Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.438
Peer-review started: October 28, 2017
First decision: November 14, 2017
Revised: December 1, 2017
Accepted: December 4, 2017
Article in press: December 4, 2017
Published online: January 21, 2018
Processing time: 83 Days and 21.2 Hours
Non-selective beta-blockers are the mainstay of medical therapy for portal hypertension in liver cirrhosis. Inhibitors of phosphodiesterase-5 (PDE-5-inhibitors) reduce portal pressure in the acute setting by > 10% which may suggest a long-term beneficial effect. Currently, there is no available data on long-term treatment of portal hypertension with PDE-5-inhibitors. This case of a patient with liver cirrhosis secondary to autoimmune liver disease with episodes of bleeding from esophageal varices is the first documented case in which a treatment with a PDE-5-inhibitor for eight years was monitored. In the acute setting, the PDE-5-inhibitor Vardenafil lowered portal pressure by 13%. The portal blood flow increased by 28% based on Doppler sonography and by 16% using MRI technique. As maintenance medication the PDE-5-inhibitor Tadalafil was used for eight consecutive years with comparable effects on portal pressure and portal blood flow. There were no recurrence of bleeding and no formation of new varices. Influencing the NO-pathway by the use of PDE-5 inhibitors may have long-term beneficial effects in compensated cirrhosis.
Core tip: Non-selective beta-blockers are the mainstay of medical therapy for portal hypertension in liver cirrhosis. Inhibitors of phosphodiesterase-5 (PDE-5) reduce portal pressure in the acute setting by > 10%. This is the first report of a patient with liver cirrhosis showing that in long-term treatment with a PDE-5-inhibitor the positive effect on liver hemodynamics is maintained thus preventing further variceal bleeding.
- Citation: Deibert P, Lazaro A, Stankovic Z, Schaffner D, Rössle M, Kreisel W. Beneficial long term effect of a phosphodiesterase-5-inhibitor in cirrhotic portal hypertension: A case report with 8 years follow-up. World J Gastroenterol 2018; 24(3): 438-444
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.438
Portal hypertension in liver cirrhosis is caused by several factors[1-3]. Structural changes (i.e., regenerative nodules and fibrosis) lead to an intrahepatic outflow obstruction which is aggravated by an increased portal blood flow (at least in early stages of cirrhosis) as a consequence of excessive extrahepatic nitric oxide (NO) production. About a quarter of the portal pressure is due to a functional intrahepatic component: the imbalance between constricting and dilating factors within the sinusoids and the dysregulation of the nitric oxide - cyclic guanosine monophosphate (NO-cGMP) system lead to an overactivity of stellate cells/myofibroblasts and contraction of sinusoids. Phosphodiesterase-5-inhibitors (PDE-5-inhibitors) inhibit the conversion of cGMP to 5’-GMP[2] thus, increasing the level of cGMP, the second messenger, which may lead to dilation of sinusoids. There are conflicting data from preclinical and clinical studies whether or not PDE-5-Inhibitors lower elevated portal pressure in cirrhosis[4-6]. However, a recent proof-of-concept study showed that the long-acting PDE-5-inhibitor Udenafil lowered portal pressure at doses of 75-100 mg by approximately 20% in the acute testing[7].
A decrease of hepatovenous pressure gradient (HVPG) by ≥ 20% from baseline or to a value of ≤ 12 mm Hg after an application of non-selective beta-blockers for more than two weeks is accepted as a good predictor of beneficial clinical response[8-10]. Meanwhile, several studies demonstrated that a decrease of HVPG by ≥ 10% in the acute setting after intravenous administration of propranolol (0.15 mg/kg) is an adequate predictor of a clinical response as well[11-13]. Therefore, it may be surmised that a decrease of HVPG by ≥ 10% in the acute setting after application of a PDE-5-inhibitor may be an indicator of a long-term beneficial effect in liver cirrhosis with portal hypertension.
In this case report of a patient with liver cirrhosis who had multiple episodes of bleeding esophageal varices we aimed to answer the following questions: (1) Are the decrease of portal pressure and increase of portal venous blood flow induced by a PDE-5-inhibitor long-lasting? (2) Does the long-term use of a PDE-5-inhibitor have a beneficial clinical effect of reducing the risk of bleeding from esophageal varices? And (3) Do we see any compound-specific adverse effects in the long-term?
In February 2009 a 53-year-old female patient was admitted to the University Hospital in Freiburg, Germany for further evaluation. The patient was initially diagnosed with autoimmune hepatitis/primary biliary cholangitis (AIH/PBC) overlap syndrome (positive for anti-nuclear antibodies, antibodies to smooth muscle cells, antibodies to pyruvate decarboxylase E2 subunit, and to soluble liver antigen) in 1989. Since histology and clinical chemistry showed that the AIH component was predominant without cholestasis, the prescribed therapy was a combination of corticosteroids and azathioprine.
In 2006 the patient was previously admitted in the Hospital of Norden, Germany with the first episode of acute variceal bleeding with hemodynamic instability. Banding of varices was performed. Sonography of the abdominal organs showed signs of liver cirrhosis including enlarged spleen. No other abnormalities were found. Within the following 3 years she bled three times per year from these varices. In 2008 she had two episodes of bleeding from rectal varices which were treated with rubber band ligation. Medical therapy of portal hypertension with propranolol was initiated but had to be stopped even at the low dose of 20 mg twice daily due to intolerable cardiovascular side effects (i.e., bradycardia and hypotension). The patient was regularly monitored in the liver transplant center of the University Hospital in Hannover (Medizinische Hochschule Hannover). She was waitlisted for liver transplantation and the implantation of TIPS was scheduled. She was referred to our hepatological unit in order to check whether the application of an inhibitor of the enzyme phosphodiesterase-5 could be an option to lower portal pressure and reduce the risk for bleeding from esophageal varices.
The patient was in a good clinical condition upon admission. The physical examination findings were normal except for the palpable liver and minor bilateral varicose veins of the lower extremities. Examination of heart and lungs was unremarkable. No bipedal edema was present. There were no signs of hepatic encephalopathy. Blood pressure was 154/86 mmHg and the heart rate was 78/min. ECG and echocardiography findings were normal except for a slight tricuspid valve insufficiency. Systolic pulmonary artery pressure was not increased (26 mmHg). Laboratory results showed no pathological values except a slight increase in total bilirubin (1.6 mg/dL) and thrombocytopenia of 105.000/μL, establishing the case as Child A cirrhosis (i.e., normal bilirubin, normal serum albumin, normal INR, no ascites, no hepatic encephalopathy). In esophago-gastro-duodenoscopy scarring transformation of two ligated varices was observed. Three small varices of grade 1-2 were visible. At this point, the daily medication consisted of azathioprine 75 mg, prednisolone 5 mg, pantoprazole 20 mg, and calcium 500 mg.
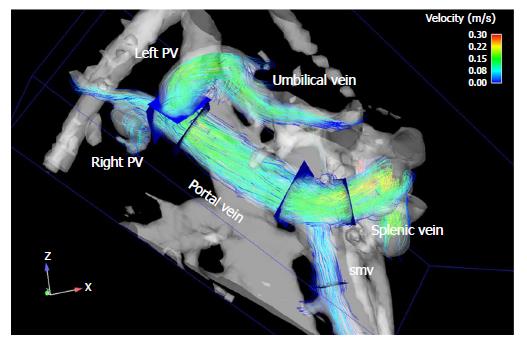
To test the effect of a PDE-5-inhibitor on portal hemodynamics in February 2009 10 mg of Vardenafil were administered orally. Wedged hepatic vein pressure (WHVP) and free hepatic vein pressure (FHVP) were measured in triplicate before and one hour after the drug administration. The HVPG, defined as WHVP - FHVP, decreased by 14% from 10.5 to 9.0 mmHg. Duplex sonography showed an increase in portal flow by 28% (0.97 L/min to 1.24 L/min) 60 min after drug intake. Systemic blood pressure changed from 130/87 to 121/75 mmHg one hour after drug administration, while heart rate changed from 65 to 61/min. Portal flow monitored by flow-sensitive 3D magnetic resonance imaging increased by 16 % (0.85 L/min to 0.99 L/min). Maximal flow velocity remained constant, at 24 cm/s in duplex sonography and 17 cm/s in 3D MRI. No relevant effect was observed on systemic blood pressure. Visualization of the portal venous system by MRI (Figure 1) confirmed the duplex sonographic findings of a prograde blood flow in the portal vein and the two main intrahepatic branches and a recanalized umbilical vein originating from the left main branch of the portal vein.
We discussed an experimental therapeutic approach of the application of a PDE-5-inhibitor with the patient. The patient was informed that this was an off-label use and gave written informed consent. After having verified that the PDE-5-inhibitor Vardenafil decreased HVPG and that it led to an increase of portal venous blood flow confirmed by two independent methods we decided to start a long-term therapy with 5 mg Tadalafil/day, as this PDE-5-inhibitor has a longer half-life than Vardenafil. In June 2009 an HVPG one hour after oral administration of 5 mg Tadalafil of 10.5 mm Hg was measured. Portal venous blood flow remained elevated at 1.21 L/min. Systemic blood pressure showed no clinically relevant changes (124/79 mmHg, heart rate 88/min to 127/77 mmHg, heart rate 64/min) in the acute setting. The next evaluation was performed in October 2009 wherein after 10 mg Tadalafil the HVPG decreased by 15% from 12.0 to 10.0 mm Hg. Portal venous blood flow remained markedly elevated (1.28 L/min in Duplex sonography and 0.99 L/min as measured by MRI). In March 2010 HVPG was 10.5 mm Hg, while portal venous blood flow remained about 30% higher than the initial reading as verified by the two methods. Notably, the flow in the umbilical vein was not influenced by the PDE-5-inhibitor. The results of the measurements are shown in Table 1. No relevant changes in heart rate or blood pressure occurred. In April 2010 a second-grade esophageal varix was injected with acryl-glue prophylactically. In March 2011 the last measurement of portal blood flow using duplex sonography and the MRI method was performed. It showed that the portal venous blood flow remained constant at a level above than at the start of treatment. Further invasive portal pressure measurements were not performed.
| Time | Flow portal vein (L/min) | Flow umbi-lical vein (L/min) | HVPG (mmHg) | Blood pressure (mmHg) | Heart rate (beats/min) | ||
| Duplex | MRI | MRI | |||||
| Start | 02/2009 | 0.97 | 0.85 | 0.53 | 10.5 | 130/87 | 69 |
| 1 h post Vardenafil 10 mg | 02/2009 | 1.24 | 0.99 | 0.56 | 9.0 (-14%) | 121/75 | 61 |
| 4 mo Tadalafil 5 mg/d | 06/2009 | 1.21 | 0.56 | 10.5 | 127/77 | 64 | |
| Without PDE-5-I | 10/2009 | 12 | |||||
| 1h post Tadalafil 5 mg | 10/2009 | 1.28 | 0.99 | 0.53 | 10.0 (-15%) | 110/70 | 84 |
| 11 mo Tadalafil 5 mg/d | 03/2010 | 1.32 | 1.33 | 10.5 | 120/80 | 70 | |
| 20 mo Tadalafil 5 mg/d | 10/2010 | 1.34 | 115/75 | 77 | |||
| 26 mo Tadalafil 5 mg/d | 04/2011 | 1.26 | 1,34 | 125/80 | 72 | ||
| 32 mo Tadalafil 5 mg/d | 10/2011 | 1.25 | 117/70 | 66 | |||
| 46 mo Tadalafil 5 mg/d | 12/2012 | 1.20 | 131/85 | 70 | |||
| 53 mo Tadalafil 5 mg/d | 07/2013 | 1.20 | 110/70 | 68 | |||
| 60 mo Tadalafil 5 mg/d | 02/2014 | 1.20 | 115/75 | 78 | |||
| 70 mo Tadalafil 5 mg/d | 12/2014 | 1.20 | 110/70 | 70 | |||
| 81 mo Tadalafil 5 mg/d | 11/2015 | 1.40 | 135/85 | 65 | |||
| 84 mo Tadalafil 5 mg/d | 12/2016 | 1.061 | 135/75 | 85 | |||
| 93 mo Tadalafil 5 mg/d | 09/2017 | 1.29 | 110/70 | 80 | |||
The clinical course of the patient remained stable. The portal venous blood flow determined by duplex sonography was constant within the range of 1.2 and 1.4 L/min. Endoscopic monitoring every six months showed scarring in the distal esophagus and with no signs of new esophageal varices. The varices remained closed by thrombosis. Two episodes of upper gastrointestinal bleeding occurred in 02/2016 and 04/2016. Upper endoscopy excluded bleeding from varices or portal hypertensive gastropathy. The bleeding from the small visible erosions in the duodenum was attributed to NSAID use which the patient had taken due to headache. After administration of a proton pump inhibitor no further bleeding occurred. In 12/2016 portal flow was quantified to be 1.06 L/min. However, this was measured with a different sonographic device. The next sonographic examination in 09/2017 with the original device revealed a constant portal blood flow of 1.29 L/min.
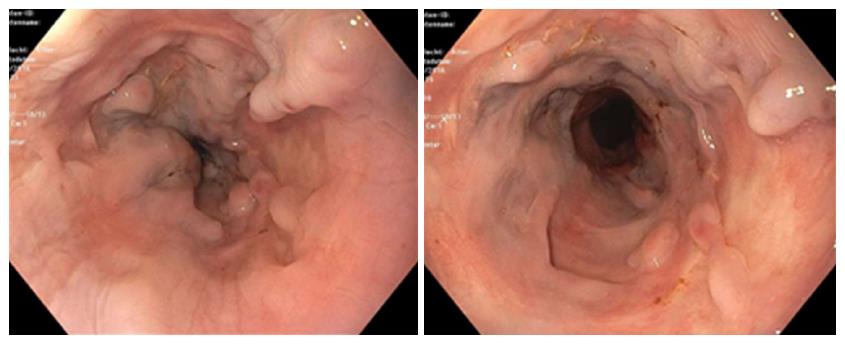
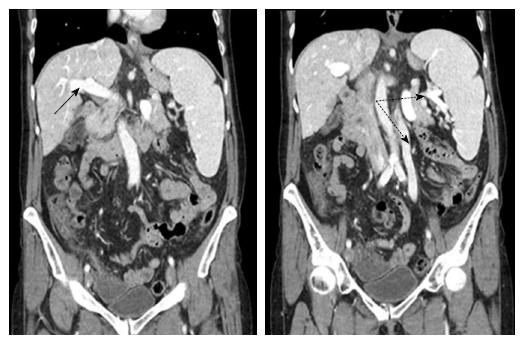
Figure 2 shows the distal aspect of the esophagus with visible scars caused by sclerosing and banding, with the old varices remained thrombosed. Figure 3 shows an abdominal computer tomographic angiography. The portal vein (solid arrow) was perfused in a prograde direction, the diameter was enlarged. There were collateral veins at the splenic hilus and an enlarged left ovarian vein (dashed arrows).
The patient continued the daily intake of 5 mg Tadalafil. Other medications consisted of 75 mg Azathioprine per day and 2.5 mg Prednisone per day. Because of the stable clinical condition the patient is no longer on the list of liver transplant candidates. During the time of follow-up the number of varicose veins of the lower extremities increased and a local therapy was suggested.
This case report describes a consistent beneficial effect of a PDE-5-inhibitor in a patient with portal hypertension due to AIH/PBC overlap syndrome who had bled from esophageal varices. HVPG decreased by 14% at the initial hemodynamic test and by 15% a few months later. Portal venous flow increased by 28% as measured by Doppler ultrasound and by 16% as measured by four-dimensional flow MRI[14,15]. These measurements persisted for more than eight years and were accompanied by a beneficial clinical effect. Since the start of a therapy with 5 mg Tadalafil per day no further esophageal variceal bleeding occurred for eight years. Compound-specific adverse effects were not observed. In particular, no clinically significant side effects to systemic hemodynamics were detected.
For several years the question whether or not an inhibition of the enzyme PDE-5 lowers portal hypertension in patients with liver cirrhosis remained unanswered. The idea to use a PDE-5-inhibitor in portal hypertension has mainly been derived from theoretical consideration based on known or assumed biochemical mechanisms involved in portal hypertension[16,17]. Meanwhile, the results of a proof-of-concept study were published showing that the long-acting PDE-5-inhibitor Udenafil lowers portal pressure in liver cirrhosis in a dose dependent-manner in the acute setting[8]. The authors tried to explain the conflicting data obtained from other working groups and suggested that the effect of a PDE-5-inhibitor is dose-dependent and that the most pronounced effect could be seen in early to middle stages of liver cirrhosis, when the regulation of the sinusoidal tonus could still be influenced. However, the potential for a beneficial or detrimental effect of PDE-5 inhibitors may depend on the stage of liver disease and the extension of portal collaterals as it has been postulated for nitrates[18].
According to recently published articles, a decrease of HVPG by > 10% after acute administration of propranolol might be sufficient to predict a beneficial clinical effect on rate of rebleeding[11-13]. In this case it was shown that using the short acting PDE-5-inhibitor Vardenafil and the long-acting PDE-5-inhibitor Tadalafil the portal pressure decreased by 14% and by 15%, respectively. These data are consistent with other published reports[4,7]. It was therefore interesting to monitor the effect of the PDE-5-inhibitor on clinical outcome measures in this patient. It was possible to observe the effect of Tadalafil on HVPG for one year. If a PDE-5-inhibitor lowers portal pressure this may be achieved by dilation of sinusoids which leads to a concomitant increase of portal venous blood flow[4]. Table 1 shows that the PDE-5-inhibitor induced an increase of portal venous flow. Within a two-year duration this increase was verified by two independent methods (Doppler sonography and MRT).
This first documented case of a long-term application of PDE-5 inhibitors in cirrhotic portal hypertension could initiate the discussion about this group of drugs as novel adjunct therapy in this setting. Further clinical studies must be conducted before PDE-5 inhibitors can be safely recommended for this indication. In this patient the effect of Tadalafil on portal blood flow persisted for eight years. Presently, there is still no generally accepted non-invasive marker for portal pressure[19]. The quantification of liver hemodynamics with Doppler sonography has been shown to correlate with the HVPG to some degree[20,21]. The effect on portal blood flow may therefore be a further surrogate marker for the influence of a drug on portal pressure in portal hypertension. It can be supposed that an increase of portal flow induced by a PDE-5-inhibitor in liver cirrhosis may indicate a preserved reactivity of the sinusoids and lowering of portal pressure by the drug. However, the reliability of the sonographic data depends on the expertise of the examiner. In this case, the MRI measurement showed an effect consistent with the Doppler sonographic results. Therefore, it is probable that during the long-term therapy with Tadalafil the positive effect on portal pressure persisted.
Another notable observation was that during the eight years of follow-up no signs of deterioration of liver function were found. AIH, PBC, and AIH/PBC overlap syndrome have unfavourable prognosis once cirrhosis developed and even more after bleeding from esophageal varices[22-25]. This raises the question whether PDE-5-inhibitors could improve or stabilize the function in a diseased liver. In thioacetamide-induced liver fibrosis/cirrhosis in animals Sildenafil was shown to induce a reversal of liver damage and fibrosis[26], and Udenafil had a positive effect on degree of fibrosis in rats[27]. It may be speculated whether some kind of remodelling in a diseased liver may be induced by long-term administration of a PDE-5-inhibitor by influencing hepatic stellate cells which are suggested as key mediators of fibrosis[28]. In the presented case the diagnosis of cirrhosis had been done in 1989. The course of disease was relatively stable under immunosuppressive therapy for 20 years. However, episodes of upper gastrointestinal bleeding occurred. It is unlikely that after such a time period a spontaneous improvement in portal hypertension would occur. Echocardiographic examinations in the year 2009, 2013 and 2017 revealed constant findings. Taken together with stable values of blood pressure and heart rate no significant changes in systemic circulation were detected during the years of follow-up. Therefore, the improvement in portal hemodynamics could be attributed to the application of the PDE-5-inhibitors.
In conclusion, in this case of a female patient with liver cirrhosis due to overlap-syndrome of AIH and primary biliary cholangitis the application of Vardenafil or Tadalafil induced a decrease of portal pressure by 14% and 15%, respectively, and an increase of portal vein blood flow by 28% (Doppler sonography) and 16% (MRT). The use of a long-acting PDE-5-inhibitor led to a long-lasting improvement of portal blood flow. During the eight years of follow-up no further bleeding from esophageal varices occurred.
This is the first report of long-term treatment of portal hypertension with PDE-5 inhibitors.
Variceal bleeding in liver cirrhosis due to autoimmune hepatitis.
Diagnosis of cirrhosis was done 20 years before start of treatment, so the decline in portal hypertension is probably attributable to PDE-5-Inhibitor-treatment rather than spontaneous improvement.
Improvement of portal hypertension was verified by invasive measurement of hepatovenous pressure gradient.
MRI and duplex sonographic images verified improvement in portal hemodynamics.
Liver cirrhosis was histologically confirmed 20 years prior to PDE-5-Inhibitor treatment.
Oral treatment with Tadalafil 5mg once daily.
Targeting the nitric oxide (NO)-cGMP-pathway in other diseases like pulmonary hypertension or erectile dysfunction.
Non-selective beta-blockers are the mainstay of medical therapy for portal hypertension in liver cirrhosis.
Influencing the NO-pathway by the use of PDE-5 inhibitors may have long-term beneficial effects in compensated cirrhosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Gallo P, Karagiannakis DS, Konishi H, Risso A S- Editor: Chen K L- Editor: A E- Editor: Huang Y
| 1. | Laleman W, Landeghem L, Wilmer A, Fevery J, Nevens F. Portal hypertension: from pathophysiology to clinical practice. Liver Int. 2005;25:1079-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Shah V, Lyford G, Gores G, Farrugia G. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126:903-913. [PubMed] |
| 3. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rössle M, Kreisel W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther. 2006;23:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Halverscheid L, Deibert P, Schmidt R, Blum HE, Dunkern T, Pannen BH, Kreisel W. Phosphodiesterase-5 inhibitors have distinct effects on the hemodynamics of the liver. BMC Gastroenterol. 2009;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Tandon P, Inayat I, Tal M, Spector M, Shea M, Groszmann RJ, Garcia-Tsao G. Sildenafil has no effect on portal pressure but lowers arterial pressure in patients with compensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8:546-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, Neagu M, Rössle M, Zipprich A, Caca K. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2291] [Article Influence: 229.1] [Reference Citation Analysis (3)] |
| 9. | Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 10. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 11. | Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer JM, Alvarez-Urturi C, Torras X, Balanzó J, Guarner C. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 12. | La Mura V, Abraldes JG, Raffa S, Retto O, Berzigotti A, García-Pagán JC, Bosch J. Prognostic value of acute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J Hepatol. 2009;51:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | de-Madaria E, Palazón JM, Hernández FT, Sánchez-Paya J, Zapater P, Irurzun J, de España F, Pascual S, Such J, Sempere L. Acute and chronic hemodynamic changes after propranolol in patients with cirrhosis under primary and secondary prophylaxis of variceal bleeding: a pilot study. Eur J Gastroenterol Hepatol. 2010;22:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Stankovic Z, Csatari Z, Deibert P, Euringer W, Blanke P, Kreisel W, Abdullah Zadeh Z, Kallfass F, Langer M, Markl M. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology. 2012;262:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Stankovic Z. Four-dimensional flow magnetic resonance imaging in cirrhosis. World J Gastroenterol. 2016;22:89-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 16. | Davies NA, Hodges SJ, Pitsillides AA, Mookerjee RP, Jalan R, Mehdizadeh S. Hepatic guanylate cyclase activity is decreased in a model of cirrhosis: a quantitative cytochemistry study. FEBS Lett. 2006;580:2123-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Loureiro-Silva MR, Iwakiri Y, Abraldes JG, Haq O, Groszmann RJ. Increased phosphodiesterase-5 expression is involved in the decreased vasodilator response to nitric oxide in cirrhotic rat livers. J Hepatol. 2006;44:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Angelico M, Lionetti R. Long-acting nitrates in portal hypertension: to be or not to be? Dig Liver Dis. 2001;33:205-211. [PubMed] |
| 19. | Bolognesi M, Di Pascoli M, Sacerdoti D. Clinical role of non-invasive assessment of portal hypertension. World J Gastroenterol. 2017;23:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Iranpour P, Lall C, Houshyar R, Helmy M, Yang A, Choi JI, Ward G, Goodwin SC. Altered Doppler flow patterns in cirrhosis patients: an overview. Ultrasonography. 2016;35:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Tasu JP, Rocher L, PEletier G, Kuoch V, Kulh E, Miquel A, Buffet C, BlEry M. Hepatic venous pressure gradients measured by duplex ultrasound. Clin Radiol. 2002;57:746-752. [PubMed] |
| 22. | Imam MH, Lindor KD. The natural history of primary biliary cirrhosis. Semin Liver Dis. 2014;34:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Floreani A, Franceschet I, Cazzagon N. Primary biliary cirrhosis: overlaps with other autoimmune disorders. Semin Liver Dis. 2014;34:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 25. | Kirstein MM, Metzler F, Geiger E, Heinrich E, Hallensleben M, Manns MP, Vogel A. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology. 2015;62:1524-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Said E, Said SA, Gameil NM, Ammar EM. Modulation of thioacetamide-induced liver fibrosis/cirrhosis by sildenafil treatment. Can J Physiol Pharmacol. 2013;91:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Choi SM, Shin JH, Kim JM, Lee CH, Kang KK, Ahn BO, Yoo M. Effect of udenafil on portal venous pressure and hepatic fibrosis in rats. A novel therapeutic option for portal hypertension. Arzneimittelforschung. 2009;59:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Cohen-Naftaly M, Friedman SL. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap Adv Gastroenterol. 2011;4:391-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |