Published online Sep 14, 2018. doi: 10.3748/wjg.v24.i34.3919
Peer-review started: July 3, 2018
First decision: July 18, 2018
Revised: July 25, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: September 14, 2018
Processing time: 74 Days and 3.6 Hours
To evaluate the National Cancer Institute (NCI) Colorectal Cancer (CRC) Risk Assessment Tool as a predictor for the presence of adenomatous polyps (AP) found during screening or surveillance colonoscopy.
This is a retrospective single center observational study. We collected data of adenomatous polyps in each colonoscopy and then evaluated the lifetime CRC risk. We calculated the AP prevalence across risk score quintiles, odds ratios of the prevalence of AP across risk score quintiles, area under curves (AUCs) and Youden’s indexes to assess the optimal risk score cut off value for AP prevalence status.
The prevalence of AP gradually increased throughout the five risk score quintiles: i.e., 27.63% in the first and 51.35% in the fifth quintile. The odd ratios of AP prevalence in the fifth quintile compared to the first and second quintile were 2.76 [confidence interval (CI): 1.71-4.47] and 2.09 (CI: 1.32-3.30). The AUC for all patients was 0.62 (CI: 0.58-0.66). Youden’s Index indicated the optimal risk score cutoff value discriminating AP prevalence status was 3.60.
Patients with the higher NCI risk score have higher risk of AP and subsequent CRC; therefore, measures to increase the effectiveness of CRC detection in these patients include longer withdrawal time, early surveillance colonoscopy, and choosing flexible colonoscopy over other CRC screening modalities.
Core tip: Due to health, financial and social burden of colorectal cancer (CRC), it is necessary to assess the risk of cancer development earlier. National Cancer Institute (NCI) CRC risk prediction model helps identifying people who are at increased risk of developing CRC. Our study demonstrated that NCI CRC risk prediction tool could also estimate the risk of having Adenomatous polyps (AP) in patients undergoing screening or surveillance colonoscopy. The results revealed that the odds ratios of AP prevalence increase progressively throughout the five quintiles of risk scores. Therefore, measures to increase the effectiveness of CRC screening in these patients should be implemented using longer withdrawal times, early surveillance colonoscopy, and choosing flexible colonoscopy over other CRC screening modalities.
- Citation: Tariq H, Kamal MU, Patel H, Patel R, Ameen M, Elona S, Khalifa M, Azam S, Zhang A, Kumar K, Baiomi A, Shaikh D, Makker J. Predicting the presence of adenomatous polyps during colonoscopy with National Cancer Institute Colorectal Cancer Risk-Assessment Tool. World J Gastroenterol 2018; 24(34): 3919-3926
- URL: https://www.wjgnet.com/1007-9327/full/v24/i34/3919.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i34.3919
Colorectal cancer (CRC) is the third most common cancer diagnosed in men and women in the United States. The lifetime risk for developing CRC is 1 in 22 for men and 1 in 24 for women. Estimates for new cases of CRC amount to 140000 yearly, and approximately 50000 people will die of CRC in 2018 alone[1]. CRC arises from colonic polyps, specifically ‘adenomas’, which result from either a sporadic mutation or a DNA mismatch repair within the mucosal lining of the intestine. Adenomas may grow in size and progress from low-grade dysplasia to high-grade dysplasia, to carcinoma-in-situ and eventually invasive carcinoma[2].
Studies have identified both genetic and environmental factors for developing CRC[3]. Currently in clinical practice many strategies are available to screen for CRC. Screening colonoscopies among them are known to decrease the incidence and mortality of CRC by identifying adenomas in asymptomatic individuals and surveillance colonoscopies at predefined intervals are employed to monitor them[4].
Given the financial and social impact of CRC on society, it is imperative to quantitatively assess the risk of developing CRC in individuals. Many tools are widely available that help calculate future risk of CRC, however they tend to be limited to specific patient groups, or be based on selected populations of patients, and have relatively poor discrimination or are not validated and/or published[5].
The Colorectal Cancer Risk Assessment Tool by the National Cancer Institute (NCI) is a validated tool that was developed using cancer incidence data from 13 NCI Surveillance, Epidemiology, and End Results (SEER) registries, and from national mortality rates. The tool uses the respondent’s answers about risk and preventive factors to calculate that person’s absolute risk of colorectal cancer for a specific time period (5-year, 10-year and lifetime risk)[6].
We conducted this study with the aim of evaluating the NCI Colorectal Cancer Risk Assessment Tool as a predictor of the presence of adenomatous polyps found during screening or surveillance colonoscopy.
This is a retrospective single center observational study. The period of study was 6 mo between January 1st, 2017 and June 30th, 2017. The study was performed according to the Declaration of Helsinki and was approved by the Institution Review Board (IRB) of Bronx Lebanon hospital center.
The data was collected from the electronic medical records of patients and tabulated in Microsoft Excel® (Microsoft Corp, Redmond, WA, United States). Findings at colonoscopy were extracted from final procedure reports, and pathology information was extracted from final pathology reports. Asymptomatic patients between 50 and 80 years old, undergoing screening colonoscopy or surveillance colonoscopy who had either excellent or good preparation with complete examination were included in the study population. Symptomatic patients, patients with indications for therapeutic or diagnostic colonoscopy, like for example, rectal bleeding, Iron-deficiency Anemia, Inflammatory Bowel Disease, CRC, Chronic diarrhea, Abnormal Imaging were excluded from participation. Incomplete colonoscopy examinations and patients with missing information/data were excluded from the study. Patients who met the above criteria were interviewed over the phone, and their lifetime NCI CRC Risk-Assessment Tool score was calculated. Patients with missing NCI colorectal cancer risk score were excluded as well. We choose adenomatous polyps (AP) over other kind of polyps in our study because these are more commonly associated with colorectal cancer. Additionally, we did not have other polyps like serrated polyps reported in our study group and hence were not reported in the study. All Authors had access to the study data and had reviewed and approved the final manuscript.
The predictors included in the NCI CRC Risk-Assessment Tool for men are number of relatives with CRC, body mass index, servings of vegetables per day, aspirin and nonsteroidal anti-inflammatory drug use, usual number of cigarettes smoked per day and years of smoking in current and former smokers, prior negative sigmoidoscopy and/or colonoscopy, polyp history and current vigorous leisure time activity[4].
The predictors included in the NCI CRC Risk-Assessment Tool for women are number of relatives with CRC, body mass index, servings of vegetables per day, aspirin and nonsteroidal anti-inflammatory drug use, an age indicator, estrogen status within the last 2 years, prior negative sigmoidoscopy and/or colonoscopy, polyp history and current vigorous leisure time activity[4].
In the original publication, estimated 10-year and 20-year CRC risks were presented. The tool is now available on the Internet (http://www.cancer.gov/colorectalcancerrisk/). The tool provides 5-year, 10-year and lifetime estimates. We used the predicted lifetime CRC risk. The data on the presence of adenomatous polyps and the numbers in each colonoscopy were collected.
Demographic information including age, gender, race, and if the appointment was screening or surveillance were reported and were stratified across AP status. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The associations between categorical variables and AP status were tested by Pearson’s chi square tests. The associations between continuous variables and AP status were assessed by ANOVA tests. The frequency and percentage of AP prevalence were stratified across risk score quintiles. The AP prevalence trend across risk score quintiles were assessed by asymptotic linear by linear association test. The odds ratios (ORs) of the prevalence of AP across risk score quintiles and their 95% confidence intervals were computed. Receiver operating characteristic (ROC) curves were plotted to assess the discriminatory accuracy of the risk score for all patients and by genders. Area under curves (AUCs) and their confidence intervals were reported. Youden’s indexes were used to assess the optimal risk score cut off value for AP prevalence status. Various disease statuses (AP prevalence, three and more AP prevalence) were stratified across risk score categories based on the optimal risk score cutoff value. Frequencies and percentages were reported. Pearson’s chi square tests were applied to assess the associations between outcome diseases and risk score categories. All analyses were conducted on all patients and on screening and follow up patients separately.
The values were considered statistically significant if P-value was < 0.05 and values were considered more significant if P-values were < 0.01. Analyses were performed in R 1.0.153.
The prevalence of AP increased progressively through the five quintiles of risk scores: 27.63% in the first and lowest quintile, 33.53% in the second quintiles, 46.31% in the third quintiles, 52.21% in the fourth quintile, and 51.35% in the fifth and highest quintile. The ORs of AP prevalence in the third quintile compared to the first and second quintile were 2.26 [confidence interval (CI): 1.40-3.65] and 1.71 (CI: 1.08-2.70). The ORs of AP prevalence in the fourth quintile compared to the first and second quintile were 2.86 (CI: 1.75-4.67) and 2.16 (CI:1.36-3.45). The ORs of AP prevalence in the fifth quintile compared to the first and second quintile were 2.76 (CI: 1.71-4.47) and 2.09 (CI: 1.32-3.30).
The demographic composition of the sample population was indicated in Table 1. There were 749 patients who met the inclusion criteria. The mean age of the study population was 59.00 ± 7.38. Most of the sample population was female (436, 58.2%), Hispanics (501, 67%), and screening colonoscopy patients (606, 80.9%). The mean age of patients without AP was slightly lower than the mean age of patients with AP (58.66 vs 59.76, P = 0.043).
| Adenoma absent | Adenoma present | Total | P value | |
| Total | 436 (58.2) | 313 (41.79) | 749 (100) | |
| Age | 0.043 | |||
| Mean ± SD | 58.656 ± 7.321 | 59.764 10 ± 7.417 | 59.119 ± 7.376 | |
| Gender | 0.798 | |||
| Male | 180 (41.3) | 133 (42.5) | 313 (41.8) | |
| Female | 256 (58.7) | 180 (57.5) | 436 (58.2) | |
| Race | 0.243 | |||
| White | 29 (6.7) | 33 (10.6) | 62 (8.3) | |
| African american | 104 (23.9) | 74 (23.7) | 178 (23.8) | |
| Asian | 5 (1.1) | 2 (0.6) | 7 (0.9) | |
| Hispanic | 298 (68.3) | 203 (65.1) | 501 (67.0) | |
| Indication of colonoscopy | 0.279 | |||
| Surveillance | 77 (17.7) | 66 (21.1) | 143 (19.1) | |
| Screening | 359 (82.3) | 247 (78.9) | 606 (80.9) |
Table 2 indicates the prevalence of AP by risk score quintiles and the ORs of AP prevalence compared across score quintiles. The prevalence of AP increased progressively through the five quintiles of risk scores: 27.63% in the first and lowest quintile, 33.53% in the second quintiles, 46.31% in the third quintiles, 52.21% in the fourth quintile, and 51.35% in the fifth and highest quintile. The ORs of AP prevalence in the third quintile compared to the first and second quintile were 2.26 (CI: 1.40-3.65) and 1.71 (CI: 1.08-2.70). The ORs of AP prevalence in the fourth quintile compared to the first and second quintile were 2.86 (CI: 1.75-4.67) and 2.16 (CI: 1.36-3.45). The ORs of AP prevalence in the fifth quintile compared to the first and second quintile were 2.76 (CI: 1.71-4.47) and 2.09 (CI: 1.32-3.30).
| NCI lifetime risk score quintile | Score range | Number of individuals with adenomas (%) | OR of an adenoma (95%CI): row quintile vs column quintile | |||
| Q1 | Q2 | Q3 | Q4 | |||
| Q1 lowest | (1.1, 1.9) | 42 (27.63) | - | - | - | - |
| Q2 | (1.9, 2.8) | 55 (33.54) | 1.32 (0.82, 2.14) | - | - | - |
| Q3 | (2.8, 4.0) | 69 (46.31) | 2.26 (1.40, 3.65) | 1.71 (1.08, 2.70) | - | - |
| Q4 | (4.0, 5.4) | 71 (52.21) | 2.86 (1.75, 4.67) | 2.16 (1.36, 3.45) | 1.27 (0.79, 2.02) | - |
| Q5 highest | (5.4, 12.3) | 76 (51.35) | 2.76 (1.71, 4.47) | 2.09 (1.32, 3.30) | 1.22 (0.78, 1.93) | 0.97 (0.61, 1.54) |
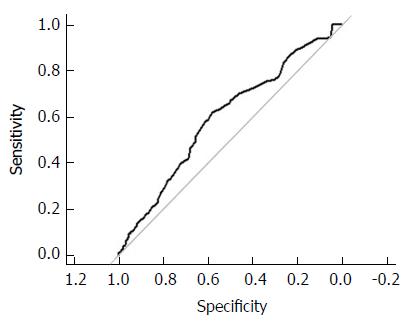
The ROC curves assessing the discriminatory accuracy of the risk score for all patients and by gender were presented in Figures 1 and 2. The AUC for all patients was 0.62 (CI: 0.58-0.66). For Female, the AUC is 0.60 (CI: 0.55-0.66). For male, the AUC is 0.63 (CI: 0.57-0.69). Youden’s Index indicated the optimal risk score cutoff value discriminating AP prevalence status was 3.60 based on data on all patients.
Table 3 indicates diseases prevalence stratified by risk score optimal cutoff value. The percentage of AP prevalence patients was smaller for patients with risk score lower than 3.6 compared to the patients with risk score 3.6 and above (30.8% vs 52.7%, P < 0.001). The percentage of patients having three and more adenomas is smaller for patients with risk score less than 3.6 compared to patients with risk score 3.6 and above (4.8% vs 14.6%, P < 0.001).
| < 3.6 (n = 373) | ≥ 3.6 (n = 376) | Total (n = 749) | P value | |
| Adenoma present | 115 (30.8) | 198 (52.7) | 313 (41.8) | < 0.001 |
| Adenoma (number) | < 0.001 | |||
| < 3 | 355 (95.2) | 321 (85.4) | 676 (90.3) | |
| ≥ 3 | 18 (4.8) | 55 (14.6) | 73 (9.7) | |
| Serrated adenoma present | 1 (0.3) | 2 (0.5) | 3 (0.4) | 1.000 |
| Hyperplastic polyp > 10 mm present | 7 (1.9) | 10 (2.7) | 17 (2.3) | 0.632 |
To strengthen the results, we studied screening and surveillance patients separately. The prevalence of AP increased progressively in screening patients among the 5 quintiles (Table 4): 28.69% in the first and lowest quintile, 33.58% in the second quintile, 43.70% in the third quintile, 51.67% in the fourth quintile, and 47.75% in the fifth and highest quintile. The OR of having AP in the third quintile compared to the first quintile was 1.93 (CI: 1.13-3.29). The OR of having AP in the fourth quintile compared to the first and second quintiles were 2.66 (CI: 1.56-4.52) and 2.11 (CI: 1.27-3.51). The OR of having AP in the fifth quintile compared to the first and second quintiles were 2.27 (CI: 1.32-3.90) and 1.81 (CI: 1.08-3.03). The AUC discriminating risk score and AP prevalence was 0.60 (CI: 0.55-0.64) (Supplementary Figure 1). The optimal risk score cutoff value indicated by Youden’s Index was 3.60. The percentage of patients with AP prevalence is lower for patients with risk score lower than 3.6 compared to patients with risk score 3.6 and above (31.1% vs 50.3%, P < 0.001). The percentage of patients with three and more AP is lower for patients with risk score lower than 3.6 compared to patients with risk score 3.6 and above (4.6% vs 12.8%, P < 0.001) (Supplementary Table 1).
| NCI lifetime risk score quintile | Score range | Number of individuals with adenomas (%) | OR of an adenoma (95%CI): row quintile vs column quintile | |||
| Q1 | Q2 | Q3 | Q4 | |||
| Q1 lowest | (1.1, 1.9) | 35 (28.69) | - | - | - | - |
| Q2 | (1.9, 2.8) | 45 (33.58) | 1.26 (0.74, 2.14) | - | - | - |
| Q3 | (2.8, 4.0) | 52 (43.70) | 1.93 (1.13, 3.29) | 1.53 (0.92, 2.56) | - | - |
| Q4 | (4.0, 5.5) | 62 (51.67) | 2.66 (1.56, 4.52) | 2.11 (1.27, 3.51) | 1.38 (0.83, 2.29) | - |
| Q5 highest | (5.5, 12.3) | 53 (47.75) | 2.27 (1.32, 3.90) | 1.81 (1.08, 3.03) | 1.18 (0.70, 1.98) | 0.85 (0.51, 1.43) |
The prevalence of AP among surveillance patients mostly increased progressively among the five quintiles (Supplementary Table 2): 23.33% in the first and lowest quintile, 33.33% in the second quintile, 56.67% in the third quintile, 50.00% in the fourth quintile, and 68.97% in the fifth and highest quintile. The OR of having AP in the third quintile compared to the first quintile was 4.3 (CI: 1.41-13.07). The OR of having AP in the fourth quintile compared to the first quintile was 3.29 (CI: 1.03-10.53). The OR of having AP in the fifth quintile compared to the first and second quintiles were 7.3 (CI: 2.30-23.18) and 4.44 (CI: 1.49-13.26). The AUC discriminating risk score and AP prevalence was 0.70 (CI: 0.62-0.79) (Supplementary Figure 2). The optimal risk score cutoff value indicated by Youden’s Index was 3.50. The percentage of AP prevalent patients is smaller for patients with risk score less than 3.5 compared to patients with risk score 3.5 and above (29.6% vs 62.5%, P < 0.001). The percentage of patients with three and more AP is smaller for patients with risk score less than 3.5 compared to patients with risk score 3.5 and above (5.6% vs 22.2%, P = 0.009) (Supplementary Table 3).
The results of our study suggest that the NCI’s Risk Assessment Tool is a reasonable option for recognizing patients who are at a higher risk for the presence of adenomatous polyps, having a moderately good discriminatory accuracy for the presence of AP.
The primary endpoint of our study was the presence of any adenomatous polyp, whereas the secondary endpoint highlighted the presence of ≥ 3 adenomatous polyps (high risk adenoma). In our study, a NIH risk score of 3.6, as calculated based on Youden’s index, was the best cut off value as a predictor of AP. The NCI risk score of more than 3.6 as compared to NCI risk score of 3.6 or less had significantly higher chance of finding an adenomatous polyp (30.8% vs 52.7%, P < 0.001) or high-risk adenoma (4.8% vs 14.6%, P < 0.001).
The prevalence of AP increased progressively throughout the five quintiles of risk scores and plateaued on the fifth quintile in our study: 27.63% in the lowest quintile, and 51.35% in highest quintile. The AUC, which reflects the overall discriminatory accuracy of the risk-prediction tool, was 0.62 (0.60 for females, and 0.63 for males), similar to those observed in the validation study of the NCI CRC Risk-Assessment Tool using data from the NIH-AARP diet and health study with incident CRC as the outcome, in which the AUCs were 0.61 (95%CI: 0.59-0.62) for women and 0.61 (95%CI: 0.60-0.62) for men, thus demonstrating a moderately good range for the given AUCs (Figures 1 and 2, Supplementary Figures 1 and 2)[7].
These findings suggest that the NCI’s Risk Assessment Tool has a moderate to good predictive value for estimating the risk for adenomatous polyps as well as future risk of CRC and can be utilized for such predictions. This dual risk estimation makes it a very effective tool for increasing the yield of colonoscopy.
Adenomas are well known precursors of CRC. The adenoma-carcinoma sequence has suggested genetic alterations and chromosomal instability as the underlying mechanism for colorectal tumorigenesis[8]. Genes such as the APC, K-Ras, p53 and others, have been identified and implicated in the development of CRC[4]. It is often observed that CRC has a slower development in most cases via the adenoma-carcinoma sequence, which can take years. The estimated annual transition rates in both men and women from the advanced neoplasia to CRC was about 2.5%-3% among the groups of 55-64 years and about 5% to 5.5% in age groups of 70-79 years. This slower development gives physicians the potential for reducing the burden of the disease by early detection and subsequent removal of adenomas thereby haltering progression to CRC. Studies relate a 53% reduction in mortality due to CRC when adenomas are identified and removed during colonoscopy[9].
Adenomas that are greater than 1 cm, contain a substantial (> 25%) villous component, or have high-grade dysplasia are commonly referred to as advanced adenomas and carry an increased cancer risk[10]. However, a recent meta-analysis reported inconsistencies in detecting advanced adenomas based on size[11]. These discrepancies are due to a lack of standard methods for estimating adenoma size and the inter-observer variability and different pathology measurements[12-13]. It was seen that the utilization of an open biopsy forceps reported precise measurement only 37% of the time[14]. Sometimes the polyps are removed piecemeal and often break during collection[2]. Therefore, accuracy based on size in not reliable and should be used with caution for surveillance colonoscopies.
Calderwood et al[2] revealed that there in inconsistent association of villous histology of polyps and risk of advanced neoplasia in different studies. In contrast to European and United States guidelines, the British guidelines doesn’t include advanced neoplastic features of polyps for recommendations of surveillance colonoscopies[2]. Based on this the British guidelines recommended one year follow up for 5 small adenomas or 3 adenomas if one is ≥ 10 mm in size vs 3 years in European and United States guidelines for patients with ≥ 3 adenomas or any adenoma ≥ 10 mm size or with high grade dysplastic features or villous histology[15-17]. Due to these reasons and inconsistencies, we did not include advanced adenomas in our analysis.
In 2009, Freedman et al[4] developed the CRC risk assessment tool for white men and women without known susceptibility. This model was validated by Park et al[7] with recommendation that the model has a modest discriminatory power for the assessment of an individual’s risk of CRC.
More than 50 proposed risk scores for colon cancer that have the potential to identify individuals at high risk[18]. A recent systematic review showed there is no clear improvement in discrimination as increasing numbers of variables are added to the risk assessment tools[19]. The two most commonly used and validated scores are the Cleveland Clinic test and the NCI test; which are both self-completed questionnaire. The Cleveland clinic test can only provide a suggested 10-year risk assessment, whereas the NCI test provides a 5-year and lifetime risk as well. Because the NCI tool has been subjected to external validation and because the predictors included in other risk scores are often a subset of the NCI tool’s multiple predictors, we designed this study to explore the NCI tool.
We recommend the use of this score to help the patient in an informed decision-making; with patients having a higher NIH score should opt for the colonoscopy as compared to other available modalities. This is because colonoscopy has the additional advantage of clearing the colon of polyps and detecting cancerous lesions early in these patients. It also saves the time and cost of doing other tests which ultimately may require colonoscopy in such group of patients[20-22]. As the higher score is consistent with ≥ 3 AP, it is reasonable to screen with colonoscopy to detect synchronous lesions throughout the entire colon.
It has been suggested by many studies that for adequate adenoma detection, the withdrawal time should be at least 6-9 min on average[10-11]. Preferably, an average withdrawal time for normal colonoscopies of 9 min is essential of adenoma detection. A higher adenoma (33.6%) detection rate was observed with withdrawal time of 9 min as compared to a lower adenoma (23.8%) detection rate with withdrawal time of 6 min[12]. We recommend that in patients having a higher NCI risk score, a higher withdrawal time should be used as it will increase the yield of adenomas and decrease the future risk of CRC in these patients.
Sanduleanu et al[23] defined interval CRC (iCRC) as “colorectal cancer diagnosed after a colorectal screening examination or test in which no cancer is detected, and before the date of the next recommended exam”. Various factors contribute towards the development of iCRC which include, missed lesions, incomplete polypectomy or rapid progression[24]. We suggest that the use of NCI score should be incorporated in clinical practice to make a decision about the duration before next colonoscopy to help decrease the incidence of interval CRC, though this has to be validated in prospective trials[25].
There are a few limitations of our study. It’s a retrospective study mainly on minority group of patients. Majority of our study patient population included Hispanics and African Americans and a small number of Asians and white Americans. Therefore, it’s unclear whether generalizability to the more diverse United States population will be effective.
We didn’t estimate the CRC risk based on the effect of location (proximal versus distal) of adenomas in this study. It is mentioned in previous studies that proximal lesions have a greater risk of adenoma detection as compared to the distal ones. These important clinical strategies need to be designed based on the location of the adenomas[26].
In the last two decades, screening and surveillance colonoscopy guidelines implementation has remarkably decreased the CRC incidence and mortality. Our study intends to further decrease the morbidity and mortality associated with CRC.
Therefore, we recommended prospective trials to validate our hypothesis in a larger and heterogeneous population group with more diverse racial and ethnic backgrounds. This will help characterize the risk of future CRC risk and AP based on NCI score and assist the patients and provider decision-making about timing and mode of screening.
Patients with the higher NCI risk score have higher risk of AP and subsequent CRC; therefore, measures to increase the effectiveness of CRC detection in these patients include longer withdrawal, early surveillance, and choosing colonoscopy over other CRC screening modalities.
Colorectal cancer (CRC) is the third most common cancer diagnosed in men and women in the United States. The lifetime risk for developing CRC is 1 in 22 for men and 1 in 24 for women. Estimates for new cases of CRC amount to 140000 yearly, and approximately 50000 people will die of CRC in 2018 alone. CRC arises from colonic polyps, specifically ‘adenomas’, which result from either a sporadic mutation or a DNA mismatch repair within the mucosal lining of the intestine. Adenomas may grow and progress from low-grade dysplasia to high-grade dysplasia, to carcinoma-in-situ and eventually invasive carcinoma. It is there essential to diagnose the pre-cancerous and cancerous lesions are earlier stage.
Due to the financial and social impact of CRC on society, it is imperative to quantitatively assess the risk of developing CRC in individuals. Many tools are widely available that help calculate future risk of CRC, however they tend to be limited to specific patient groups, or be based on selected populations of patients, and have relatively poor discrimination or are not validated and/or published. Therefore, it is needed to developed tools which help predict future risk of CRC more specifically.
National Cancer Institute Colorectal Cancer Risk Assessment tool provides 5-year, 10-year and lifetime estimates and currently helps predict lifetime CRC risk. We conducted this study with the aim of evaluating the NCI Colorectal Cancer Risk Assessment Tool as a predictor of the presence of adenomatous polyps found during screening or surveillance colonoscopy.
This is a retrospective single center observational study over a period of 6 mo duration. The data was collected from the electronic medical records of patients and tabulated in Microsoft Excel. Findings at colonoscopy were extracted from final procedure reports, and pathology information was extracted from final pathology reports. Asymptomatic patients between 50 and 80 years old, undergoing colonoscopy were included in the study population. Patients who met the above criteria were interviewed over the phone, and their lifetime NCI CRC Risk-Assessment Tool score was calculated. The predictors included in the NCI CRC Risk-Assessment Tool are number of relatives with CRC, body mass index, servings of vegetables per day, aspirin and nonsteroidal anti-inflammatory drug use, usual number of cigarettes smoked per day and years of smoking in current and former smokers, prior negative sigmoidoscopy and/or colonoscopy, polyp history and current vigorous leisure time activity etc.The authors in original paper estimated 10-year and 20-year CRC risks. This tool is available on the Internet and provides 5-year, 10-year and lifetime estimates. We used the predicted lifetime CRC risk. The data on the presence of adenomatous polyps and the numbers in each colonoscopy were collected.
After data analysis, it was noticed that the prevalence of AP increased progressively through the five quintiles of risk scores: 27.63% in the first and lowest quintile, 33.53% in the second quintiles, 46.31% in the third quintiles, 52.21% in the fourth quintile, and 51.35% in the fifth and highest quintile. The odd ratios (ORs) of AP prevalence in the third quintile compared to the first and second quintile were 2.26 (CI: 1.40-3.65) and 1.71 (CI: 1.08-2.70). The ORs of AP prevalence in the fourth quintile compared to the first and second quintile were 2.86 (CI: 1.75-4.67) and 2.16 (CI: 1.36-3.45). Youden’s Index indicated the optimal risk score cutoff value discriminating AP prevalence status was 3.60 based on data on all patients. The percentage of AP prevalence patients was smaller for patients with risk score lower than 3.6 compared to the patients with risk score 3.6 and above (30.8% vs 52.7%, P < 0.001). The percentage of patients having three and more adenomas is smaller for patients with risk score less than 3.6 compared to patients with risk score 3.6 and above (4.8% vs 14.6%, P < 0.001).
According to this study, patients with the higher NCI risk score have higher risk of AP and subsequent CRC. Our findings propose that patients who are categorized as high risk according to the NCI CRC risk assessment tool should undergo colonoscopy for the screening of CRC. Our study also revealed that the NCI CRC risk assessment tool can predict about the presence of AP, in addition to the lifetime risk of CRC. In these high risk patients, the measures to increase the effectiveness of CRC detection in these patients include longer withdrawal time, early surveillance colonoscopy, and choosing flexible colonoscopy over other CRC screening modalities. This study characterized the risk of future CRC risk and AP based on NCI score and will assist the patients and providers with informed decision-making about timing and mode of screening.
In the last two decades, screening and surveillance colonoscopy guidelines implementation has remarkably decreased the CRC incidence and mortality. Our study was conducted to further decrease the morbidity and mortality associated with CRC. The tool can be used to predict the risk of future CRC and AP and therefor can assist the patients and providers with informed decision-making about timing and mode of screening. To further validate the results of our study, there is a need to conduct prospective trials in a larger and heterogeneous population group with more diverse racial and ethnic backgrounds and involving multiple center.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hoensch HP, Luo HS S- Editor: Gong ZM L- Editor: A E- Editor: Bian YN
| 1. | Key Statistics for Colorectal Cancer. Accessed March 23, 2018. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html. |
| 2. | Calderwood AH, Lasser KE, Roy HK. Colon adenoma features and their impact on risk of future advanced adenomas and colorectal cancer. World J Gastrointest Oncol. 2016;8:826-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 3. | CDC. What Are the Risk Factors for Colorectal Cancer? Accessed March 23. 2018; Available from: https://www.cdc.gov/cancer/colorectal/basic_info/risk_factors.htm. |
| 4. | Freedman AN, Slattery ML, Ballard-Barbash R, Willis G, Cann BJ, Pee D, Gail MH, Pfeiffer RM. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations to estimate survival in patients with colorectal cancer: cohort study. BMJ. 2017;357:j2497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Colorectal Cancer Risk Assessment Tool. Accessed March 23, 2018. Available from: https://www.cancer.gov/colorectalcancerrisk/about-tool.aspx. |
| 7. | Park Y, Freedman AN, Gail MH, Pee D, Hollenbeck A, Schatzkin A, Pfeiffer RM. Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol. 2009;27:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 462] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 9. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2285] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 10. | Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 303] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 11. | Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1-9, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Moug SJ, Vernall N, Saldanha J, McGregor JR, Balsitis M, Diament RH. Endoscopists’ estimation of size should not determine surveillance of colonic polyps. Colorectal Dis. 2010;12:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997;46:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Rex DK, Rabinovitz R. Variable interpretation of polyp size by using open forceps by experienced colonoscopists. Gastrointest Endosc. 2014;79:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1445] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 16. | Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 17. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 18. | Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 434] [Cited by in RCA: 392] [Article Influence: 49.0] [Reference Citation Analysis (11)] |
| 19. | Usher-Smith JA, Walter FM, Emery JD, Win AK, Griffin SJ. Risk Prediction Models for Colorectal Cancer: A Systematic Review. Cancer Prev Res (Phila). 2016;9:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 20. | Wong MC, Ching JY, Chan VC, Sung JJ. The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs. colonoscopy. Sci Rep. 2015;5:13568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Janz T, Lu K, Povlow MR, Urso B. A Review of Colorectal Cancer Detection Modalities, Stool DNA, and Fecal Immunochemistry Testing in Adults Over the Age of 50. Cureus. 2016;8:e931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Wheeler SB, Kuo TM, Meyer AM, Martens CE, Hassmiller Lich KM, Tangka FK, Richardson LC, Hall IJ, Smith JL, Mayorga ME. Multilevel predictors of colorectal cancer testing modality among publicly and privately insured people turning 50. Prev Med Rep. 2016;6:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, Valori R, Young GP, Schoen RE; Expert Working Group on ‘Right-sided lesions and interval cancers’, Colorectal Cancer Screening Committee, World Endoscopy Organization. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1467] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 25. | Shaukat A, Rector TS, Church TR, Lederle FA, Kim AS, Rank JM, Allen JI. Longer Withdrawal Time Is Associated With a Reduced Incidence of Interval Cancer After Screening Colonoscopy. Gastroenterology. 2015;149:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 26. | Elhanafi S, Ortiz AM, Yarlagadda A, Tsai C, Eloliby M, Mallawaarachchi I, Dwivedi A, Zuckerman MJ, Othman MO. Estimation of the Adenoma Detection Rate From the Polyp Detection Rate by Using a Conversion Factor in a Predominantly Hispanic Population. J Clin Gastroenterol. 2015;49:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |