Published online Sep 14, 2018. doi: 10.3748/wjg.v24.i34.3898
Peer-review started: May 22, 2018
First decision: May 30, 2018
Revised: June 11, 2018
Accepted: June 25, 2018
Article in press: June 25, 2018
Published online: September 14, 2018
Processing time: 117 Days and 22.3 Hours
To investigate the effects of different levels of expression of CDK5RAP3 and DDRGK1 on long-term survival of patients undergoing radical gastrectomy.
The expression of CDK5RAP3 and DDRGK1 was detected by immunohistochemistry in 135 patients who received standard gastrectomy were enrolled in the study. Western Blot was used to detect the expression of CDK5RAP3 and DDRGK1 in gastric cancer and its adjacent tissues and cell lines. The correlations between the expression of CDK5RAP3 and DDRGK1 and clinicopathological factors were analyzed, and the value of each parameter to the prognosis of the patients was compared. Receiver operating characteristic analysis was used to compare the accuracy of the prediction of clinical outcome by the parameters.
CDK5RAP3 and DDRGK1 expression was down-regulated in the gastric cancer compared to its respective adjacent non-tumor tissues. The expression of CDK5RAP3 was closely related to the age of the patients (P = 0.035) and the T stage of the tumor (P = 0.017). The expression of DDRGK1 was correlated with the sex of the patients (P = 0.080), the degree of tumor differentiation (P = 0.036), the histological type (P = 0.036) and the N stage of the tumor (P = 0.014). Low expression CDK5RAP3 or DDRGK1 is a poor prognostic factor for gastric cancer patients. Prognostic analysis showed that the co-expression of CDK5RAP3 and DDRGK1 was an independent prognostic factor correlating with the overall survival of gastric cancer patients. Combined expression analysis of CDK5RAP3 and DDRGK1 may provide a more accurate prognostic value for overall survival.
The co-expression of CDK5RAP3 and DDRGK1 is an independent prognostic factor for gastric cancer, which can provide a more accurate model for the long-term prognosis.
Core tip: The expression of CDK5RAP3 and DDRGK1 was down-regulated in gastric cancer tissues. Low expression CDK5RAP3 or DDRGK1 is a poor prognostic factor for gastric cancer patients. The co-expression of CDK5RAP3 and DDRGK1 is an independent prognostic factor for the overall survival of patients with gastric cancer. Moreover, we also found that co-expression of CDK5RAP3 and DDRGK1 can provide a more accurate model for the long-term prognosis of gastric cancer.
- Citation: Lin JX, Xie XS, Weng XF, Zheng CH, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Li P, Huang CM. Low expression of CDK5RAP3 and DDRGK1 indicates a poor prognosis in patients with gastric cancer. World J Gastroenterol 2018; 24(34): 3898-3907
- URL: https://www.wjgnet.com/1007-9327/full/v24/i34/3898.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i34.3898
Although the morbidity and mortality of primary gastric cancer has declined in recent decades, it is still the third most common cause of cancer-related deaths worldwide[1-3]. At present, the etiology and pathogenesis of gastric cancer has not yet been fully clarified. There is also a lack of specific and highly effective therapeutic drugs available for use in clinical practice. The symptom specificity of early gastric cancer is not obvious, so most patients are already in advanced stages before receiving medical treatment, which seriously affects the prognosis of patients. Therefore, searching for molecular markers that can be used as an independent prognostic factor for gastric cancer is of great significance for the early diagnosis and targeted treatment of gastric cancer.
The cyclin-dependent kinase 5 activating binding protein (CDK5RAP3, also called C53) was first identified as a binding protein of the cyclin-dependent kinase 5 (CDK5) activators P35 and P39[4]. In recent years, an increasing number of studies have been conducted on the role of CDK5RAP3 in tumors, but its expression and role in different tumors has been found to be different. An et al[5] reported that CDK5RAP3 inhibited the phosphorylation and activation of p38 by promoting the binding of p38 and p53-induced protein phosphatase 1 to inhibit tumor proliferation. However, Stav et al[6] found that the expression of CDK5RAP3 in most cancer tissues was increased, which is of great significance in the diagnosis of lung cancer. The expression and function of CDK5RAP3 are also controversial in the same types of tumors. Mak et al[7] found that CDK5RAP3 was highly expressed in hepatocellular cancer and that it could promote the metastasis of hepatoma cancer cell by activating p21-activated protease 4 and down-regulating the expression of tumor suppressor gene p14. However, Zhao et al[8] showed that the expression of CDK5RAP3 protein was down-regulated in hepatocellular cancer and that down-regulation of CDK5RAP3 expression was associated with a poor prognosis.
Recent studies have shown that DDRGK1 interacts with CDK5RAP3[9]. DDRGK1 was cloned from human liver in 2010 by Lemaire et al[10] .DDRGK1 is located on the short arm of chromosome 20 (20p13), also known as UFBP1, C20orf116, or dJ1187M17. The DDRGK1 sequence is highly conserved and exists in many tissues and organs. Its N-terminal 1-28 amino acid residue region is highly hydrophobic and is an endoplasmic reticulum anchor sequence; 65-69 amino acid residues are nuclear localization signals. The 229-273 amino acid residues near the C-terminus are the protein PCI domain[11]. Studies have shown that proteins containing a PCI domain are primarily responsible for the construction and assembly of protein complexes[12]. Xi et al[13] found that DDRGK1 interacts with IkBa and regulates its stability, thereby regulating the transcriptional activity of NF-κB. At present, there are few studies on the co-expression of CDK5RAP3 and DDRGK1 in gastric cancer and its impact on prognosis. In this study, we examined the expression of CDK5RAP3 and DDRGK1 in 135 cases of gastric cancer, and analyzed their correlation with clinicopathological features and long-term prognosis of the patients.
The gastric cancer specimens were obtained from 135 patients with gastric adenocarcinoma, who had undergone D2 lymph node dissection and gastrectomy for gastric cancer at the Department of Gastric Surgery, Fujian Medical University Union Hospital (Fujian, China) with available detailed clinic pathologic parameters, between January 2013 and June 2015. All patients received their first diagnosis of gastric cancer and received no other treatment, such as chemotherapy, before surgery. All diagnoses were confirmed by pathology after surgery. Gastric cancer was confirmed by hematoxylin and eosin (H&E) staining in all cases. The clinicopathological data of the 135 GC patients included age, sex, size of the primary tumor, location of the primary tumor, degree of differentiation, histological type, Borrmann type, depth of invasion, lymph node metastasis, distant metastasis and TNM stage. The pathologic stage of the tumor was re-assessed according to the TNM classification of gastric cancer (eighth edition) of the International Union against Cancer (2016).The clinical and pathological data were recorded prospectively for the retrospective analysis. This study was approved by the ethics committee of Fujian Medical University Union Hospital and written consent was obtained from all patients involved.
Paraffin sections containing sufficient formalin fixed tumor tissue were sectioned continuously at a thickness of 4 μm and were mounted on silage coated slides for immunohistochemical analysis. The slices were deparaffinized with xylene and rehydrated in 95%, 85% and 75% ethanol. Antigen retrieval was performed by subjecting the slides to high-pressure sterilization at 121 °C for 2 min in 0.01 mol/L sodium citrate buffer solutions (pH 6.0). Endogenous peroxidase activity was blocked by incubating the slides with 3% H2O2 at room temperature for 10 min. The slices were then washed in phosphate buffered saline (PBS) solution and blocked in 10% goat serum (Zhongshan Biotechnology Co. Ltd.) for 30 min. Next, the sections were incubated with diluted rabbit anti-human CDK5RAP3 (ab157203, 1:200 dilution; Abcam) or DDRGK1 (21445-1-AP, 1.50 dilution; Proteintech) overnight in a humidified chamber at 4 °C. After three washes in PBS, the sections were incubated with the secondary antibody conjugated to horseradish peroxidase at room temperature for 30 min. The signal was developed with diaminobenzidine solution, which was followed by counterstaining in 20% hematoxylin. Finally, all slides were dehydrated and mounted on cover glass. For negative controls, non-specific antibody diluent was substituted for the primary antibody.
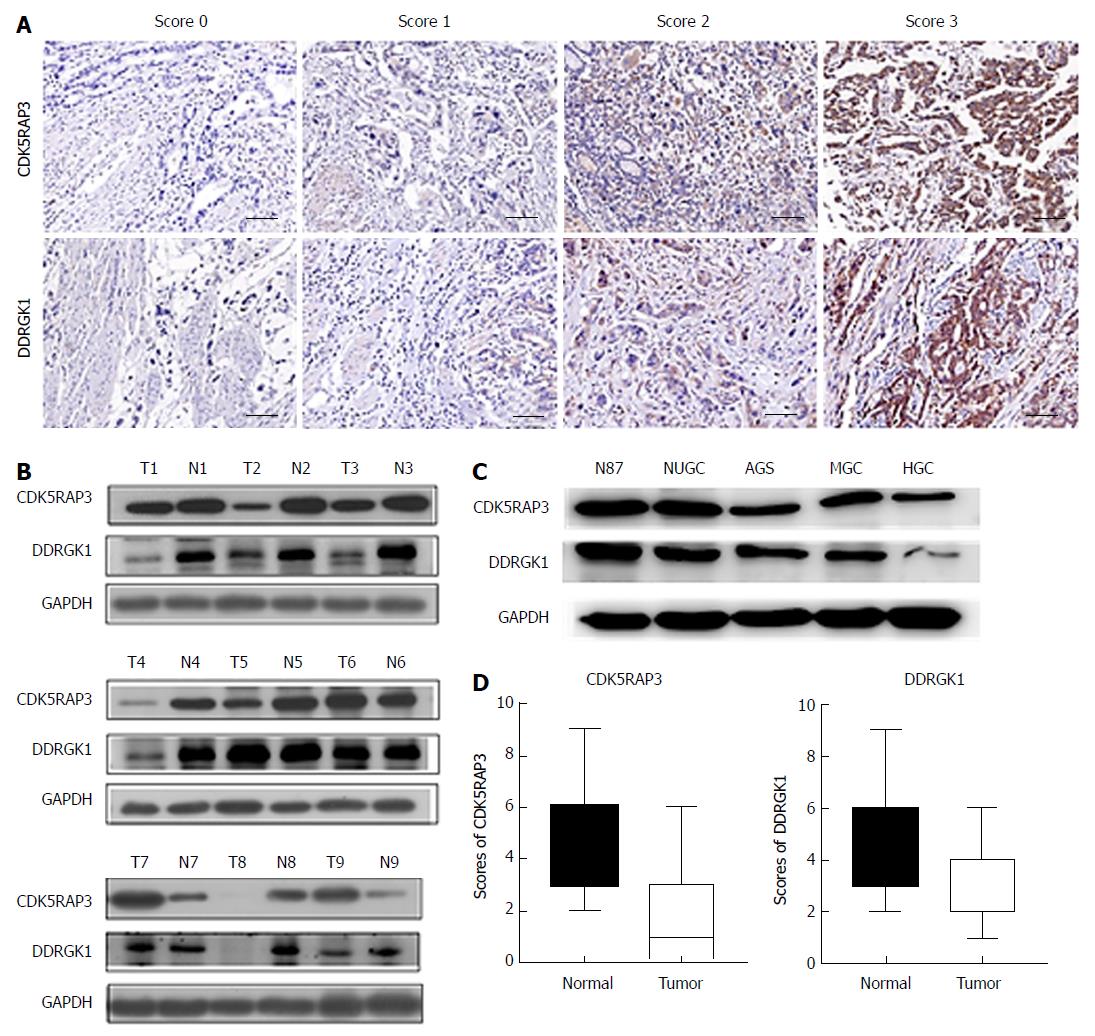
The immunohistochemistry (IHC) of the tissue sections were examined by two experienced pathologists, who scored the slides according to the intensity of cell staining and the proportion of positively stained tumor cells. The definition for the evaluation of CDK5RAP3 and DDRGK1 staining intensity was as follows: no staining (score of 0), weak staining (light yellow, score of 1), moderate staining (yellow brown, score of 2) and strong staining (brown, score of 3). The positive proportion of stained tumor cells was scored as follows: ≤ 5% positive cells (score of 0), 6% to 25% positive cells (score of 1), 26% to 50% positive cells (score of 2), ≥ 51% positive cells (score of 3). If the total score (intensity x percentage score) was less than 3, the protein expression was considered low, however, if the score was 4 or higher, the protein expression was considered high (Figure 1A).
After the cells grew to 90%-100% confluence, the cells were flushed twice with pre-cooled PBS and then extracted with RIPA lysis solution (Thermo Fisher Scientific, Waltham, MA, United States) containing a 10% cocktail (Roche, South San Francisco, CA, United States).Protein samples (40 μg per lane) were separated on 10% polyacrylamide gels by the SDS-PAGE method and transferred to PVDF membranes. Then, at room temperature, 5% skim milk was used to block the PVDF membrane for 1 h. The membrane was then incubated at 4 °C with the primary anti-CDK5RAP3 (ab157203, 1:1000 dilution; Abcam), anti-DDRGK1 (21445-1-AP, 1:1000 dilution; Proteintech), or anti-GAPDH (ab8245, 1:5000 dilution; Abcam) and washed with TBS-T 3 times, 5 min each time, then incubated at room temperature with the HRP secondary antibody (Cell Signaling Technology) for 1 h. GAPDH was used as an internal control. Finally, the membrane was washed with TBS-T for 30 min and the protein bands were detected by an enhanced chemiluminescence method (Amersham Corporation, Arlin-gton Heights, IL, United States).
All patients were followed up once every three months for the first two years and were then followed up every six months for the next three to five years. The last follow-up time point was January 2018. Follow-up routine examinations, including a physical examination, laboratory tests (CA19-9, CEA and CA72-4), chest X-ray, abdominal CT, B ultrasound, and gastroscopy were performed each year. The total survival time was defined as the time from surgery to the last follow-up, or the time of death, or the expiration of the follow-up database (e.g., lost to follow-up, death from other diseases, etc.)
All of the data were processed by the SPSS23.0 statistical software package. Appropriate test methods, such as the χ2 test or Fisher’s exact test, were selected according to the type of variables and the purpose of comparison. The survival rate was calculated by the Kaplan-Meier method, and the subsequent survival curve was plotted. The log-rank test was used to compare the survival rates. Cox regression was used to analyze the independent factors that affected the prognosis. The area under the ROC curve was used to compare the prognostic ability of different indexes. The difference was statistically significant when P < 0. 05.
Of 135 patients with primary gastric cancer, 109 patients (80.7%) had low expression of CDK5RAP3 and 26 patients (19.3%) had high expression. The DDRGK1 immunohistochemical score showed low expression in 98 cases (72.6%) and high expression in 37 cases (27.4%) (Table 1). Western blotting was used to detect the expression of CDK5RAP3 and DDRGK1 in the tumor and adjacent tissues of nine patients with gastric cancer. It was found that the expression of CDK5RAP3 and DDRGK1 in six patients was higher in respective adjacent non-tumor tissues than that in gastric cancer tissues (Figure 1B). In addition, the expression of CDK5RAP3 and DDRGK1 in gastric cancer cell lines decreased with the decrease of differentiation degree of the gastric cancer cell lines (Figure 1C). We also found that the histological scores of CDK5RAP3 and DDRGK1 in adjacent tissues were higher than those in cancer tissues, with a statistically significant difference (Figure 1D).
| Variables | Total | CDK5RAP3 expression | DDRGK1 expression | ||||||
| Low | High | χ2 | P value | Low | High | χ2 | P value | ||
| Gender | 1.659 | 0.198 | 3.057 | 0.080 | |||||
| Male | 107 | 84 | 23 | 74 | 33 | ||||
| Female | 28 | 25 | 3 | 24 | 4 | ||||
| Age (yr) | 4.441 | 0.035 | 0.719 | 0.397 | |||||
| > 60 | 91 | 78 | 13 | 64 | 27 | ||||
| ≤ 60 | 44 | 31 | 13 | 34 | 10 | ||||
| Tumor size(cm) | 0.125 | 0.723 | 0.043 | 0.835 | |||||
| > 5 | 53 | 42 | 11 | 39 | 14 | ||||
| ≤ 5 | 72 | 67 | 15 | 59 | 23 | ||||
| Location of tumor | 3.860 | 0.277 | 1.537 | 0.674 | |||||
| Lower 1/3 | 55 | 48 | 7 | 37 | 18 | ||||
| Middle 1/3 | 20 | 15 | 5 | 16 | 4 | ||||
| Upper 1/3 | 45 | 36 | 9 | 34 | 11 | ||||
| More than 1/3 | 15 | 10 | 5 | 11 | 4 | ||||
| Borrmann type | 0.285 | 0.593 | 1.312 | 0.252 | |||||
| I + II Type | 31 | 24 | 7 | 25 | 6 | ||||
| III + IV Type | 104 | 85 | 19 | 73 | 31 | ||||
| Degree of differentiation | 0.187 | 0.666 | 4.414 | 0.036 | |||||
| Well/moderate | 57 | 47 | 10 | 36 | 21 | ||||
| Poor and not | 78 | 62 | 16 | 62 | 16 | ||||
| Histological type | 1.271 | 0.736 | 8.547 | 0.036 | |||||
| Papillary | 58 | 48 | 10 | 39 | 19 | ||||
| Tubular | 42 | 32 | 10 | 27 | 15 | ||||
| Mucinous | 10 | 9 | 1 | 9 | 1 | ||||
| Signet-ring cell | 25 | 20 | 5 | 23 | 2 | ||||
| Depth of invasion | 5.674 | 0.017 | 1.428 | 0.232 | |||||
| T1 + T2 | 21 | 13 | 8 | 13 | 8 | ||||
| T3 + T4 | 114 | 96 | 18 | 85 | 29 | ||||
| Lymph node metastasis | 0.008 | 0.927 | 6.023 | 0.014 | |||||
| Negative | 20 | 16 | 4 | 10 | 10 | ||||
| Positive | 115 | 93 | 22 | 88 | 27 | ||||
| TNM stage | 1.383 | 0.240 | 2.632 | 0.105 | |||||
| I + II | 44 | 33 | 11 | 28 | 16 | ||||
| III + IV | 91 | 76 | 15 | 70 | 21 | ||||
| Distant metastasis | 1.761 | 0.184 | 0.639 | 0.424 | |||||
| Negative | 128 | 102 | 26 | 92 | 36 | ||||
| Positive | 7 | 7 | 0 | 6 | 1 | ||||
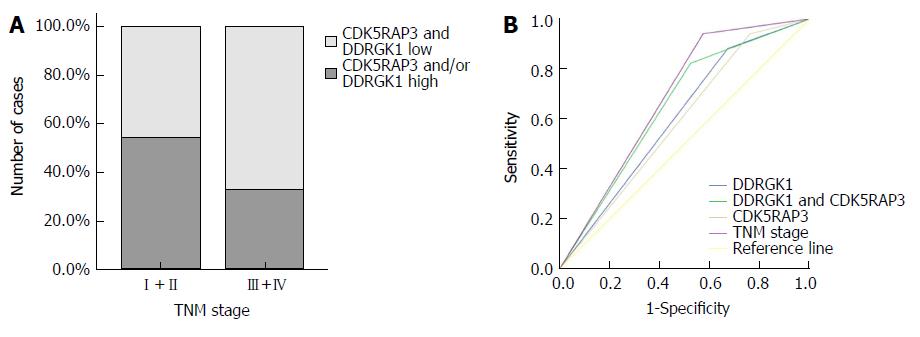
We analyzed the relationship between CDK5RAP3 and DDRGK1 protein expression in gastric cancer tissues and various clinicopathological data. The expression of CDK5RAP3 in gastric cancer was correlated with the age (P = 0.035) and T stage of the tumor (P = 0.017). However, the expression of DDRGK1 in gastric cancer was closely correlated with the differentiation degree (P = 0.036), the histological type (P = 0.036) and N stage of tumor (P = 0.014), as shown in Table 1. The co-expression level of CDK5RAP3 and DDRGK1 was related to sex (P = 0.024), T stage (P = 0.026), N stage (P = 0.048) and TNM stage (P = 0.016), as shown in Table 2.
| Variables | Total | C53 and DDRGK1 low expression | C53 and/or DDRGK1 high expression | χ2 | P value |
| Gender | |||||
| Male | 107 | 59 | 48 | 5.077 | 0.024 |
| Female | 28 | 22 | 6 | ||
| Age (yr) | |||||
| > 60 | 91 | 55 | 36 | 0.022 | 0.881 |
| ≤ 60 | 44 | 26 | 18 | ||
| Tumor size (cm) | |||||
| > 5 | 53 | 32 | 21 | 0.005 | 0.943 |
| ≤ 5 | 82 | 49 | 33 | ||
| Location of tumor | |||||
| Lower 1/3 | 55 | 33 | 22 | 1.481 | 0.687 |
| Middle 1/3 | 20 | 12 | 8 | ||
| Upper 1/3 | 45 | 29 | 16 | ||
| More than 1/3 | 15 | 7 | 8 | ||
| Borrmann type | |||||
| I + II Type | 31 | 20 | 11 | 0.342 | 0.559 |
| III + IV Type | 104 | 61 | 43 | ||
| Degree of differentiation | |||||
| Well/moderate | 57 | 31 | 26 | 1.296 | 0.255 |
| Poor and not | 78 | 50 | 28 | ||
| Histological type | |||||
| Papillary | 57 | 33 | 25 | 4.415 | 0.220 |
| Tubular | 42 | 22 | 20 | ||
| Mucinous | 10 | 8 | 2 | ||
| Signet-ring cell | 25 | 18 | 7 | ||
| Depth of invasion | |||||
| T1 + T2 | 21 | 8 | 13 | 4.972 | 0.026 |
| T3 + T4 | 114 | 73 | 41 | ||
| Lymph node metastasis | |||||
| Negative | 20 | 8 | 12 | 3.913 | 0.048 |
| Positive | 115 | 73 | 42 | ||
| TNM stage | |||||
| I + II | 44 | 20 | 24 | 5.754 | 0.016 |
| III + IV | 91 | 61 | 30 | ||
| Distant metastasis | |||||
| Negative | 126 | 75 | 53 | 2.034 | 0.154 |
| Positive | 7 | 6 | 1 |
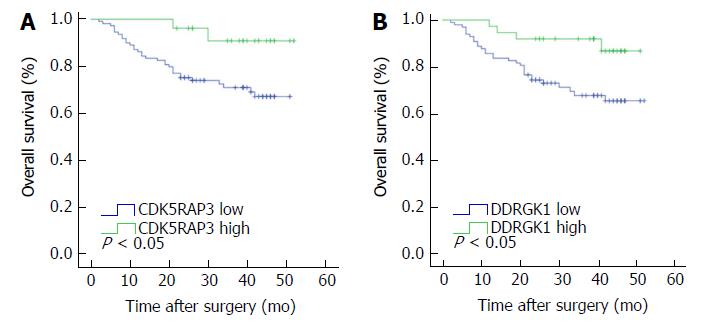
The median follow-up time was 30.0 mo, and the three-year survival rate was 70.7%. Survival analysis showed that the three-year survival rate of gastric cancer patients with low expression of CDK5RAP3 was 71.1%, which was lower than that of patients with high expression of CDK5RAP3 (90.8%, Figure 2A). The survival time of gastric cancer patients with low expression of DDRGK1 was significantly lower than that of patients with high expression of DDRGK1 (67.7% vs 91.9%, Figure 2B). When combining analysis of CDK5RAP3 and DDRGK1, the three-year survival rate of gastric cancer patients with low expression of CDK5RAP3 and DDRGK1 was 64.2%, which was significantly lower than that of the patients with high expression of CDK5RAP3 and DDRGK1 (Figure 3A). We compared the prognostic value of high expression of CDK5RAP3 and low expression of DDRGK1 to low expression of CDK5RAP3 and high expression of DDRGK1, and there were no significant differences between these survival curves (Supplementary Figure 1). So we combined the two groups into a group for further analysis. Further stratification analysis showed that the prognosis was the best when CDK5RAP3 and DDRGK1 were both highly expressed, and the prognosis was the worse when either CDK5RAP3 or DDRGK1 was highly expressed, while the worst prognosis was correlated with low expression of both CDK5RAP3 and DDRGK1 (Figure 3B). In addition, we used immunoprecipitation combined with mass spectrometry (in an HGC cell line) to find CDK5RAP3 binding protein and potential downstream targets. The results of string analysis show that CDK5RAP3 can bind DDRGK1 (Supplementary Figure 2).
Univariate analysis showed that the overall survival was correlated with the T status (P = 0.026), N status (P = 0.031), M status (P = 0.005), TNM stage (P = 0.001), and the expression level of CDK5RAP3 (P = 0.023) and DDRGK1 (P = 0.015) in gastric cancer tissues and the co-expression level of CDK5RAP3 and DDRGK1 in gastric cancer tissues (P = 0.001) (Table 3). Multivariate Cox prognostic analysis showed that the co-expression levels of CDK5RAP3 and DDRGK1 (P = 0.009) and the TNM stage (P = 0.007) were both independent prognostic factors in gastric cancer patients (Table 4).
| Clinicopathological parameters | Three-year cumulative survival rate | Log-rank test | P value |
| Gender | |||
| Male | 75.4 | 0.395 | 0.53 |
| Female | 71.4 | ||
| Age (yr) | |||
| > 60 | 75.4 | 0.163 | 0.686 |
| ≤ 60 | 73.8 | ||
| Tumor size (cm) | |||
| > 5 | 68.3 | 0.988 | 0.32 |
| ≤ 5 | 79.3 | ||
| Location of tumor | |||
| Lower 1/3 | 78.2 | 3.438 | 0.329 |
| Middle 1/3 | 83.6 | ||
| Upper 1/3 | 63.3 | ||
| More than 1/3 | 86.7 | ||
| Borrmann type | |||
| I + II | 76.9 | 0.373 | 0.541 |
| III + IV | 74 | ||
| Degree of differentiation | |||
| Well/moderate | 75.5 | 0.111 | 0.739 |
| Poor and not | 74.2 | ||
| Histological type | |||
| Papillary | 70 | 4.386 | 0.223 |
| Tubular | 85.6 | ||
| Mucinous | 70 | ||
| Signet-ring cell | 69.1 | ||
| Depth of invasion | |||
| T1 + T2 | 91.7 | 4.95 | 0.026 |
| T3 + T4 | 71.7 | ||
| Lymph node metastasis | |||
| Negative | 95 | 4.629 | 0.031 |
| Positive | 71.2 | ||
| TNM stage | |||
| I + II | 92.3 | 11.424 | 0.001 |
| III + IV | 66.3 | ||
| Distant metastasis | |||
| Negative | 76.7 | 8.015 | 0.005 |
| Positive | 42.9 | ||
| CDK5RAP3 expression | |||
| Low | 71.1 | 5.168 | 0.023 |
| High | 90.8 | ||
| DDRGK1 expression | |||
| Low | 67.7 | 5.971 | 0.015 |
| High | 91.9 | ||
| CDK5RAP3/DDRGK1 expression | |||
| CDK5RAP3 and/or DDRGK1 high | 90.1 | 10.415 | 0.001 |
| CDK5RAP3 and DDRGK1 low | 64.2 |
| Covariates | Coefficient | Standard error | HR | 95%CI for HR | P value |
| CDK5RAP3 expression (high vs low) | -1.226 | 0.735 | 0.294 | 0.070-1.239 | 0.095 |
| DDRGK1 expression (high vs. low) | -0.979 | 0.536 | 0.376 | 0.131-1.074 | 0.068 |
| CDK5RAP3 and DDRGK1 expression (low/low vs. high and/or high) | 1.178 | 0.453 | 3.247 | 1.336-7.891 | 0.009 |
| Depth of invasion (T3,T4 vs T1,T2) | 1.071 | 1.045 | 2.920 | 0.376-22.635 | 0.305 |
| Lymph node metastasis (positive vs negative) | 1.538 | 1.020 | 1.974 | 0.631-34.367 | 0.132 |
| Distant metastasis (positive vs negative) | 0.861 | 0.544 | 2.365 | 0.815-6.8631 | 0.113 |
| TNM stage (stage III and IV vs I and II) | -1.630 | 0.608 | 7.195 | 0.060-0.645 | 0.007 |
As shown in Figure 4, we established a ROC curve to compare the expression of CDK5RAP3 or DDRGK1 alone and the expression of CDK5RAP3 and DDRGK1 together with TNM stage in gastric cancer prognostication. The results showed that the area under the curve of the combination of CDK5RAP3 and DDRGK1 (AUC: 0.649, 95%CI: 0.548-0.751, P = 0.009) was larger than that of CDK5RAP3 or DDRGK1 expression alone (CDK5RAP3: AUC: 0.589, 95%CI: 0.486-0.693, P = 0.120, DDRGK1: AUC: 0.605, 95%CI: 0.501-0.708, P = 0.069). In addition, the prognostic value of the combined expression of CDK5RAP3 and DDRGK1 was closer to that of the TNM stage (AUC: 0.683, 95%CI: 0.591-0.776, P = 0.001).
In recent years, although some progress has been made in the treatment of gastric cancer, the prognosis of gastric cancer patients is still not optimistic because the majority of patients are only diagnosed in moderate or advanced stages, and the effect of adjuvant therapy is limited. Therefore, finding new biomarkers will help to improve earlier diagnosis and treatment of gastric cancer. DDRGK1 is not only a target protein of ufmylation, but is also an integral component of the ufmylation modification system. Ufmylation mediated by DDRGK1 plays an important role in carcinogenesis [14,15]. Shiwaku et al[16]found that the amino acid sequence of CDK5RAP3 contained an ubiquitin protein ligase binding region. Wu’s study[9] found that CDK5RAP3 can interacted with DDRGK1 and UFL1 (called RCAD in their study) and regulate the stability of CDK5RAP3 and DDRGK1. Therefore, based on these previous reports and our finding, we speculate that the function of CDK5RAP3 and DDRGK1 is related. However, the expression of CDK5RAP3 and DDRGK1 in gastric cancer and their influence on clinicopathological characteristics and prognosis have not previously been reported.
CDK5RAP3 is widely expressed in various tissues and cells of the whole body, including the heart, brain, skeletal muscle, placenta, lung, liver, kidney and pancreas[17]. In early embryonic development, CDK5RAP3 regulates cell cycle progression, epidermal cell adhesion and migration[18]. Recent studies have suggested that CDK5RAP3 plays an important role in various cancers such as lung cancer, liver cancer, and head and neck cancer[6,19,20]. Our study found that the three year survival rate of patients with low expression of CDK5RAP3 was lower than that of patients with high expression of CDK5RAP3 (P < 0.05), and the expression of CDK5 RAP3 was correlated with tumor T stage, suggesting that CDK5RAP3 is involved in gastric cancer. It may play a role in suppressing cancer, which is also consistent with our previous research results[21]. In the case of DDRGK1, some studies have shown that DDRGK1 is a tumor suppressor. The ufmylation of DDRGK1 itself is essential for its combination with UFL1 and activation of the UFL1 ubiquitin ligase. If DDRGK1 is unable to undergo ufmylation, it cannot bind and activate UFL1 activity, thereby blocking the ufmylation of the nuclear receptor co-activator ASC1 and, inhibiting the binding of ASC1 and the transcription factors p300 and SRC1 to the downstream target genes of the estrogen receptor ERα[11,14].
In this study, the survival of gastric cancer patients with low expression of DDRGK1 was significantly shorter than that of patients with high expression of DDRGK1 (P < 0.05), and the expression of DDRGK1 was related to tumor differentiation, histological type and N stage. It has also been suggested that DDRGK1 may play a role in suppressing the progression of gastric cancer.
In addition, we found that patients with low expression of CDK5RAP3 and DDRGK1 had the worst prognosis while patients with high expression of both proteins had the best prognosis, and the other patients were between them. Further analysis showed that the accuracy of prognostication with a combination of CDK5RAP3 and DDRGK1 was higher than that of CDK5 RAP3 or DDRGK1 alone. We showed that the combined expression of CDK5RAP3 and DDRGK1 had a better ability to predict the overall survival rate of gastric cancer patients. Xi et al[13] found that DDRGK1 interacted with IkBa and regulated its stability, thereby regulating the transcriptional activity of NF-κB and its target gene expression. However, Wang’s study[9] of CDK5RAP3 found that down-regulation of CDK5RAP3 increased cell invasiveness and increased the transcriptional activity of NF-κB. CDK5RAP3 binds to RelA to inhibit its phosphorylation and increase the binding of HDAC to RERA, thereby inhibiting the transcriptional activity of NF-κB. CDK5RAP3 and DDRGK1 can interact with each other, and their roles in the NF-κB pathway are similar. Therefore, we hypothesized that their impact on prognosis may be related to the overlapping of the two tumor suppressing effects. However, the interaction between CDK5RAP3 and DDRGK1 in gastric cancer has not been fully elucidated. Further manipulation of gene expression in different gastric cancer cell lines and investigation of the characteristics and mechanism of these genes effects on gastric cancer are needed in additional studies.
In summary, low expression of CDK5RAP3 and DDRGK1 are closely related to the prognosis of gastric cancer, and the co-expression of CDK5RAP3 and DDRGK1 is an independent prognostic factor correlated with the overall survival of gastric cancer patients.
Although the morbidity and mortality of the primary gastric cancer has declined in recent decades, it is still the third most common cause of cancer-related deaths worldwide. The symptoms of early gastric cancer are not highly specific. Therefore, misdiagnosis and missed diagnosis may occur. Therefore, finding new biomarkers will help to improve earlier diagnosis and treatment of gastric cancer. In recent years, an increasing number of studies on the prognostic indicators of gastric cancer have been published. However, the expression of CDK5RAP3 and DDRGK1 in gastric cancer and its influence on prognosis have not yet been reported.
At present, the etiology and pathogenesis of gastric cancer has not yet been fully clarified. There is also a lack of specific and highly effective therapeutic drugs available for use in clinical practice. The symptom specificity of early gastric cancer is not obvious, so most patients are already in advanced stages before receiving medical treatment, which seriously affects the prognosis of patients. Therefore, searching for molecular markers that can be used as an independent prognostic factor for gastric cancer is of great significance for the early diagnosis and targeted treatment of gastric cancer. A series of studies on tumor prognostic factors is expected to provide a new target for the treatment of gastric cancer while providing new targets for the treatment of gastric cancer. The expression of CDK5RAP3 and DDRGK1 in gastric cancer and their influence on clinicopathological characteristics and prognosis have not previously been discussed.
The aim of this study is to identify novel effective biomarkers to classify patients with low or high survival. This would provide a guide to clinicians to select therapeutic strategies for patients and provide personalized therapy according to the predicted survival rate. In this study, we investigated two interacting proteins, CDK5RAP3 and DDRGK1, which may help determine patient management strategies.
We used immunohistochemistry to detect the expression of CDK5RAP3 and DDRGK1 in gastric cancer and adjacent tissues. Western Blot was used to detect the expression of CDK5RAP3 and DDRGK1 in gastric cancer and its adjacent tissues and cell lines. According to immunohistochemistry scores, the patients were divided into CDK5RAP3 high expression group and CDK5RAP3 low expression group, DDRGK1 high expression group and DDRGK1 low expression group, and the relationship between the expression level and clinicopathological data was analyzed. Furthermore, based on the combined expression of CDK5RAP3 and DDRGK1, we classified the patients into three subtypes: CDK5RAP3 and DDRGK1 high (n = 9), CDK5RAP3 or DDRGK1 low (n = 45) and CDK5RAP3 and DDRGK1 low (n = 81). Then, we used the Kaplan-Meier method to analyze the effect of different expression patterns on prognosis.
Our research found that the expression of CDK5RAP3 and DDRGK1 was down-regulated in gastric cancer. Low expression of CDK5RAP3 or DDRGK1 is a poor prognostic factor for gastric cancer patients. Moreover, prognostic analysis showed that the co-expression of CDK5RAP3 and DDRGK1 was an independent prognostic factor correlating with the overall survival of gastric cancer patients. Combined expression analysis of CDK5RAP3 and DDRGK1 may provide a more accurate prognostic value for overall survival. This study presents two interacting proteins, which may be useful to determine patient management strategies. These makers may predict the prognosis of gastric cancer patients through an analysis of CDK5RAP3 and DDRGK1 protein expression in preoperative biopsy and tumor specimens.
This study found that low expression of CDK5RAP3 and DDRGK1 are closely related to the poor prognosis of gastric cancer patients, and the co-expression of CDK5RAP3 and DDRGK1 is an independent prognostic factor correlated with the overall survival of gastric cancer patients. These two interacting proteins, CDK5RAP3 and DDRGK1, may be helpful in determining patient management strategies, and to predict the prognosis of gastric cancer patients.We hypothesized that CDK5RAP3 and DDRGK1 were key genes which may participate in the biological regulation of gastric cancer. The mechanism of their role in gastric cancer has not been fully elucidated and further studies are needed. With advances in technology, humans may find more effective and new indicators in the future to guide treatment, improve prognosis, and reduce the recurrence rate and mortality of patients with gastric cancer.
This study found the prognostic value of two interacting proteins, CDK5RAP3 and DDRGK1, by detecting the expression of both in clinical specimens, combined with detailed clinicopathological data analysis. This study provided ideas for finding new tumor prognosis related molecules. Manipulation of both CDK5RAP3 and DDRGK1 expression in different gastric cancer cell lines, such as overexpression or knockdown, will be needed for future research. Further study is necessary to investigate the characteristics of cancer cells and explore the mechanism of CDK5RAP3 and DDRGK1 affecting the development of gastric cancer by an in vitro cell model and in vivo xenograft model.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chuang SM, Tomizawa M, Zhang J S- Editor: Gong ZM L- Editor: Filipodia E- Editor:Bian YN
| 1. | Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, Wilkinson NW. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21:3008-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004;15:1585-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Wang X, Ching YP, Lam WH, Qi Z, Zhang M, Wang JH. Identification of a common protein association region in the neuronal Cdk5 activator. J Biol Chem. 2000;275:31763-31769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | An H, Lu X, Liu D, Yarbrough WG. LZAP inhibits p38 MAPK (p38) phosphorylation and activity by facilitating p38 association with the wild-type p53 induced phosphatase 1 (WIP1). PLoS One. 2011;6:e16427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Stav D, Bar I, Sandbank J. Usefulness of CDK5RAP3, CCNB2, and RAGE genes for the diagnosis of lung adenocarcinoma. Int J Biol Markers. 2007;22:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Mak GW, Chan MM, Leong VY, Lee JM, Yau TO, Ng IO, Ching YP. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res. 2011;71:2949-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Zhao JJ, Pan K, Li JJ, Chen YB, Chen JG, Lv L, Wang DD, Pan QZ, Chen MS, Xia JC. Identification of LZAP as a new candidate tumor suppressor in hepatocellular carcinoma. PLoS One. 2011;6:e26608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Wu J, Lei G, Mei M, Tang Y, Li H. A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing Protein 1 (DDRGK1) and modulates NF-kappaB signaling. J Biol Chem. 2010;285:15126-15136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Lemaire K, Moura RF, Granvik M, Igoillo-Esteve M, Hohmeier HE, Hendrickx N, Newgard CB, Waelkens E, Cnop M, Schuit F. Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS One. 2011;6:e18517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Neziri D, Ilhan A, Maj M, Majdic O, Baumgartner-Parzer S, Cohen G, Base W, Wagner L. Cloning and molecular characterization of Dashurin encoded by C20orf116, a PCI-domain containing protein. Biochim Biophys Acta. 2010;1800:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Hofmann K, Bucher P. The PCI domain: a common theme in three multiprotein complexes. Trends Biochem Sci. 1998;23:204-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Xi P, Ding D, Zhou J, Wang M, Cong YS. DDRGK1 regulates NF-κB activity by modulating IκBα stability. PLoS One. 2013;8:e64231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Yoo HM, Kang SH, Kim JY, Lee JE, Seong MW, Lee SW, Ka SH, Sou YS, Komatsu M, Tanaka K. Modification of ASC1 by UFM1 is crucial for ERα transactivation and breast cancer development. Mol Cell. 2014;56:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Tatsumi K, Sou YS, Tada N, Nakamura E, Iemura S, Natsume T, Kang SH, Chung CH, Kasahara M, Kominami E. A novel type of E3 ligase for the Ufm1 conjugation system. J Biol Chem. 2010;285:5417-5427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Shiwaku H, Yoshimura N, Tamura T, Sone M, Ogishima S, Watase K, Tagawa K, Okazawa H. Suppression of the novel ER protein Maxer by mutant ataxin-1 in Bergman glia contributes to non-cell-autonomous toxicity. EMBO J. 2010;29:2446-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Lew J, Beaudette K, Litwin CM, Wang JH. Purification and characterization of a novel proline-directed protein kinase from bovine brain. J Biol Chem. 1992;267:13383-13390. [PubMed] |
| 18. | Mak GW, Lai WL, Zhou Y, Li M, Ng IO, Ching YP. CDK5RAP3 is a novel repressor of p14ARF in hepatocellular carcinoma cells. PLoS One. 2012;7:e42210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Wang J, An H, Mayo MW, Baldwin AS, Yarbrough WG. LZAP, a putative tumor suppressor, selectively inhibits NF-kappaB. Cancer Cell. 2007;12:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Wang JB, Wang ZW, Li Y, Huang CQ, Zheng CH, Li P, Xie JW, Lin JX, Lu J, Chen QY. CDK5RAP3 acts as a tumor suppressor in gastric cancer through inhibition of β-catenin signaling. Cancer Lett. 2017;385:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Merbl Y, Refour P, Patel H, Springer M, Kirschner MW. Profiling of ubiquitin-like modifications reveals features of mitotic control. Cell. 2013;152:1160-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |