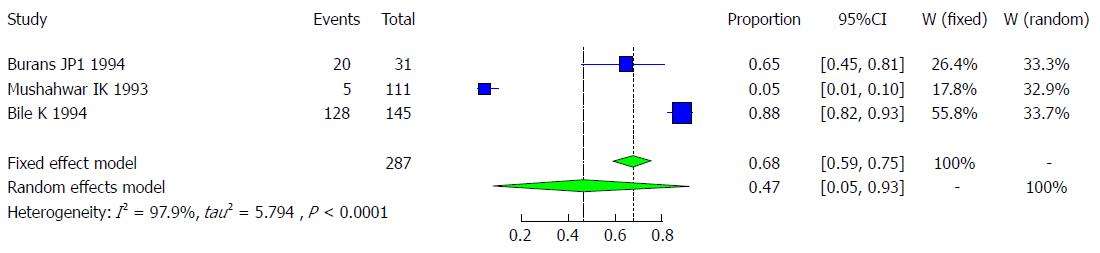
It is believed that more than 50% of hepatitis E cases in developing countries are unrelated to HAV or HBV infection, and a high proportion of these cases seems to be enterically transmitted[44]. Studies on the outbreak of an acute viral hepatitis occurring in three villages in the lower Shabeli region of southern Somalia examined the presence of antibodies to the hepatitis E antigen, and it was found that the prevalence of anti-HEV ranged from 77.8% to 94.0% among the three villages and was widely distributed among all age groups, without a preponderance in any specific age. During the onset of the outbreak, 111 samples were collected in one of the three villages, which showed that a very low prevalence (4.5% or 5/111) was positive for IgG anti-HEV[45]. In another survey of 142 villages with a population of 245312 individuals, 11413 icteric cases were recorded, 346 of whom had died, corresponding to an attack rate and a case fatality rate of 4.6% and 3.0%, respectively. The role of HEV in this epidemic was proven by documenting anti-HEV in 88.2% (128 of 154) of the sampled cases, which served as a sign of recent infection with HEV. Further, the attack rate was found to be higher with increasing age, from 5% in the 1- to 4-year-old age group to 13% in the 5- to 15-year-old age group, and to 20% for persons older than 15 years of age. A high fatality rate of 13.8% was estimated among pregnant females. Therefore, the attack rate was higher (6.0%) in villages supplied with river water, while fewer cases were recorded in those relying on wells (1.7%) or ponds (1.2%) for their water supply[46]. Burans and his colleagues conducted a study during the operation of Restore of Hobe in Somalia in 1994, finding that among 31 Somalians with acute hepatitis, 20 (65%) had IgM anti-HEV[47]. Three studies met the inclusion criteria for the meta-analysis of the prevalence of HEV infection, and a total of 287 Somali participants were examined. The quality of included studies also varied (Table 17).
We used the extracted data of the 3 studies to quantify the overall pooled prevalence of HEV infection. The pooled effect size for the prevalence of HEV infection among Somali people was 46.86% (95%CI: 5.31% to 93.28%). The heterogeneity was high (I2 = 97.9%, 95%CI: 96% to 98.9%). Another indicator of high heterogeneity was the Q-statistic [Q (degrees of freedom = 2) = 93.4, P value < 0.001]. The result of this analysis is presented in the forest plot showing the effect sizes for the individual original studies and their 95%CIs, as shown in Figure 36.
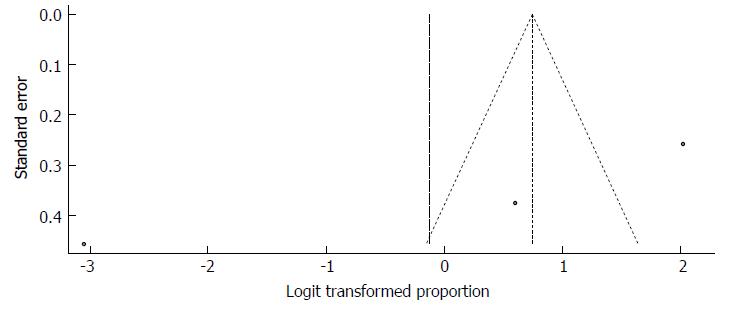
Because the significant heterogeneity, the funnel plot displayed a somewhat asymmetric spread of studies in terms of relative weight and effect size, thereby indicating evidence of publication bias (Figure 37). Notably, the total number of studies was small, and the individual studies were of variable sample size. The study by Mushahwar et al[45] is clearly an outlier.
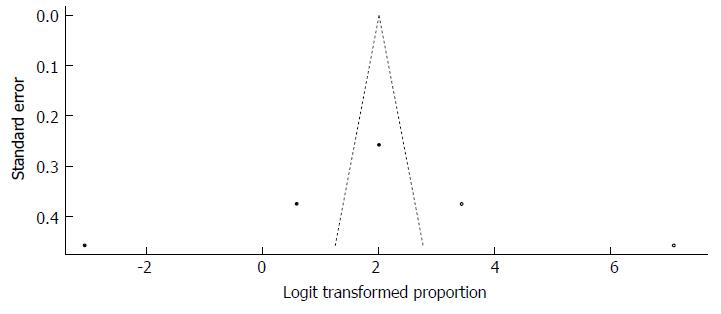
Moreover, Duval and Tweedie’s trim and fill procedure for the detection of publication bias did support the possibility of missing studies from the analysis. The effect size imputed was 88.28%, which was well above the observed effect size. The funnel plot generated via Duval and Tweedie’s trim and fill procedure indicated the absence of studies with a high prevalence if the balance was to be restored (Figure 38).
Viral hepatitis is major public health problem in the world, especially in developing countries. To our knowledge, this review is the first systematic review and meta-analysis study that has attempted to thoroughly summarize evidence on the different types of viral hepatitis infection in Somalia. The aim of this review was to understand the burden of viral hepatitis, especially HBV and HCV, in Somalia and to inform public health practitioners, researchers and policy makers in the development of national strategies for the prevention and control of viral hepatitis. The findings of this review clearly show the burden of the viral hepatitis in the country, particularly HBV. The estimation prevalence of HBV in most African countries was 5%-20%[48], and the WHO HBV endemic definition of HBsAg prevalence is 5%-7%[5]. In our review, we identified the prevalence of HBV in Somalia, as detected by HBsAg, to be high (18.9%). Somalia was categorized among countries with a high endemicity of HBV (prevalence ≥ 8%)[20,21]. The rate of global prevalence (3.61%) reported by Sweitzer and his colleagues, as well as the rate of 8.83% of the WHO African region, was significantly less than our study result[49]. In countries such the United States, Iran and Kosovo, the HBV prevalence rate has been estimated to be approximately < 0.27%, 2.14%, and 4.2%, respectively[50-52]. Therefore, this comparative information highlighted the extent of the HBV burden in Somalia. The pooled prevalence in this study was higher than that of a recent report from African countries, such as Ethiopia, Cameroon, Ghana, and Nigeria, the pooled prevalence of which was 7.4%, 11.2%, 12.3%, and 13.6%, respectively[48,53-55].
Additionally, HBV infection among blood donors (19.1%)[33] and the pooled prevalence of pregnant women (20.5%) also remain high in Somalia, which justifies the requirements of a national HBV screening program for all pregnant women in antenatal clinics and a national policy to vaccinate all pregnant women and adults who test negative for HBV, so as to prevent and reduce the risk of HBV of mother-to-child transmission. We also need to establish a national blood policy throughout Somalia for the prevention or reduction of receiving contaminated blood, the occurrence of which remains high within this HBV population. According to studies of HBV infection among children, the prevalence (5.7%) is lower than that of other groups, while that of Somali immigrants around the world and of chronic liver disease patients was 23% and 39.2%, respectively. These findings therefore demonstrate the true epidemiological burden of HBV in Somalia.
Regarding HCV, we found a high pooled prevalence of 4.84% in Somalia. This prevalence rate is considerably higher than the reported pooled prevalence in Somalia of 0.9% by Chaabna et al[56] and 1.5% by Karoney et al[57]. Meanwhile, in Djibouti and Sudan, the pooled prevalence rate was 0.3% and 1.0%, respectively[56]. We also found close prevalence rates in our study and found that the pooled prevalence rate in Ethiopia, Ghana, and the Democratic Republic of the Congo was 3.1%[48], 3.0%[58] and 2.9%[59], respectively, but the overall prevalence rate is 3.0% in all sub-Saharan African countries[60]. However, in Cameroon, the estimated prevalence of anti-HCV is 11.6%[61] and the reported prevalence rate for Egypt is 14.7%[61,62]; thus, the estimated prevalence of anti-HCV is much lower in Somalia than in these two countries. The pooled prevalence rate of sub-groups examined in this study, such as blood donors, Somali immigrants, risk groups, children and patients with chronic liver disease, was 0.8%, 3.81%, 2.43%, 1.37%,and 29.82%, respectively. These findings may emphasize that the level of chronic HCV in Somalia may be significantly high. Genotypes commonly found in Africa are 1, 4 and 5[57]. Therefore, in this review, we also present the most frequent genotype among the Somali population, which is genotype 4. Most African countries, such as Uganda, Sudan, Rwanda, Burundi, Cameroon and Egypt, also show a predominance of HCV genotype 4[57]. The next most prevalent genotype in our country is genotype 3, which is also similar to our neighboring countries, such as Ethiopia, Kenya, and Eritrea[57].Meanwhile, the next most prevalent genotype in Somalia is genotype 1.
The number of studies of HDV in Somalia among Somali patients and chronic liver disease patients was very low, but the data available thus far demonstrated a considerable prevalence of HDV infection. Our results showed that the pooled prevalence rate of HDV in Somalia was 28.99%. The pooled prevalence of Sudan and Uganda, which are other sub-Saharan African countries, was 27.8%[63] and 30.6%[63], respectively, and the pooled prevalence rate of the EMRO region is 14.74%[64]. Therefore, our result is similar to that of other countries in our region. The worldwide estimation rate also indicated that 5% of HBsAg carriers were infected with HDV[1]. The pooled prevalence rate in this review for patients with chronic liver disease, including hepatocellular carcinoma and positivity for anti-HDV, was shown to be 43.77%, and this result is similar to the prevalence rates among patients in the EMRO region with chronic liver disease (chronic hepatitis disease 27.8% and cirrhosis/hepatocellular carcinoma 36.57%)[64]. However, the previous pooled estimation in Somalia showed a similar prevalence rate as chronic hepatitis disease (47.36%) and cirrhosis/hepatocellular carcinoma (33.20%)[64]. Other countries in the EMRO region also showed results similar to our findings. For example, the prevalence of chronic hepatitis disease and cirrhosis/hepatocellular carcinoma in Pakistan was 37.38% and 53.77%, respectively, and the prevalence of the same conditions in Egypt was 24.37% and 29.6%, respectively[64]. These results indicate that HDV infection is endemic in Somalia.
Although the number of prevalence studies of hepatitis A and E virus infections among Somali patients were also very low in Somalia due to the lack of government support in the country in the last three decades, the available data for HAV and HEV still illustrated a high prevalence rate in the country. These viruses were transmitted through the fecal-oral route, and many environmental and socio-economic factors promoted the transmission routes. This systematic review showed a high pooled prevalence rate of HAV, which is 90.2%, and this prevalence rate was close to the individual estimation rates of old reports in Somalia[15,16,18,19]. We also indicate that the estimates of HEV infection in previous reports in Somalia may quite high, ranging between 4.5% and 88.2%[45-47], which is similar to estimates for the Ghanaian population of anti-HEV, with their range of 5.8% and 71.55%[65]. However, this review showed a pooled prevalence rate of HEV of 46.86%, which is also high among Somali patients. HEV infection in Africa is widespread and is predicted to be a hazard to numerous lives, in particular for pregnant women and their fetuses[2]. Serological studies from Egypt have shown that the seroprevalence of anti-HEV can reach close to 100% in the general population[2]. Another serological study from Ghana showed that anti-HEV can also reach close to 50%, and a study in Nigeria showed nearly100% of anti-HEV in the general population[2]. Meanwhile, a number of large outbreaks occurred and were reported at different times in many countries in Africa, such as Kenya, Sudan, Ethiopia, Somalia, Uganda and Chad[2,45]. The outbreak that occurred in Somalia showed a range of 77.8% to 94.0% among three villages in the country[45]. A survey from 142 villages showed 11413 jaundice patients in a population of 245312 individuals, of whom 346 persons died, with a corresponding attack rate of 4.6% and a case fatality rate of 3.0%[46]. An older report demonstrated that the attack rate was higher with increasing age, from 5%, 13% and 20% for the age groups of 1-4 years, 5-15 years and above 15 years old, respectively. Meanwhile, pregnant women had a high fatality rate, estimated to be 13.8%[46], in comparison to the rates in other African countries, such Ghana, (66.7%)[65], Sudan (31.1%)[66] and the Central African Republic (14.3%)[67]. However, the fatality rate of pregnant women was higher than that of our older report in the country[46]. This systematic review and meta-analysis study indicates that the two viruses of HAV and HEV are endemic in the country.
This systematic review and meta-analysis study had several limitations. The main limitation is that to the best of our knowledge, this review is the first that focuses on all types of viral hepatitis infection in Somalia. The second limitation of this review is the relatively small number of studies identified on all types of viral hepatitis in the country before and after the civil war. This lack of studies highlights the fact that research and knowledge of the viral hepatitis infection burden in Somalia are less developed than in other African and Arab countries. The third limitation is that the majority of our studies involved samples from only four or five regions of Somalia, especially in South Somalia, and samples of Somali immigrants around the world. Subsequently, we did not have samples from the southwestern, central, and northern regions of the country, such as Gedo, Bakol, Hiran, Galgadud, Mudug, Nugal, East, Sol, Sanag, Togder, Northwest and Awdal regions, which were included in the studies reviewed, while most studies were completed before the government collapsed, and few studies were done during and after the civil war.
In conclusions, although this systematic review and meta-analysis study is based on a limited number of studies and a small sample size, the study effects were a potential limitation of the review. However, the results of this review indicate that all types of viral hepatitis infection are common among Somalis, particularly HBV, which is more prevalent and endemic. This study has also documented a high prevalence rate of HCV infection in Somalia. The prevalence of these viruses could be understood as one of the nation’s public health problems, and urgent public health interventions are needed to reduce the infection rate of the country, because these preventable diseases, particularly HBV and HAV, can be eradicated by vaccination. Preventive measures include increasing awareness and knowledge about the transmission of all forms of viral hepatitis, screening all donated blood, targeting high-risk groups, providing treatment for affected persons, and generally improving the sanitary and living conditions. Other efforts needed to reduce the burden of the disease and strengthen the Somali health system include the establishment of a national blood policy and prevention and control viral hepatitis policy. Additionally, further studies are needed to fully understand the population factors underlying the high prevalence of all forms viral hepatitis, particularly in underrepresented regions and among adults, children, blood donors and high-risk groups, to offer better perspectives on all forms of the viral hepatitis burden in Somalia. The generation of up-to-date data is recommended, especially regarding the magnitude of HAV, HCV, and their genotypes, HDV and HEV.
Viral hepatitis is a major public health problem affecting several hundred million people globally. The most common types of viral hepatitis are six distinct types (hepatitis A, B, C, D, E, G viruses), and they may present in acute form or chronic form causes substantial morbidity and mortality (including chronic hepatitis, cirrhosis and hepatocellular carcinoma).
In the field of viral hepatitis, there is a lack of researches last three decades in country and all articles about viral hepatitis published and unpublished are scattered. In this systematic review and meta-analysis the authors aim to provide a clear understanding of viral hepatitis epidemiology and their clinical burdens in Somalia according to the related documents published and unpublished articles during the last decades.
The main objective of this study is to determine the prevalence of all viral hepatitis in Somalia especially hepatitis B virus (HBV) and hepatitis C virus (HCV), and to inform public health practitioners, researchers and policy makers, and to be a baseline data for future Hepatology researches in the country.
A systematic review and meta-analysis was conducted as Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A comprehensive literature search of published studies on viral hepatitis was performed from 1977-2016 in PubMed, Google Scholar, Science Direct, World Health Organization African Index Medicus and the Africa Journals Online databases, as well as on the Ministry of Health website. We also captured unpublished articles that were not available on online systems.
Twenty-nine studies from Somalia and Somali immigrants (United Kingdom, United States, Italy, Libya) with a combined sample size for each type of viral hepatitis were analyzed. The overall pooled prevalence rate of hepatitis A virus was 90.2%. The overall pooled prevalence of HBV was 18.9%. The overall pooled prevalence of HCV was estimated as 4.84%. The overall pooled prevalence of hepatitis D virus was 28.99%. The overall pooled prevalence of hepatitis E virus was 46.86%.
This study demonstrates a high prevalence of all forms of viral hepatitis in Somalia and it also indicates that chronic HBV was the commonest cause of chronic liver disease.
Viral hepatitis in Somalia demonstrated a high rate in its all types of hepatitis especially hepatitis B virus and C, while hepatitis B determined the common cause of chronic liver disease in Somalia. According to this systematic review and meta-analysis, further studies are needed in order to search out data about viral hepatitis in different regions of the country, risk factors and its complications.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Somalia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Esmat SM, Pokorska-Spiewak M, Wang K S- Editor: Wang JL L- Editor: A E- Editor: Bian YN
| 1. | World Health Organization. Global Hepatitis Report 2017 Accessed March 16, 2018. Available from: apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf. |
| 2. | Kim JH, Nelson KE, Panzner U, Kasture Y, Labrique AB, Wierzba TF. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect Dis. 2014;14:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653-6657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (3)] |
| 4. | Hassan-Kadle MA. The Diagnostic Challenges, Possible Etiologies and Lack of Researches of Hepatocellular Carcinoma in Somalia. Open J Gastroenterol. 2017;7:115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Global policy report on the prevention and control of viral hepatitis, 2013. Accessed March 16. 2018; Available from: apps.who.int/iris/bitstream/10665/85397/1/9789241564632_eng.pdf. |
| 6. | Central Intelligence Agency. The world fact book. Accessed March 16. 2018; Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/so.html. |
| 7. | World Bank. Somalia overview. Accessed March 16. 2018; Available from: http://www.worldbank.org/en/country/somalia/overview. |
| 8. | African Development Bank Group. Somalia Country Brief 2013-2015. Accessed March 16. 2018; Available from: http://www.afdb.org/fileadmin/uploads/afdb/Documents/Project-and-Operations/2013-2015%20-%20Somalia%20-%20Country%20Brief.pdf. |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Reprint--preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8875] [Article Influence: 554.7] [Reference Citation Analysis (0)] |
| 10. | Viechtbauer W. Package ‘metafor’. Meta-Analysis Package for R. Accessed March 16. 2018; Available from: https://cran.r-project.org/web/packages/metafor/metafor.pdf. |
| 11. | Schwarzer G. Package ‘meta’. General Package for Meta-Analysis. Accessed March 16. 2018; Available from: https://cran.r-project.org/web/packages/meta/meta.pdf. |
| 12. | Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9093] [Article Influence: 363.7] [Reference Citation Analysis (0)] |
| 14. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40538] [Article Influence: 1447.8] [Reference Citation Analysis (2)] |
| 15. | Mohamud KB, Aceti A, Mohamed OM, Pennica A, Maalin KA, Biondi R, Hagi MA, Quaranta G, Paparo SB, Celestino D. [The circulation of the hepatitis A and B viruses in the Somali population]. Ann Ital Med Int. 1992;7:78-83. [PubMed] |
| 16. | Bile K, Mohamud O, Aden C, Isse A, Norder H, Nilsson L, Magnius L. The risk for hepatitis A, B, and C at two institutions for children in Somalia with different socioeconomic conditions. Am J Trop Med Hyg. 1992;47:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Bile K, Aceti A, Hagi MA, Paparo BS, Pennica A, Celestino D, Sebastiani A. Primary hepatitis A virus infection in developing countries: decline of maternal antibodies and time of infection. Boll Ist Sieroter Milan. 1986;65:464-466. [PubMed] |
| 18. | Sebastiani A, Aceti A, Russo V, Paparo BS, Pennica A, Bile K, Farah AA, Farah AH. Age-specific prevalence of hepatitis A and hepatitis B virus infection in fluvial Somalian village. Estratto dal. 1984;30:17-23. |
| 19. | Faustini A, Franco E, Saitto C, Cauletti M, Zaratti L, Papini P, Ali’ Ahmed S, Zampieri F, Ierussi A, Panà A. Hepatitis A, B, C and D in a community in Italy of immigrants from NE Africa. J Public Health Med. 1994;16:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Averhoff F. Hepatitis B. Accessed March 16. 2018; Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/hepatitis-b. |
| 21. | Howell J, Ladep NG, Lemoin M, Thursz MR, Taylor-Robinson SD. Hepatitis B in Sub-Saharan Africa. South Sudan Medical J. 2014;7:59-61. |
| 22. | Delia S, Nuti M, Soro S. [Relations between hepatitis B surface antigen and parasitic diseases: observations in patients with ancylostomiasis and schistosomiasis]. Quad Sclavo Diagn. 1977;13:238-243. [PubMed] |
| 23. | Nuti M, Abdullhai SE, Thamer G. [E antigen (HBeAG) and surface antigen (HBsAg) in bladder schistosomiasis]. Parassitologia. 1978;20:153-159. [PubMed] |
| 24. | Nuti M, Tarabini G, Palermo P, Tarabini GL, Thamer G. Leprosy and hepatitis B virus markers: incidence of HBsAg and HBeAg in Somalian patients. Int J Lepr Other Mycobact Dis. 1979;47:580-584. [PubMed] |
| 25. | Nuti M, Harare O, Thamer G. The surface antigen (HBsAg) and the e-antigen (HBeAg) in Somalian patients with acute viral hepatitis. Trans R Soc Trop Med Hyg. 1979;73:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Nuti M, Abdullahi Elmi S, Alario C. [Diffusion of hepatitis B surface antigens in subjects with bladder schistosomiasis]. Boll Ist Sieroter Milan. 1979;58:220-223. [PubMed] |
| 27. | Sebastiani A, Aceti A, Paparo BS, Pennica A, Ilardi I, Bile K, Mohamud OM. Hepatitis B virus circulation in three different villages of Somalia. Trans R Soc Trop Med Hyg. 1985;79:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Bile KM, Aden A, Lindberg G, Nilsson L, Lidman K, Norder H, Magnius L. Epidemiology of hepatitis B in Somalia: inference from a cross-sectional survey of serological markers. Trans R Soc Trop Med Hyg. 1987;81:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Kolmos HJ, Zimakoff J. [DASPID--an interdisciplinary Danish study group for the elucidation of peritonitis among peritoneal dialysis patients]. Ugeskr Laeger. 1991;153:1068. [PubMed] |
| 30. | Aceti A, Mohamed OM, Paparo BS, Mohamud OM, Quaranta G, Maalin KA, Sebastiani A. High prevalence of anti-hepatitis delta virus antibody in chronic liver disease in Somalia. Trans R Soc Trop Med Hyg. 1991;85:541-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Aceti A, Paparo BS, Celestino D, Pennica A, Caferro M, Grilli A, Sebastiani A, Mohamud OM, Abdirahman M, Bile K. Sero-epidemiology of hepatitis delta virus infection in Somalia. Trans R Soc Trop Med Hyg. 1989;83:399-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Bile KM, Abdirahman M, Aden C, Norder H, Magnius L, Lindberg G, Nilsson LH. Minor role of hepatitis B virus in the causation of chronic liver disease in Somalia indicated by a case-control study. Trans R Soc Trop Med Hyg. 1991;85:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Nur YA, Groen J, Elmi AM, Ott A, Osterhaus AD. Prevalence of serum antibodies against bloodborne and sexually transmitted agents in selected groups in Somalia. Epidemiol Infect. 2000;124:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Bile K, Aden C, Norder H, Magnius L, Lindberg G, Nilsson L. Important role of hepatitis C virus infection as a cause of chronic liver disease in Somalia. Scand J Infect Dis. 1993;25:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Aweis D, Brabin BJ, Beeching NJ, Bunn JE, Cooper C, Gardner K, Iriyagolle C, Hart CA. Hepatitis B prevalence and risk factors for HBsAg carriage amongst Somali households in Liverpool. Commun Dis Public Health. 2001;4:247-252. [PubMed] |
| 36. | Shire AM, Sandhu DS, Kaiya JK, Oseini AM, Yang JD, Chaiteerakij R, Mettler TA, Giama NH, Roberts RO, Therneau TM. Viral hepatitis among Somali immigrants in Minnesota: association of hepatitis C with hepatocellular carcinoma. Mayo Clin Proc. 2012;87:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 37. | Olow KH, Warsame FS. Prevalence of Liver Cirrhosis Post - Hepatitis B and C Among Adults in Mogadishu Hospitals. 2011;. |
| 38. | Kadle M, Hassan AM, Yasin AM, Sheikh AH, Omar MS, Sayid AM. Frequency of Hepatocellular Carcinoma and Its Associated of Hepatitis B and C in Patients Attending Mogadishu Hospitals. Accessed March 16. 2018; Available from: https://www.researchgate.net/publication/309564485_Frequency_of_Hepatocellular_Carcinoma_and_its_associated_Hepatitis_B_and_C_in_Patients_attending_Mogadishu_hospitals. |
| 39. | Padovese V, Egidi AM, Melillo TF, Farrugia B, Carabot P, Didero D, Costanzo G, Mirisola C. Prevalence of latent tuberculosis, syphilis, hepatitis B and C among asylum seekers in Malta. J Public Health (Oxf). 2014;36:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Bile K, Abdirahman M, Mohamud O, Aden C, Isse A, Nilsson L, Norder H, Magnius L. Late seroconversion to hepatitis B in a Somali village indicates the important role of venereal transmission. J Trop Med Hyg. 1991;94:367-373. [PubMed] |
| 41. | Watts DM, Corwin AL, Omar MA, Hyams KC. Low risk of sexual transmission of hepatitis C virus in Somalia. Trans R Soc Trop Med Hyg. 1994;88:55-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Aceti A, Taliani G, Bruni R, Sharif OS, Moallin KA, Celestino D, Quaranta G, Sebastiani A. Hepatitis C virus infection in chronic liver disease in Somalia. Am J Trop Med Hyg. 1993;48:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Daw MA, El-Bouzedi A, Ahmed MO, Dau AA, Agnan MM; In association with the Libyan Study Group of Hepatitis & amp; HIV. Epidemiology of hepatitis C virus and genotype distribution in immigrants crossing to Europe from North and sub-Saharan Africa. Travel Med Infect Dis. 2016;14:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Bradley DW. Enterically-transmitted non-A, non-B hepatitis. Br Med Bull. 1990;46:442-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Mushahwar IK, Dawson GJ, Bile KM, Magnius LO. Serological studies of an enterically transmitted non-A, non-B hepatitis in Somalia. J Med Virol. 1993;40:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Bile K, Isse A, Mohamud O, Allebeck P, Nilsson L, Norder H, Mushahwar IK, Magnius LO. Contrasting roles of rivers and wells as sources of drinking water on attack and fatality rates in a hepatitis E epidemic in Somalia. Am J Trop Med Hyg. 1994;51:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Burans JP, Sharp T, Wallace M, Longer C, Thornton S, Batchelor R, Clemens V, Hyams KC. Threat of hepatitis E virus infection in Somalia during Operation Restore Hope. Clin Infect Dis. 1994;18:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 49. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1999] [Article Influence: 199.9] [Reference Citation Analysis (4)] |
| 50. | Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, Bell B. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 51. | Alavian SM, Hajarizadeh B, Ahmadzad-Asl M, Kabir A, Bagheri-Lankarani K. Hepatitis B Virus infection in Iran: A systematic review. Hepatitis monthly. 2008;8:281-294. |
| 52. | Fejza H, Telaku S. Prevalence of HBV and HCV among blood donors in Kosovo. Virol J. 2009;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Bigna JJ, Amougou MA, Asangbeh SL, Kenne AM, Noumegni SRN, Ngo-Malabo ET, Noubiap JJ. Seroprevalence of hepatitis B virus infection in Cameroon: a systematic review and meta-analysis. BMJ Open. 2017;7:e015298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Ofori-Asenso R, Agyeman AA. Hepatitis B in Ghana: a systematic review & amp;amp; meta-analysis of prevalence studies (1995-2015). BMC Infect Dis. 2016;16:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria, 2000-2013: a systematic review and meta-analysis. Niger J Clin Pract. 2015;18:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 56. | Chaabna K, Kouyoumjian SP, Abu-Raddad LJ. Hepatitis C Virus Epidemiology in Djibouti, Somalia, Sudan, and Yemen: Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0149966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Karoney MJ, Siika AM. Hepatitis C virus (HCV) infection in Africa: a review. Pan Afr Med J. 2013;14:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Agyeman AA, Ofori-Asenso R, Mprah A, Ashiagbor G. Epidemiology of hepatitis C virus in Ghana: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Muzembo BA, Akita T, Matsuoka T, Tanaka J. Systematic review and meta-analysis of hepatitis C virus infection in the Democratic Republic of Congo. Public Health. 2016;139:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Madhava V, Burgess C, Drucker E. Epidemiology of chronic hepatitis C virus infection in sub-Saharan Africa. Lancet Infect Dis. 2002;2:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1362] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 62. | Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis. 2013;13:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 63. | Mudawi HM. Epidemiology of viral hepatitis in Sudan. Clin Exp Gastroenterol. 2008;1:9-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Amini N, Alavian SM, Kabir A, Aalaei-Andabili SH, Saiedi Hosseini SY, Rizzetto M. Prevalence of hepatitis d in the eastern mediterranean region: systematic review and meta analysis. Hepat Mon. 2013;13:e8210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Ofori-Asenso R, Agyeman AA. Hepatitis E infection among Ghanaians: a systematic review. Infect Dis Poverty. 2017;6:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Boccia D, Guthmann JP, Klovstad H, Hamid N, Tatay M, Ciglenecki I, Nizou JY, Nicand E, Guerin PJ. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis. 2006;42:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Goumba CM, Yandoko-Nakouné ER, Komas NP. A fatal case of acute hepatitis E among pregnant women, Central African Republic. BMC Res Notes. 2010;3:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |