Published online Aug 7, 2018. doi: 10.3748/wjg.v24.i29.3222
Peer-review started: May 4, 2018
First decision: May 17, 2018
Revised: May 31, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: August 7, 2018
Processing time: 95 Days and 1.6 Hours
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive diseases and is characterized by high chemoresistance, leading to the lack of effective therapeutic approaches and grim prognosis. Despite increasing understanding of the mechanisms of chemoresistance in cancer and the role of ATP-binding cassette (ABC) transporters in this resistance, the therapeutic potential of their pharmacological inhibition has not been successfully exploited yet. In spite of the discovery of potent pharmacological modulators of ABC transporters, the results obtained in clinical trials have been so far disappointing, with high toxicity levels impairing their successful administration to the patients. Critically, although ABC transporters have been mostly studied for their involvement in development of multidrug resistance (MDR), in recent years the contribution of ABC transporters to cancer initiation and progression has emerged as an important area of research, the understanding of which could significantly influence the development of more specific and efficient therapies. In this review, we explore the role of ABC transporters in the development and progression of malignancies, with focus on PDAC. Their established involvement in development of MDR will be also presented. Moreover, an emerging role for ABC transporters as prognostic tools for patients’ survival will be discussed, demonstrating the therapeutic potential of ABC transporters in cancer therapy.
Core tip: Pancreatic cancer is one of the deadliest cancers due to its highly aggressive biology and resistance to broad range of therapeutics. Expression of ATP-binding cassette (ABC) transporters by cancer cells is one of the main mechanisms responsible for the lowered drug accumulation. However, the attempts made in multidrug resistance reversal by the inhibition of their activity have not provided satisfactory results in clinical trials. Nevertheless, current knowledge on the role played by ABC transporters in carcinogenesis beyond chemoresistance, could create the opportunity for the development of novel, direct targeted therapeutic strategies. Additionally, the association between ABC transporters expression and pancreatic ductal adenocarcinoma patients’ prognosis and response to applied therapies confirms their pharmacological potential.
- Citation: Adamska A, Falasca M. ATP-binding cassette transporters in progression and clinical outcome of pancreatic cancer: What is the way forward? World J Gastroenterol 2018; 24(29): 3222-3238
- URL: https://www.wjgnet.com/1007-9327/full/v24/i29/3222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i29.3222
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal diseases in western world. Although not one of the leading causes of death, PDAC is certainly to be considered amid the most unfavourable cancers, ranking at 4th place in terms of death rate, with a 7%-8% chance of 5-year survival in United States[1]. Despite the progress made in understanding the biology and in the treatment of different cancer types, the mortality of PDAC patients still nearly equals its incidence and has not changed remarkably for the last few decades. The dismal prognosis of PDAC is the result of multiple factors including an aggressive nature, chemo- and radio-resistance and the lack of effective treatments and diagnostic tools. Therefore, when diagnosed, the vast majority of PDAC patients present with metastatic disease, not susceptible for surgery[2]. Only one fifth of the patients have the tumour resected and, unfortunately, most of them eventually relapse. Post-operative chemo- and radiotherapy are usually applied in order to delay tumour recurrence; nevertheless, high resistance and the heterogeneous nature of pancreatic tumours impede its treatment[3].
Pancreatic cancer pathology is a multistep process. It arises as an accumulation of abnormalities, both genetic and physiological, progressing through 3 stages of precursor lesions called pancreatic intraepithelial neoplasias (PanINs) before transforming into a fully differentiated tumour[4]. The substantial number of genetic modifications and consequent dysregulation of the wide range of essential signalling pathways accompanying these processes make PDAC highly heterogeneous[5]. Also, the variability of mutations between patients as well as within the same tumour contributes to its high resistance to applied therapy. High heterogeneity of PDAC is expressed also phenotypically. Genetically diverse subclones, possessing different metabolic and functional characteristics, exist within a tumour. Recent evidence shows that one of the populations acquires characteristics similar to stem cells, which enables it to survive during stressful conditions and is partly responsible for cancer relapse after treatment[6]. Furthermore, PDAC cell plasticity, which plays a role in epithelial to mesenchymal transition (EMT), facilitates metastatic spread and adds to the dismal prognosis[7]. Moreover, one of the main characteristics of PDAC, responsible for therapies’ failure, is the formation of dense desmoplastic reaction, influencing cancer progression and impeding drug delivery to the tumour[8]. The interplay between tumour cells and stromal components (pancreatic stellate cells (PSCs), immune cells, cytokines or extracellular matrix proteins) influences cell metabolism, drug delivery and distribution. In addition, the existence of a rich tumour microenvironment (TME), influencing cancer cell functions and favouring chemoresistance, has been recently claimed to be an essential factor in cancer stem cell initiation and promotion[9].
On account of PDAC aggressive nature and its resistance to therapies, no successful treatment has been introduced so far[10]. In fact, until recently the gold standard in PDAC treatment was gemcitabine. Applied as a first line therapy drug since 1997, gemcitabine modestly improved patients’ perspectives, increasing overall survival (OS) for 6 mo compared to previously used fluorouracil (5-FU)[11]. Since that time, attempts have been made to increase the efficacy of PDAC treatment and prolong patient survival; however, only modest or statistically insignificant improvements have been achieved so far. In the last years, two new drug regimens, ABRAXANE and FOLFIRINOX have been introduced[12,13]. However, their application did not increase OS to a meaningful degree when compared to gemcitabine, at the same time escalating the frequency of adverse events. Nevertheless, both treatments have obtained FDA approval and currently ABRAXANE combined with gemcitabine is acknowledged as a standard first-line therapy for pancreatic cancer. Considering the high number of genes altered during PDAC progression, targeted therapies emerged as a potential therapeutic tool. Many small inhibitors have been developed as single agents or applied in combination with gemcitabine or ABRAXANE to enhance their efficacy[14-18]. However, the vast majority of them failed to improve patients’ survival in the clinical settings. Therefore, it remains pivotal to gain better knowledge on the mechanisms of PDAC chemoresistance and to find novel therapeutic strategies in order to develop more effective treatment regimens.
Among other factors, the failure of PDAC treatment has been attributed to local recurrence and liver metastasis and importantly, to its high chemoresistance, both intrinsic and acquired. The phenomenon called multi-drug resistance (MDR), which is characterized by resistance to a broad spectrum of structurally diversified compounds, has been confirmed as one of the main reasons for the inefficiency of PDAC therapies, leading to tragic health and economic consequences.
There are multiple factors contributing to the development of MDR in pancreatic cancer, such as decreased drug uptake, accelerated drug metabolism and DNA repair, blocking of apoptotic pathways, metabolic changes and the presence of highly resistant stem-like cells. Also, high heterogeneity of the tumour, dense stroma and hypoxia impairing drug delivery and constitutive activation of several signalling pathways, including K-Ras, PI3K/Akt, Notch or NF-κB, with the latter being additionally enhanced during chemo- and radiotherapy, all confer the modest response of PDAC to applied therapies[19-23]. Moreover EMT, frequently observed in PDAC tumours, has been implicated in conferring its resistance. Also, acquired mutations in targeted genes and reactivation of parallel pathways add to the therapy failing. However, in most cases the interplay between several of these processes is essential for chemoresistance development[24]. Additionally, high expression of transmembrane proteins belonging to the ATP-binding cassette (ABC) transporter family in tumour specimens is one of the major factors contributing to increased drug efflux and has been connected with MDR, adding to the poor response of PDAC to treatments[25-28]. Apart from drug extrusion, as integral membrane constituents, ABC transporters normally regulate the distribution of a wide variety of molecules, influencing different pathways and biological processes, which suggests their more direct impact on cell physiology and possibly, carcinogenesis. Therefore, the understanding of the role of ABC transporters both in healthy physiology and in cancer is crucial for the development of specific, potent and safe inhibitors that might be used in PDAC therapy.
One of the main obstacles in cancer therapy is the resistance, both constitutive and acquired to administered drugs. As aforementioned, one of the processes responsible for drug resistance is the decreased intracellular accumulation of the drugs caused by their efflux from the cells induced by the expression of membrane drug transporters belonging to the ABC family.
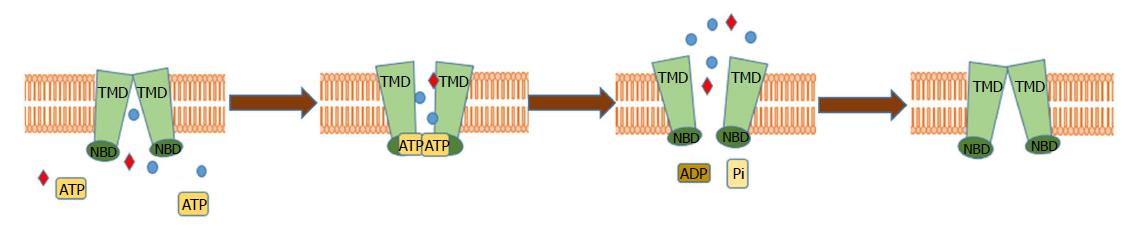
The family of ABC transporters is a highly conserved family of proteins, expressed in all organisms, which implies their relevance in many biological functions. To date, 48 human genes and one pseudogene encoding the members of ABC family have been described and grouped into 7 subfamilies (ABCA-G), based on their sequence and structural similarity[29,30]. ABC transporters are integral transmembrane proteins which, by utilizing energy obtained from ATP hydrolysis, which drives the progressive conformational changes in their domains, shuffle molecules across the plasma and intracellular membranes against their gradient[31,32] (Figure 1). The structure of ABC transporters is highly conserved and consists of two hydrophobic transmembrane domains (TMDs), which form a pore in the membrane creating substrate-binding environment linked to two hydrophilic nucleotide-binding domains (NBDs) localized in the cytosol[33,34]. ABC transporters are reported to export a wide variety of structurally diverse endogenous ligands including amino acids, peptides, vitamins, sugars, hormones, ions, lipids and xenobiotics[26,32,35-37]. For example, ABCB1 has been reported to be able to transport more than 200 structurally diversified molecules[38-41]. Additionally, ABC transporters are known to excrete toxins form kidneys, gastrointestinal tract and liver, demonstrating a protective role in those tissues[42]. Few ABC transporters, e.g., ABCC7- cystic fibrosis transmembrane conductance regulator (CFTR) or ABCC8- the sulphonyl urea receptor (SUR1), are not directly involved in transport of molecules across the membrane but use the ATP hydrolysis to regulate the activity of Cl- and K+ channels respectively[43]. In healthy physiology, ABC transporters are expressed in a wide variety of tissues, mainly associated with biological barriers (Table 1). As an example, ABCC1 is expressed in kidneys, intestine, ovaries, adrenal glands, colon, stomach, testes, lungs and blood-brain barrier and ABCB1 is mostly expressed in gastrointestinal tract, pancreas, kidneys, brain and adrenal glands, where they are involved in diverse physiological functions and in excreting toxins from the cells[40,44,45]. However, their enhanced levels have been found in different cancer types, suggesting the relevance of ABC transporters in cancer and its chemoresistance. So far, 15 of the transporters have been attributed the role of drug pumps, contributing to MDR in vitro[46]. Especially, P glycoprotein (P-gp)/ABCB1, breast cancer resistance protein (BCRP)/ABCG2, multidrug resistance protein 1 (MRP1)/ABCC1 and other members of ABCC subfamily (e.g., ABCC2, ABCC3) have been reported to be responsible for PDAC chemoresistance[47].
| ABC transporter | Tissue expression | Cancer overexpression | Correlation with PDAC survival (5-yr survival) |
| ABCA | |||
| ABCA1 | Lung, colon, liver, brain, testicles | Glioma, lung, testis, liver, colorectal, breast, renal cancer, | H: 21% |
| L: 29% | |||
| ABCA7 | Bone marrow, brain, kidney, colon, lung pancreas | Melanoma, Lung, cervical, stomach, endometrial, colorectal, pancreatic cancer | H: 38% |
| L: 0% | |||
| ABCB | |||
| ABCB1 | Brain, blood-brain barrier, colon, liver, kidney, testis, placenta, small intestine, pancreas | Ovarian, breast, colon, kidney, adrenocortical cancer, AML | H: 34% |
| L: 20% | |||
| ABCB4 | Liver | Liver, lung, renal cancer, melanoma | H: 49% |
| L: 22% | |||
| ABCC | |||
| ABCC1 | Kidney, colon, pancreas, lymph nodes, liver, testis, brain, blood-brain barrier, breasts, spleen, | Breast, lung, ovarian or prostate cancer, neuroblastoma | H: 13% |
| L: 43% | |||
| ABCC2 | Brain, lymph nodes, liver, colon, kidney, lung, testis, breasts, pancreas | Colorectal, liver, lung, gastric cancer | H: 29% |
| L: 27% | |||
| ABCC3 | Pancreas, liver, lymph nodes, lung, adrenal glands, colon, testis, spleen, small intestine | Pancreatic, liver, lung, colorectal, stomach, renal, breast cancer | H: 13% |
| L: 41% | |||
| ABCC4 | Brain, testis, colon, kidney adrenal glands, pancreas, liver, ovary, lung, spleen, breasts, skin, heart | Prostate, renal, lung, breast, ovarian, stomach cancer | H: 32% |
| L: 23% | |||
| ABCC5 | Lymph nodes, pancreas, kidney, testis, brain, colon, liver, heart, muscles | Lung, urothelial, breast, cervical, renal cancer, glioma | H: 34% |
| L: 0% | |||
| ABCG | |||
| ABCG1 | Pancreas, liver, colon, kidney, brain, lung, lymph nodes, testis | Lung, renal, breast, endometrial, prostate, colorectal, cervical, pancreatic cancer, glioma | H: 34% |
| L: 0% | |||
| ABCG2 | Intestine, testis, colon, placenta, liver, kidney, small intestine | Liver, testis, prostate, renal cancer, glioma | H: 32% |
| L: 23% | |||
| ABCG4 | Brain, endocrine, testis, colon, liver, kidney | Glioma, melanoma, thyroid, head and neck, renal, testis, ovarian, endometrial cancer | H:43% |
| L: 23% |
Up to date, most research has been focused on P-gp, a member of the ABCB subfamily of transporters[48,49]. It exports a wide variety of molecules of “amphipathic nature” including anthracyclines, HIV-protease inhibitors, calcium channel blockers, steroid hormones, antibiotics, lipids, taxanes and alkaloids[50,51]. P-gp overexpression has been observed in several cancers including ovarian, colon, kidney or adrenocortical cancer, correlating with poor prognosis[52,53]. Additionally, treatment-induced increase in ABCB1 expression has been noted in acute myeloid leukaemia (AML)[54], breast and high-grade bladder cancer[55]. ABCB1 is known to be responsible for developing drug resistance to neutral and cationic hydrophobic compounds, e.g., to anthracyclines (daunorubicin, doxorubicin), colchicines, taxanes (paclitaxel, docetaxel), vinca alkaloids (e.g., vincristine, vinblastine) and tyrosine kinase inhibitors (imatinib)[56-58].
The main role in xenobiotic transport and drug resistance in many cancers has been attributed to the ABCC subfamily of transmembrane transporters[59], with 9 out of 12 members being involved in MDR[47,59]. The most studied of MDR proteins, ABCC1 (MPR1) has been demonstrated to be expressed in several cancers, including breast, lung, ovarian and prostate cancer, showing the correlation between the expression of ABCC1 and poor patients’ outcome[60]. It has been suggested that ABCC1 expression may confer resistance to methotrexate, vinca alkaloids, anthracyclines and camptothecins[47,61,62], influencing drug resistance in plethora of cancers. Additionally, cyclic nucleotides and their analogues (e.g., gemcitabine) may be transported by ABCC4 and ABCC5[62-64], potentially contributing to their ineffectiveness in PDAC therapy.
Resistance to doxorubicin, mitoxantrone, anthracyclines and topotecan (quinolone topoisomerase inhibitor)[65] has been attributed to ABCG2 transporter[66,67], which functions mainly in the ovaries, brain, liver, prostate, placenta and small intestine[68]. Additionally, increased ABCG2 expression has been reported in pluripotent stem cells, suggesting its role in the maintenance and protection of stem cells[69].
Regardless of the remarkable increase in the knowledge on the ABC transporters structure and MDR induction achieved in the past few decades, the actual function and significance of these proteins is highly underexplored. It is known that in healthy physiology, ABC transporters are involved in drug absorption, distribution and elimination, determining bioavailability of administered drugs. Both apical and basolateral membranes of gastrointestinal tract and biological barriers, in which ABC transporter expression has been demonstrated, need to be penetrated by the drug to reach its target. Therefore, ABC transporters expression may influence pharmacokinetic characteristics of administered chemotherapeutics. Additionally, various other physiological roles have been assigned to ABC transporters such as export of fatty acids, cholesterol, peptides, sterols and xenobiotics. Many ABC transporters are involved in secretion of bioactive molecules and in the transport of signalling lipids, which contribution to cancer progression has been well established. As an example, ABCA1 is involved in reverse cholesterol transport as well as phospholipids transport to plasma membrane[70,71]. Interestingly, recent studies demonstrated ABCC1 as an active player in progression of ovarian and prostate cancer[72,73], by extrusion of lipids (lysophosphatidylinositol, sphingosine 1-phosphate) that have been previously attributed a crucial role in carcinogenesis[73,74]. The changes in cancer cell proliferation, migration, invasion and resistance to apoptosis mediated by the activity of ABC transporters have been also widely documented[75]. Considering that information, attention has been brought to the pivotal role played by ABC transporters in carcinogenesis beyond chemoresistance and to the correlation between their expression with cancer progression and aggressiveness. Nevertheless, this area is still overlooked and more studies need to be focused on this aspect of ABC transporters’ activity in order to fully elucidate their role in cancer.
There have been very limited studies on the role and expression of ABC transporters in pancreatic cancer; however, strong correlation between few of their members and PDAC has been recently suggested. On the basis of mRNA analysis, the expression of ABCC1, ABCC3, ABCC4, ABCC5 and ABCG2 in both pancreatic cancer samples and in healthy pancreas has been demonstrated[76,77] and was correlated with cell resistance to commonly applied chemotherapeutics[78]. At the same time, ABCC6, ABCC8 and ABCC9 could not be detected in any of the studied pancreatic cancer cell lines[79]. Furthermore, more in depth analysis showed that although ABCG2, ABCC1 and ABCC4 levels did not differ significantly between tumour and healthy tissues, ABCC3 and ABCC5 were found to be remarkably overexpressed in PDAC specimens. Moreover, although expression of none of them could be coupled with cancer stage, the differentiation status and tumour grading were related with increased ABCC3 levels and correlated with poor survival, whereas no such correlation could be found for ABCC5.
ABCC3 transporter is involved in transporting of bile salts and organic ions[80,81]. It has been also implicated in mediation of drug resistance, e.g., to vincristine, methotrexate or etoposide; compounds used in clinical studies for PDAC treatments, which demonstrated only marginal effects[82]. Moreover, its expression levels have been correlated with survival of patients after resection, suggesting possible predictive aspect of ABCC3 expression in PDAC.
ABCC5 is involved in transport of nucleotide analogues; therefore, it is tempting to speculate its involvement in excessive efflux of nucleotide analogues-based drugs, such as 5-FU or gemcitabine. In fact, although still controversial, it has been shown that ABCC5 is responsible for gemcitabine resistance in pancreatic cancer[64,79,83]. Analysis of PDAC specimens demonstrated overexpression of ABCC5 transporter in samples resistant to gemcitabine, suggesting its involvement in the decreased efficiency of the drug. Furthermore, exposure of different PDAC cell lines to gemcitabine, as well as 5-FU/gemcitabine combination significantly increased the expression of ABCC5 demonstrating drug induced mechanism of PDAC cell resistance to the treatment[79,84]. Therefore, although not directly associated with PDAC progression, the importance of ABCC5 in PDAC chemoresistance, both inherent and acquired, makes it a valuable drug target for the enhancement of the efficacy of applied therapies.
While the role of ABC transporters in mediating chemoresistance is well established, little is known about their direct, drug-efflux independent contribution to pancreatic cancer progression. Nevertheless, intensive studies in recent years suggest that beyond their role in drug resistance, the biological functions of ABC transporters are more complex. It has been proposed that tumour-promoting functions of ABC transporters are based on their ability to export active signalling molecules and hormones, which by autocrine or paracrine regulation activate cancer cells as well as tumour environment. Increasing interest in this area has demonstrated the significant impact of these proteins on invasion, migration and differentiation of malignant cells[75]. Also, changes in metabolism as well as redox status, characteristics pivotal in PDAC tumorigenesis, may be induced by ABC transporters-released molecules.
One of the major events in PDAC development is the metabolic switch, which occurs in response to decreased nutrient and oxygen supply[85-87]. Increased glucose dependence and use or aerobic glycolysis for energy production, known as Warburg effect, allows quickly proliferating PDAC cells to survive under harsh conditions and is considered as one of the hallmarks of cancer[88]. Additionally, glutamine dependence and increased protein breakdown add to cancer cell high proliferative abilities. However, a small population of cells with stem-like characteristics, which reside the areas of the tumour lacking oxygen and glucose supply, are known to rely on mitochondrial oxidative phosphorylation rather than glycolysis, which results in increased ATP production. This phenomenon may add to increased activity of ABC transporters observed in cancer cells. Therefore, low oxygen and nutrient supply may contribute to PDAC resistance by increase of the ABC transporters levels and their ATP-dependent substrate transport, suggesting a possible mechanism of hypoxia-induced chemoresistance, tumour maintenance and cancer progression.
Apart from glucose and glutamine addiction, increased lipid metabolism and demand has been recently demonstrated for PDAC[89,90]. Bioactive phospholipids are directly involved in the induction of cancer cell proliferation and thereby, cancer progression[91]. Increase in the levels of saturated lipids helps cancer cells to acquire additional resistance to oxidative stress by consolidating the membranes. Both, de novo lipid synthesis and their increased uptake have been reported in PDAC[92,93]. Moreover, enzymes involved in lipolysis and lipogenesis are overexpressed in PDAC and are usually correlated with poor prognosis[90]. It has been demonstrated by our work that, in prostate and ovarian cancer, ABCC1-transported lysophosphatidylinositol activates GPR55 receptor forming an autocrine loop, which activation triggers signalling cascade inducing cell proliferation[72]. Phospholipids transport has been also reported for another member of ABC transporter family, ABCG1. Therefore, it is tempting to suspect the existence of a similar mechanism, involving ABC transporter-mediated phospholipid activation of cancer cells in PDAC. An essential factor in PDAC cell survival is also cholesterol availability. As a component of lipid rafts, it influences membrane composition and integrity and interacts with membrane-bound proteins, facilitating activation of phosphorylation cascades[90]. The essential role played by cholesterol in PDAC tumorigenesis limits the growth and division of PDAC cells, depending on its availability[94]. A recent study by Mohelnikova-Duchonova et al[95] showed an upregulation in transcript levels of several ABC transporters in PDAC compared to non-neoplastic tissues. Particularly, upregulation of 2 members of ABCA family, ABCA1 and ABCA7 involved in cholesterol export, together with expression of ABCG1 transporting phosphatidylserine, phosphatidylcholine and sphingomyelin, suggests their involvement in cellular cholesterol imbalance in the disease[95]. Another of the characteristics of PDAC is the highly inflammatory environment, which actively promotes cancer cell proliferation and survival, angiogenesis and assists the metastatic spread[96]. Chronic inflammation, that aids the tumorigenesis and at the same time is one of the main factors contributing to its initiation, is mediated by prostaglandin-mediated pathways. Therefore, the main inflammatory molecules- prostaglandins and leukotrienes are considered as significant players in PDAC development. The prostaglandin-mediated PDAC progression may involve activation of PI3K-Akt signalling pathway, a major player in PDAC progression, increase in VEGFA expression and stimulation of angiogenesis and support of the inflammatory environment[97]. It is now known that several ABC transporters, mainly belonging to the ABCC subfamily (ABCC1, ABCC2, ABCC4) are involved in prostaglandins efflux outside of the cells, enabling the activation of the G protein-coupled receptors, triggering cancer progression[75,98]. Therefore, the manipulation of ABC transporter activity blocking prostaglandin signalling represents an additional potential therapeutic tool. Additionally, due to the proved contribution of leukotriene C4 (LTC4) to PDAC progression[99], its induced pathways have been widely studied as potential drug targets. Regarding the involvement of ABC transporters in leukotriene release, their inhibition presents an additional possibility for LCT4-signalling blockade, influencing cancer development.
Elevated levels of reactive oxygen species (ROS), inducing oxidative stress are also implicated in PDAC initiation and progression[100]. One of the molecules responsible for the maintenance of redox status in homeostasis is glutathione (GSH)[101], which transport is activated in response to oxidative stress. It is also involved in several signalling processes regulating cell proliferation, apoptosis or immune response. Several members of ABCC family (ABCC1, ABCC2, ABCC3, ABCC4, ABCC5, ABCC7) and ABCG2 mediate glutathione transport, suggesting their involvement in cellular response to the oxidative stress. Also, ABCB10 has been implicated in cellular protection from oxidative stress[102]. Moreover, oxidative stress induces the activation of NF-κB and Nrf2 signalling, which in turn enhances expression of ABCB1, ABCG2 and ABCC2, additionally contributing to cancer cell resistance[103,104]. Therefore, manipulation of the activity of ABC transporters in cancer cells might potentially increase their antioxidant capacity, which has been shown to provide additional anti-tumorigenic protection[105].
Additionally, tumour environment and its engagement in cancer progression and metastatic spread has emerged as key player in carcinogenesis. Considering the significant role of stroma in PDAC progression and in the development of tumour chemoresistance, targeting its components presents a tempting approach in the development of novel therapies. However, the attempts to deplete stroma have not provided satisfactory results so far. The most promising combination of gemcitabine and Hedgehog inhibitor IPI-926-03 tested by Olive et al[106] has failed due to high toxicity and lack of effectiveness in clinical trials[107]. Currently, molecules targeting hyaluronic acid, combined with chemotherapy, are being tested in phase II and III clinical trials[108]. Nevertheless, investigation of new approaches to target stroma in order to increase chemotherapy efficiency, as well as restraining tumour expansion remains essential. Recently, expression of several of ABC transporters in PDAC stroma has been reported. One of the main stromal components- macrophages- have been demonstrated to express several of the drug transporters, inter alia ABCC1 and ABCC3, contributing to both chemoresistance and tumour progression[109]. Therefore, considering the involvement of ABC transporters in chemoresistance and an emerging role in tumorigenesis, therapies targeting ABC transporters might prove to be useful in depleting or reprograming cancer stroma and reversing cancer resistance to applied drugs. Additionally, expression of few of ABC transporters in non-neoplastic tissues has been recently reported to influence PDAC progression and to be predictive of patients’ overall response.
Finally, the most aggressive tumours are composed of non-differentiated cells possessing highly proliferative abilities[75], called cancer stem cells (CSCs). In particular, the existence and high importance of CSCs in cancer resistance to chemotherapy and its involvement in disease recurrence has been suggested for PDAC. Interestingly, high expression of ABC transporters has been reported in less differentiated tumour zones, conferring them a more aggressive phenotype[110-112], also in PDAC[76]. Therefore recently, the interest in CSCs as drivers of resistance and aggressive nature has emerged in PDAC[113,114]. A noticeable characteristic of cancer stem cells is the high expression of members of the ABC transporters family compared to more differentiated cells[115]. Also, it is speculated that their expression profile may be considered as the indicator of stem cell formation and carcinogenic potential of the tissue[116]. Considering the association of cell differentiation levels with its proliferative potential, the overexpression of ABC transporters in cancer stem cells highly supports their contribution to the more aggressive nature of the PDAC. Overexpression of ABC transporters in cancer stem cells may assist in their survival by efflux of xenobiotics, exhibiting protective roles, sustaining their proper performance and maintaining self-renewal characteristics. Additionally, their enhanced expression and activity in cancer cells and especially in CSCs, suggests an additional role in maintaining cancer cells aggressive biology and makes them an attractive therapeutical target.
Although the investigation on the role of ABC transporters in PDAC is still in its outset, the initial analysis suggests their probable contribution to PDAC development and points at potential beneficial clinical consequences. Database analysis showed that the high importance and the potential of ABC transporters as pharmacological targets in PDAC is reflected in the association of the expression of its individual members with the prognosis of patients’ survival[117]. Notable correlation between observed 5-year survival and expression of a majority of ABC transporters has been observed (Table 1); however, this discovered association is not uniform. Significant reduction in survival probability has been attributed to high expression of e.g., ABCA1, ABCA12, ABCB1, ABCC1, ABCC3 or ABCC7. Expression of few other ABC transporters showed similar trend, nonetheless, their relationship with the OS was not remarkably pronounced. On the other hand, higher expression of a substantial number of ABC transporter genes has been correlated with increased chance of PDAC patients’ survival. Among others, the expression of ABCA2, ABCA7, ABCB6 ABCB8, ABCC5 or AGCG1 in PDAC tissues most markedly correlated with prolonged 5-year survival, suggesting their-mediated release of molecules of anti-tumorigenic characteristics and favourable prognostic potential.
Considering the elevated expression of multiple ABC transporters in a vast majority of cancers and their redundancy in substrate specificity and activity, determination of their expression profiles and their clustering in prognostic groups, rather than analysis of individual members, also raised a lot of interest in the last years. The existence of ABC transporters expression signatures in PDAC and their correlation with clinic-pathological characteristics of the tumours has been studied by Mohelnikova-Duchonova et al[95], and dysregulation of expression of several members of ABC family has been observed. Upregulation of ABCB4, ABCB11, ABCC1, ABCC3, ABCC5, ABCC10 and ABCG2 has been noted in PDAC, compared to non-neoplastic tissues. Surprisingly however, expression of few ABC transporters in non-neoplastic tissues also could be correlated with tumour progression and survival. Moreover, higher levels of T3 and T4 stages were associated with ABCA1 and ABCB3 upregulation and ABCG1 and ABCG2 downregulation. In contrast, smaller size tumours were connected with the cluster, in which ABCA8, ABCB5, ABCA9, ABCA10 and ABCC9 were upregulated, while downregulation of ABCA12, ABCA13, ABCC3, ABCC7 and ABCC13 has been noted. Similarly, ABCB9 and ABCC4 upregulation correlated with N1 status, while ABCA3, ABCD1 overexpression and ABCA6 and ABCC10 downregulation corresponded with increased angioinvasion.
This and previous studies demonstrated the correlation of ABC transporter expression in tumour specimens with clinic-pathological features in different cancer types[118]. Nevertheless, the high importance of tumour microenvironment and its proposed involvement in PDAC progression, suggests that ABC transporter expression in non-neoplastic tissues might have important clinical implications. Following the analysis of 27 non-neoplastic pancreatic tissues and pairing them with 32 PDAC samples, 4 different clusters could be distinguished based on the gene expression profiles in cancer vs normal specimens. PN1 and PN2 clusters were characterized by upregulation of the majority of ABC transporters genes and correlated with significantly shorter patients’ overall survival (OS) than patients grouped into PN3 and PN4 clusters, in which significant downregulation of genes or heterogeneous gene expression has been observed[119]. Especially, ABCA2, ABCA4, ABCA5, ABCC2 and ABCD4 signatures showed significant difference in patients’ survival when comparison between upregulated and downregulated genes was carried out. Additionally, tumour-node-metastasis, age, gender, disease stage, margin status, therapy and survival have been analysed; however, no significant correlation between those features and ABC profiles could be established. Although the study presented few limitations, such as small group size or the distance between collected tumours and control tissue, created expression clusters could be successfully implemented into clinical practice. Moreover, reduction of the analysed genes to the limited group showing most distinct expression, did not have any impact on the statistical significance of observed clinic-pathological correlations, creating more practical and convenient clinical prognostic tools.
Looking at the key role played by ABC transporters in cancer chemoresistance and the emerging knowledge on their crucial contribution to tumorigenesis, the development of targeted therapies, aiming to block or modulate their activity has become a crucial area in cancer research. Inhibition of transporter activity, arrest of the transcription factors regulating their expression or blockade of the transporter-induced signalling pathways represent the options for impeding ABC transporters activity[120]. So far, 3 generations of ABC transporters modulators, directed mainly against ABCB1, have been developed[120,121] (Table 2). The first generation inhibitors, such as verapamil, quinine or cyclosporine A, compounds previously established for other conditions, in spite of promising in vitro activity[122,123], showed significant toxicity, unacceptable for further usage[123,124]. Lack of potency and specificity, combined with pharmacokinetic complications restrained their further investigation[125]. Structural modifications of existing inhibitors, aiming to enhance their efficacy and specificity, at the same time decreasing observed adverse effects, also did not provide satisfactory results. Valspodar (cyclosporine A derivative), a second generation ABCB1 inhibitor, demonstrated enhanced efficiency accompanied by decreased toxicity[126]. However, it showed unsatisfactory results in the majority of clinical trials, in which its co-administration with chemotherapeutics, e.g., carboplatin, paclitaxel or doxorubicin did not exhibit any benefits, and in some cases deteriorated patients’ outcome[127,128]. Likewise, application of dofequidar or biricodar citrate (VX-710)[129] did not result to be favourable, as their use has been restricted by the potential interactions with anti-cancer therapeutics (vincristine or paclitaxel)[130]. All these limitations led to the development of a third generation of inhibitors which potency, due to the rational QSAR design, has been described as 200-fold higher than the previously developed anti-ABCB1 molecules, greatly enhancing drug accumulation[131]. Additionally, only minimal drug-drug interactions have been reported. Clinical trials have been commenced for zosuquidar (LY335979)[132], elacridar (F12091)[133], mitotane (NSC-38721)[134], annamycin[135] or tarquidar (XR9576)[136]. Nevertheless disappointingly, most of the clinical trials testing their applicability have been discontinued due to lack of significant positive response and off-site effects.
| ABC transporter | Inhibitor | MDR | Ref. |
| ABCB1 | I generation:Cyclosporine A, Verapamil | Daunorubicin, epirobicin, doxorubicin, colchicines, paclitaxel, docetaxel, vincristine, vinblastine, imatinib | [46,145,146,171] |
| II generation: Valspodar, zosuquidar | |||
| III generation: Tariquidar, OC144-093 | |||
| ABCC1 | MK571, probenecid, ibrutinib, 3ATA | Anthracyclines, vinca alkaloids, camptothecins, daunorubicin, imatinib, etoposide, vincristine, vinblastine, methotrexate | [46,145,146,172] |
| ABCC2 | Metothrexate, cyclosporine A, fluorescein, MK571 | Doxorubicin, cisplatin, irinotecan, epirubicin, vinblastine | [46,145,146,171,173] |
| ABCC3 | Indomethacin, sufinpyrazone, probenecid, benzmromarone | Etoposide, methotrexate, teniposide | [46,145,146,171,173] |
| ABCC5 | Curcumin, trequensin, sildenafil | Gemcitabine, methotrexate, 6-mercaptopurine | [46,145,146,171,173] |
| ABCG2 | Fumitremorgin C, Ko143, GF120918 | Daunorubicin, doxorubicin, irinotecan, mitoxantrone, methotrexate, epirubicin, etoposide | [46,145,146,171] |
There are several reasons for the lack of success of the ABC transporters inhibition. Increased toxicity caused by off-target action in healthy tissues, as well as their high doses were the main reasons for the discontinuation of the trials for first and second generation inhibitors[42]. Increasing evidence of substrate similarities between ABC transporters and CYP450, enzyme involved in drug metabolism, suggests interactions of tested compounds with the enzyme, which influences pharmacokinetic properties of co-administrated chemotherapeutics, changing their activity, lowering the efficacy and, as a consequence, increasing the toxicity[137]. Therefore single-agent application of ABC transporters inhibitors should be considered in future research. Another reason for high toxicity of these modulators has been attributed to decreased clearance of anticancer agents and natural xenobiotics caused by unspecific blockade of the transporters. As an example, ABCB1 inhibition, apart from cancer cells may also result in its blockade in canalicular membrane in healthy liver or kidney, reducing the clearance of chemotherapeutics[42,138]. The involvement of some of the ABC transporters (mostly ABCB and ABCC subfamilies) in the immune system is another obstacle, as disruption of its proper functioning may result in undesirable deterioration in anti-cancer immune responses[139]. The ineffectiveness of targeted therapies may also lay in the functional redundancy of several ABC transporters, highly impairing full efficiency of the blockade of individual protein. Another limitation in the presented approach has been the fact that the vast majority of studies have been focused on ABCB1. Nevertheless, with increasing evidence of the role of other ABC transporters in cancer, the inhibitors of ABCC1 (e.g., probenecid, sulindac, biricodar, BAY-u9773 or MK571)[129,140-142], ABCG2 (Ko143, fumitremorgin C, genistein, biochanin A)[143,144] or ABCC3 (indomethacin or sulfinpyrazone)[145,146] have been considered (Table 2). However, some of them similarly to ABCB1 blockers, exhibited unfavourable toxicity levels when combined with chemotherapy. Additionally, several non-selective ABCB1 inhibitors have been tested for their activity towards other ABC transporters[146]. Nonetheless, as the interest in ABC transporters increased only recently, the efficacy of the abovementioned therapeutic approach still needs to be evaluated. Also, the majority of the studies conducted so far have been focused on the reversal of chemoresistance rather than influencing cancer progression. However, current knowledge on the additional, or maybe principal role of ABC transporters in tumorigenesis might shed more light on the basis of current inhibitors toxicity as well as could allow for exploration of novel more specific molecules, aiming at slowing down cancer progression, rather than reversing MDR.
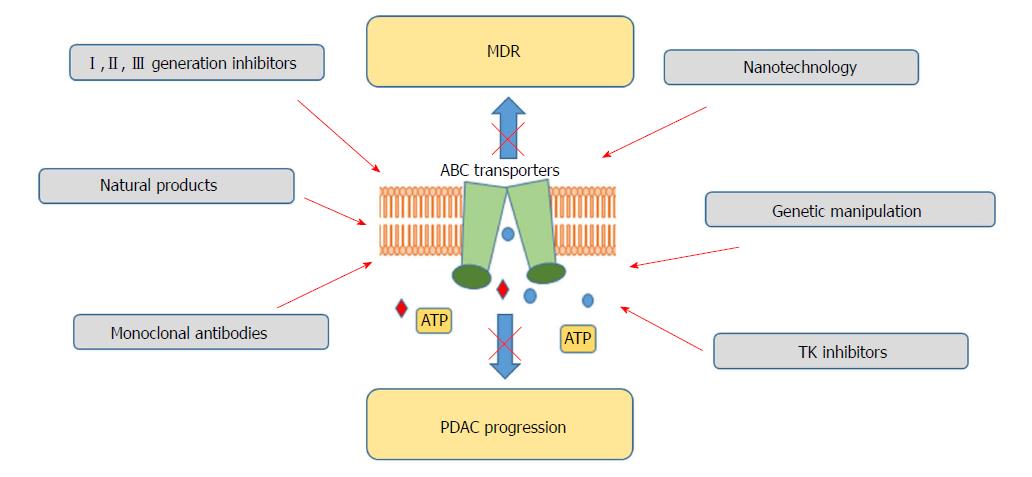
Considering the marginal effectiveness of ABC transporters inhibitors achieved so far, alternative concepts for ABC transporters targeting are being tested (Figure 2). RNA interference, use of monoclonal antibodies, antisense oligonucleotides or the use of transcription regulators is currently under consideration[147-150]. miRNA use has been also claimed as a possible way for ABC transporter regulation and reversal of chemoresistance[151,152]. As crucial players in carcinogenesis, also confirmed in PDAC, miRNA regulation has been proposed as an interesting therapeutic tool[153]. To date, several miRNAs have been reported to inhibit the expression of different ABC transporters, having chemotherapeutic effects[154-156]. Moreover, it has been demonstrated that tyrosine kinase inhibitors may block ABC transporters by binding to their transmembrane domain at substrate-binding sites[157]. Imatinib, nilotinib, sunitinib or lapatinib, drugs tested for the PDAC therapy independently of their ABC-inhibiting properties, have been demonstrated to block ABCB1, ABCC2 or ABCC10[158-161]. However, this approach also needs further evaluation.
Currently, the use of nanoparticles for the delivery of therapeutics to the target cells has emerged as a growing area of interest[162,163]. Their small size, together with increased surface area, enhances the stability and solubility of the administered drugs, improving their bioavailability[164]. Additionally, controlled, prolonged release and protection form degradation present further advantages of that therapeutic approach. Co-delivery of the inhibitors of ABC transporters and chemotherapeutics with the use of nanoparticles is also applied to minimize observed side effects occurring as a result of drug-drug interactions. Nevertheless, the emerging field of the manipulation of ABC transporter activity for therapeutic purposes is still in its outset and more studies are needed to fully assess their pharmacological potential.
In the last years, ABC transporters have attracted remarkable attention of researchers from different scientific areas. The role of ABC transporters in different physiological and pathological conditions, including cancer, has been widely reported, increasing the interest in the development of their specific inhibitors. Especially, the well-known involvement of ABC transporters in the development of multi-drug resistance (MDR) led to the investigation of the potential of its reversal by blocking ABC transporter activity. Clinical relevance of several ABC transporters in multi-drug resistance reversal has been primarily attributed to P-gp, ABCG2, ABCB4 and 4 members of ABCC subfamily- ABCC1, ABCC2, ABCC3 and ABCC4[165]. Therefore, the main focus so far has been placed on these proteins in terms of their pharmacological potential. However, in spite of the initial enthusiasm regarding ABCB1 inhibitors, their efficacy in clinical settings has failed to provide any improvements, leading to the early closure of the trials[166,167]. Considerably high toxicity caused by lack of specificity and changes in pharmacokinetic of co-applied chemotherapeutics, decreasing their efficacy were some of the reasons for the disappointing results[168]. The successful implementation of developed inhibitors was strongly impeded by the complexity of ABC transporters functioning. The correlation between cancer chemoresistance and ABC transporters expression is two-sided and forms a specific loop, which may increase cancer resistance to applied therapies. On one hand, their expression contributes to enhanced drug efflux from the cells, diminishing their efficacy, on the other hand, many studies have reported increased expression of the transporters, induced by drugs application, complementing formed loop. Therefore, in spite of the enhancement of drug accumulation and reversal of induced chemoresistance demonstrated in vitro, little success has been reported during clinical trials. Also, increased toxicity and insufficient potency observed during clinical trials restrained the majority of tested compounds from the clinical use. Additionally, the majority of carried clinical trials were performed on patients previously treated with several anticancer therapeutics. Therefore, the assessment of the protein levels might have been misevaluated due to drug-induced enhancement of expression of ABC transporters. Moreover, several of the studies were designed without proper patient stratification for ABC transporters expression. As an example, little success rate in ovarian cancer patients, might be explained by low expression rate of P-gp in this tumour type[127]. Although reversal of the drug resistance was the principal goal of ABC-targeted therapies, considering the increasing awareness of the pivotal role of ABC transporters beyond chemoresistance, their specific inhibition might not only aid to increase the activity of other therapeutics, but directly balk tumour development and progression[56], encouraging their further exploration. Therefore, the repertoire of ABC transporters against which inhibitors are being developed should be expanded for those playing an active role, not only in MDR, but in the expulsion of bioactive molecules. Looking at the wide variety of substrates transported by ABC transporters, together with their increased expression in cancer cells and especially cancer stem cells, the role of these proteins in the transport of signalling molecules, which activity promotes cancer progression, has become an area of interest. High impact of bioactive lipids, including phospholipids, sphingolipids or cholesterol on PDAC tumorigenesis and an emerging role of ABC transporters in their release presents a novel opportunity for targeting the disease. It has been previously demonstrated that one of the hallmarks of PDAC is lipid-dependence and that the decrease of the lipids levels may reduce cancer progression. Accordingly, aiming to block specific ABC transporters responsible for their extrusion, mainly members of ABCA and ABCC subfamilies, and depriving cancer cells of the necessary fuel may highly contribute to slowing down PDAC development. In fact, it has been demonstrated in several cancers that targeting of ABC transporters involved in lipid transport (e.g., ABCC1 in prostate or ovarian cancer or ABCC4 in neuroblastoma) showed significant improvement in in vitro and in vivo models[75], slowing down cancer progression. Therefore, single-agent therapies based on ABC transporter inhibition should be considered to target cancer progression. Moreover, patients’ treatment with ABC transporters single inhibitors would eliminate the risk of drug-drug interactions, reducing the risk of adverse events.
Importantly, the expression of ABC transporters may not only be explored in terms of their pro-tumorigenic activity but may also serve as prediction of therapy efficiency and patients’ outcome. Database analysis demonstrated strong influence of the expression of the transporters e.g., ABCC3 or ABCC1 on reported 5-year survival. However, positive association of other transporters (e.g., ABCC5 or ABCA7) with the increased survival demonstrates the complexity of the role of ABC transporters in PDAC tumorigenesis. It also shows the necessity for enhanced research in this area to fully understand and explore the therapeutic potential of these transmembrane proteins in PDAC therapy. The enhancement of chemotherapy efficacy, e.g., by ABCC5 blocking, has been demonstrated for the gemcitabine-based therapies. However, considering the favourable association of this transporter with PDAC patients’ survival, it is tempting to speculate that its inhibition might interfere with some of the protective functions that ABCC5 might exhibit and, as a consequence, deteriorate patients’ outcome. Also, despite being overexpressed in a majority of cancer types, the role of ABC transporters is not uniform. Negative or positive correlation of the protein expression and survival observed in PDAC patients, is not invariably reflected in other cancer types. As an example ABCA7, expressed at a similar level in pancreatic and lung cancer, although positively correlated with 5-year survival in the PDAC (38% high expression vs 0% low expression), has no statistically significant effect in the latter case (48% vs 43%)[117]. Therefore, studying the context accompanying ABC transporters expression and functioning is of high importance in order to stratify their individual members in context of their pharmacological potential in diverse cancers. Additionally, the focus of research should not be placed only on the potential of the inhibition of ABC transporters that have undermining roles in carcinogenesis. Hence, the investigation of the characteristics of the ABC transporters that favour the survival of PDAC patients should be also explored to study the mechanisms and molecules responsible for their protective function.
Finally, ABC transporters profiling in cancer has proven to provide a potent tool in estimation of patients’ response to applied therapies. As an example, analysis of 21 breast cancer specimens before and after neoadjuvant treatment showed different expression of several ABC transporters[169]. Similarly, 6 ABC transporters genes in AML samples allowed for their organization in two expression groups, correlated with resistance and patients’ prognosis[170]. Correspondingly, generation of ABC transporter expression profiles in PDAC has allowed for creation of clusters, characterized by differentiated expression of their individual members. Correlation of each cluster with a variety of disease parameters (e.g., number of metastases or drug response) and more importantly, with patients’ survival suggested the gene profiling for ABC transporters expression as a clinically relevant prognostic tool.
Although a lot of advancement has been achieved in the identification of new druggable targets involved in PDAC progression and chemoresistance, no significant improvement in transferring that knowledge into clinical practice has been accomplished, leaving PDAC patients with grim prognosis. As critical players in PDAC chemoresistance and disease development, ABC transporters seem a promising target for the development of novel targeted therapies. However, despite their remarkable pharmacological potential demonstrated in vitro, acquired knowledge has not been successfully implemented in the clinic yet. Nevertheless, the knowledge learnt from previous mistakes and the potential reasons for the failed implementation of the inhibitors should be considered in the development of new studies and treatments. In the light of recent data, the potential of few ABC transporters beyond MDR reversal should be further explored to fully scrutinize the applicability of ABC transporter inhibition for clinical practice. More emphasis on the ABC transporters involvement in PDAC progression should be placed in prospective studies, leading to the determination of the proteins with the most pharmacological potential followed by design of single-agent treatment. The knowledge on the involvement of ABC transporters in cancer metabolic swift, their role in tumour-microenvironment cross-talk should be additionally expanded. Animal models of pancreatic cancer should be implemented in the development of new potential inhibitors to investigate their impact on abovementioned processes. In conclusion, proper study design and patients stratification regarding ABC transporters expression leading to tailored therapies should be elucidated in order to add to the efficiency of administered drugs.
The authors acknowledge the infrastructure and staff support provided by the School Of Pharmacy and Biomedical Sciences and CHIRI, Faculty of Health Sciences Curtin University. MF is supported by Avner Pancreatic Cancer Foundation and by Keith and Ann Voughan Foundation. AA is supported by the Curtin International Postgraduate Research Scholarship (CIPRS)/Health Sciences Faculty International Research Scholarship (HSFIRS).
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Muhammad JS, Tang Y, Zhang Q S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | ASCO. org CN. Pancreatic Cancer: Statistics. Available from: https://www.cancer.net/cancer-types/pancreatic-cancer/statistics. |
| 2. | Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 442] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 3. | Vaccaro V, Sperduti I, Vari S, Bria E, Melisi D, Garufi C, Nuzzo C, Scarpa A, Tortora G, Cognetti F. Metastatic pancreatic cancer: Is there a light at the end of the tunnel? World J Gastroenterol. 2015;21:4788-4801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 5. | Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253-5260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Wang S, Huang S, Sun YL. Epithelial-Mesenchymal Transition in Pancreatic Cancer: A Review. Biomed Res Int. 2017;2017:2646148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1024] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 9. | Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 399] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 10. | Adamska A, Domenichini A, Falasca M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci. 2017;18:pii: E1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 428] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 11. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4174] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 12. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5628] [Article Influence: 402.0] [Reference Citation Analysis (1)] |
| 13. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4877] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 14. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2774] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 15. | Li Y, Sun J, Jiang Z, Zhang L, Liu G. Gemcitabine and S-1 combination chemotherapy versus gemcitabine alone for locally advanced and metastatic pancreatic cancer: a meta-analysis of randomized controlled trials in Asia. J Chemother. 2015;27:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 17. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 18. | Momenta Pharmaceuticals Inc. M402 in combination with nab-Paclitaxel and gemcitabine in pancreatic cancer. 2017;ClinicalTrials.gov [Internet]. Avaliable from: https://clinicaltrials.gov/ct2/show/NCT01621243. Accessed on: 15.05.2017. |
| 19. | Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1455] [Cited by in RCA: 1581] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 20. | DI C, Zhao Y. Multiple drug resistance due to resistance to stem cells and stem cell treatment progress in cancer (Review). Exp Ther Med. 2015;9:289-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Durand RE, Olive PL. Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods Cell Biol. 2001;64:211-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2012;64:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Gillet JP, Gottesman MM. Overcoming multidrug resistance in cancer: 35 years after the discovery of ABCB1. Drug Resist Updat. 2012;15:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 4146] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 26. | Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 2822] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 27. | Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cell Mol Life Sci. 2008;65:3145-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 28. | Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr Drug Targets. 2011;12:621-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 534] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 31. | Locher KP. Structure and mechanism of ABC transporters. Curr Opin Struct Biol. 2004;14:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda). 2007;22:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 33. | Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 831] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 34. | Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1547] [Cited by in RCA: 1520] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 35. | Albrecht C, Viturro E. The ABCA subfamily--gene and protein structures, functions and associated hereditary diseases. Pflugers Arch. 2007;453:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Takahashi K, Kimura Y, Nagata K, Yamamoto A, Matsuo M, Ueda K. ABC proteins: key molecules for lipid homeostasis. Med Mol Morphol. 2005;38:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 464] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 38. | Esser L, Zhou F, Pluchino KM, Shiloach J, Ma J, Tang WK, Gutierrez C, Zhang A, Shukla S, Madigan JP. Structures of the Multidrug Transporter P-glycoprotein Reveal Asymmetric ATP Binding and the Mechanism of Polyspecificity. J Biol Chem. 2017;292:446-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Callaghan R. Providing a molecular mechanism for P-glycoprotein; why would I bother? Biochem Soc Trans. 2015;43:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 41. | Didziapetris R, Japertas P, Avdeef A, Petrauskas A. Classification analysis of P-glycoprotein substrate specificity. J Drug Target. 2003;11:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Ueda K. ABC proteins protect the human body and maintain optimal health. Biosci Biotechnol Biochem. 2011;75:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Soucek P, Hlavac V, Elsnerova K, Vaclavikova R, Kozevnikovova R, Raus K. Whole exome sequencing analysis of ABCC8 and ABCD2 genes associating with clinical course of breast carcinoma. Physiol Res. 2015;64 Suppl 4:S549-S557. [PubMed] |
| 44. | Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 752] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 45. | Sarkadi B, Homolya L, Szakács G, Váradi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 559] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 46. | Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1063] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 47. | Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537-7552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 442] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 48. | Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2204] [Cited by in RCA: 2221] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 49. | Riordan JR, Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979;254:12701-12705. [PubMed] |
| 50. | Sharom FJ, Liu R, Qu Q, Romsicki Y. Exploring the structure and function of the P-glycoprotein multidrug transporter using fluorescence spectroscopic tools. Semin Cell Dev Biol. 2001;12:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Borgnia MJ, Eytan GD, Assaraf YG. Competition of hydrophobic peptides, cytotoxic drugs, and chemosensitizers on a common P-glycoprotein pharmacophore as revealed by its ATPase activity. J Biol Chem. 1996;271:3163-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Callaghan R, Crowley E, Potter S, Kerr ID. P-glycoprotein: so many ways to turn it on. J Clin Pharmacol. 2008;48:365-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Penson RT, Oliva E, Skates SJ, Glyptis T, Fuller AF Jr, Goodman A, Seiden MV. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol Oncol. 2004;93:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Han K, Kahng J, Kim M, Lim J, Kim Y, Cho B, Kim HK, Min WS, Kim CC, Lee KY. Expression of functional markers in acute nonlymphoblastic leukemia. Acta Haematol. 2000;104:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Chung HC, Rha SY, Kim JH, Roh JK, Min JS, Lee KS, Kim BS, Lee KB. P-glycoprotein: the intermediate end point of drug response to induction chemotherapy in locally advanced breast cancer. Breast Cancer Res Treat. 1997;42:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr Drug Targets. 2006;7:893-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Avendaño C, Menéndez JC. Inhibitors of multidrug resistance to antitumor agents (MDR). Curr Med Chem. 2002;9:159-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci USA. 1987;84:3004-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 764] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 59. | Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1245] [Cited by in RCA: 1209] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 60. | Berger W, Setinek U, Hollaus P, Zidek T, Steiner E, Elbling L, Cantonati H, Attems J, Gsur A, Micksche M. Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: prognostic implications. J Cancer Res Clin Oncol. 2005;131:355-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Zurita AJ, Diestra JE, Condom E, García Del Muro X, Scheffer GL, Scheper RJ, Pérez J, Germà-Lluch JR, Izquierdo MA. Lung resistance-related protein as a predictor of clinical outcome in advanced testicular germ-cell tumours. Br J Cancer. 2003;88:879-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Kruh GD, Zeng H, Rea PA, Liu G, Chen ZS, Lee K, Belinsky MG. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr. 2001;33:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Schuetz JD, Connelly MC, Sun D, Paibir SG, Flynn PM, Srinivas RV, Kumar A, Fridland A. MRP4: A previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 401] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 64. | Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 65. | Noguchi K, Katayama K, Mitsuhashi J, Sugimoto Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 2009;61:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118-E133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 67. | Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665-15670. [PubMed] |
| 68. | Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337-5339. [PubMed] |
| 69. | Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 547] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 70. | Santamarina-Fojo S, Remaley AT, Neufeld EB, Brewer HB Jr. Regulation and intracellular trafficking of the ABCA1 transporter. J Lipid Res. 2001;42:1339-1345. [PubMed] |
| 71. | Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000;11:253-260. [PubMed] |
| 72. | Ruban EL, Ferro R, Arifin SA, Falasca M. Lysophosphatidylinositol: a novel link between ABC transporters and G-protein-coupled receptors. Biochem Soc Trans. 2014;42:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Piñeiro R, Maffucci T, Falasca M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene. 2011;30:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 74. | Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394-16399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 75. | Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 800] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 76. | König J, Hartel M, Nies AT, Martignoni ME, Guo J, Büchler MW, Friess H, Keppler D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer. 2005;115:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193-201. [PubMed] |
| 78. | Hagmann W, Jesnowski R, Faissner R, Guo C, Löhr JM. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Hagmann W, Faissner R, Schnölzer M, Löhr M, Jesnowski R. Membrane drug transporters and chemoresistance in human pancreatic carcinoma. Cancers (Basel). 2010;3:106-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3). J Biol Chem. 1999;274:15181-15185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 81. | Zeng H, Liu G, Rea PA, Kruh GD. Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res. 2000;60:4779-4784. [PubMed] |
| 82. | Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA. 1999;96:6914-6919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 450] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 83. | Hagmann W, Jesnowski R, Löhr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Nambaru PK, Hübner T, Köck K, Mews S, Grube M, Payen L, Guitton J, Sendler M, Jedlitschky G, Rimmbach C. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos. 2011;39:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Falasca M, Kim M, Casari I. Pancreatic cancer: Current research and future directions. Biochim Biophys Acta. 2016;1865:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 86. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47059] [Article Influence: 3361.4] [Reference Citation Analysis (5)] |
| 87. | Sousa CM, Kimmelman AC. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 2014;35:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 88. | Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1565] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 89. | Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1227] [Cited by in RCA: 1522] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 90. | Swierczynski J, Hebanowska A, Sledzinski T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J Gastroenterol. 2014;20:2279-2303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 140] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 91. | Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci USA. 2013;110:8882-8887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 573] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 92. | Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117-8126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 545] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 93. | Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 588] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 94. | Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112:2473-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 95. | Mohelnikova-Duchonova B, Brynychova V, Oliverius M, Honsova E, Kala Z, Muckova K, Soucek P. Differences in transcript levels of ABC transporters between pancreatic adenocarcinoma and nonneoplastic tissues. Pancreas. 2013;42:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 96. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8317] [Article Influence: 489.2] [Reference Citation Analysis (0)] |
| 97. | Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 98. | Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, Wijnholds J, Borst P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA. 2003;100:9244-9249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 397] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 99. | Knab LM, Grippo PJ, Bentrem DJ. Involvement of eicosanoids in the pathogenesis of pancreatic cancer: the roles of cyclooxygenase-2 and 5-lipoxygenase. World J Gastroenterol. 2014;20:10729-10739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 100. | Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1197] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 101. | Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 887] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 102. | Liesa M, Qiu W, Shirihai OS. Mitochondrial ABC transporters function: the role of ABCB10 (ABC-me) as a novel player in cellular handling of reactive oxygen species. Biochim Biophys Acta. 2012;1823:1945-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 103. | Demicco EG, Kavanagh KT, Romieu-Mourez R, Wang X, Shin SR, Landesman-Bollag E, Seldin DC, Sonenshein GE. RelB/p52 NF-kappaB complexes rescue an early delay in mammary gland development in transgenic mice with targeted superrepressor IkappaB-alpha expression and promote carcinogenesis of the mammary gland. Mol Cell Biol. 2005;25:10136-10147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci. 2014;34:8585-8593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 105. | Cabello CM, Bair WB 3rd, Wondrak GT. Experimental therapeutics: targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022-1037. [PubMed] |
| 106. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2538] [Article Influence: 158.6] [Reference Citation Analysis (0)] |
| 107. | Olive K. Clinical Trial: IPI-926-03 for metastatic pancreatic ductal adenocarcinoma patients who have not been treated with other chemotherapy. Available from: https://www.olivelab.org/ipi-926-03.html. |
| 108. | Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2016;22:2848-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 109. | Moreau A, Le Vee M, Jouan E, Parmentier Y, Fardel O. Drug transporter expression in human macrophages. Fundam Clin Pharmacol. 2011;25:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Vander Borght S, Komuta M, Libbrecht L, Katoonizadeh A, Aerts R, Dymarkowski S, Verslype C, Nevens F, Roskams T. Expression of multidrug resistance-associated protein 1 in hepatocellular carcinoma is associated with a more aggressive tumour phenotype and may reflect a progenitor cell origin. Liver Int. 2008;28:1370-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 111. | Weinstein RS, Jakate SM, Dominguez JM, Lebovitz MD, Koukoulis GK, Kuszak JR, Klusens LF, Grogan TM, Saclarides TJ, Roninson IB. Relationship of the expression of the multidrug resistance gene product (P-glycoprotein) in human colon carcinoma to local tumor aggressiveness and lymph node metastasis. Cancer Res. 1991;51:2720-2726. [PubMed] |
| 112. | Filipits M, Suchomel RW, Dekan G, Haider K, Valdimarsson G, Depisch D, Pirker R. MRP and MDR1 gene expression in primary breast carcinomas. Clin Cancer Res. 1996;2:1231-1237. [PubMed] |
| 113. | Lee CJ, Li C, Simeone DM. Human pancreatic cancer stem cells: implications for how we treat pancreatic cancer. Transl Oncol. 2008;1:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 115. | Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2777] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 116. | Barbet R, Peiffer I, Hutchins JR, Hatzfeld A, Garrido E, Hatzfeld JA. Expression of the 49 human ATP binding cassette (ABC) genes in pluripotent embryonic stem cells and in early- and late-stage multipotent mesenchymal stem cells: possible role of ABC plasma membrane transporters in maintaining human stem cell pluripotency. Cell Cycle. 2012;11:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | The Human Protein Atlas. Available from: https://www.proteinatlas.org/. |
| 118. | Dvorak P, Pesta M, Soucek P. ABC gene expression profiles have clinical importance and possibly form a new hallmark of cancer. Tumour Biol. 2017;39:1010428317699800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 119. | Dvorak P, Hlavac V, Mohelnikova-Duchonova B, Liska V, Pesta M, Soucek P. Downregulation of ABC Transporters in Non-neoplastic Tissues Confers Better Prognosis for Pancreatic and Colorectal Cancer Patients. J Cancer. 2017;8:1959-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Kathawala RJ, Gupta P, Ashby CR Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015;18:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 558] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 121. | Falasca M, Linton KJ. Investigational ABC transporter inhibitors. Expert Opin Investig Drugs. 2012;21:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 122. | Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967-1972. [PubMed] |
| 123. | Kelly RJ, Draper D, Chen CC, Robey RW, Figg WD, Piekarz RL, Chen X, Gardner ER, Balis FM, Venkatesan AM. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clin Cancer Res. 2011;17:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 124. | Pennock GD, Dalton WS, Roeske WR, Appleton CP, Mosley K, Plezia P, Miller TP, Salmon SE. Systemic toxic effects associated with high-dose verapamil infusion and chemotherapy administration. J Natl Cancer Inst. 1991;83:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 125. | Yu M, Ocana A, Tannock IF. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer Metastasis Rev. 2013;32:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 126. | Tidefelt U, Liliemark J, Gruber A, Liliemark E, Sundman-Engberg B, Juliusson G, Stenke L, Elmhorn-Rosenborg A, Möllgård L, Lehman S. P-Glycoprotein inhibitor valspodar (PSC 833) increases the intracellular concentrations of daunorubicin in vivo in patients with P-glycoprotein-positive acute myeloid leukemia. J Clin Oncol. 2000;18:1837-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 127. | Lhommé C, Joly F, Walker JL, Lissoni AA, Nicoletto MO, Manikhas GM, Baekelandt MM, Gordon AN, Fracasso PM, Mietlowski WL. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol. 2008;26:2674-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 128. | Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, Dugan K, Lum B, Chin DL, Dewald G. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995). J Clin Oncol. 2004;22:1078-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 129. | Minderman H, O’Loughlin KL, Pendyala L, Baer MR. VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res. 2004;10:1826-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 130. | Gottesman MM, Ludwig J, Xia D, Szakács G. Defeating drug resistance in cancer. Discov Med. 2006;6:18-23. [PubMed] |
| 131. | Tang R, Faussat AM, Perrot JY, Marjanovic Z, Cohen S, Storme T, Morjani H, Legrand O, Marie JP. Zosuquidar restores drug sensitivity in P-glycoprotein expressing acute myeloid leukemia (AML). BMC Cancer. 2008;8:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 132. | Kemper EM, Cleypool C, Boogerd W, Beijnen JH, van Tellingen O. The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. Cancer Chemother Pharmacol. 2004;53:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 133. | Dörner B, Kuntner C, Bankstahl JP, Bankstahl M, Stanek J, Wanek T, Stundner G, Mairinger S, Löscher W, Müller M. Synthesis and small-animal positron emission tomography evaluation of [11C]-elacridar as a radiotracer to assess the distribution of P-glycoprotein at the blood-brain barrier. J Med Chem. 2009;52:6073-6082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 134. | Bates SE, Shieh CY, Mickley LA, Dichek HL, Gazdar A, Loriaux DL, Fojo AT. Mitotane enhances cytotoxicity of chemotherapy in cell lines expressing a multidrug resistance gene (mdr-1/P-glycoprotein) which is also expressed by adrenocortical carcinomas. J Clin Endocrinol Metab. 1991;73:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Consoli U, Priebe W, Ling YH, Mahadevia R, Griffin M, Zhao S, Perez-Soler R, Andreeff M. The novel anthracycline annamycin is not affected by P-glycoprotein-related multidrug resistance: comparison with idarubicin and doxorubicin in HL-60 leukemia cell lines. Blood. 1996;88:633-644. [PubMed] |
| 136. | Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 137. | Patel J, Mitra AK. Strategies to overcome simultaneous P-glycoprotein mediated efflux and CYP3A4 mediated metabolism of drugs. Pharmacogenomics. 2001;2:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004;25:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 399] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 139. | van de Ven R, Scheffer GL, Scheper RJ, de Gruijl TD. The ABC of dendritic cell development and function. Trends Immunol. 2009;30:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 140. | Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 313] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 141. | Kim HS, Min YD, Choi CH. Double-edged sword of chemosensitizer: increase of multidrug resistance protein (MRP) in leukemic cells by an MRP inhibitor probenecid. Biochem Biophys Res Commun. 2001;283:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | O’Connor R, O’Leary M, Ballot J, Collins CD, Kinsella P, Mager DE, Arnold RD, O’Driscoll L, Larkin A, Kennedy S. A phase I clinical and pharmacokinetic study of the multi-drug resistance protein-1 (MRP-1) inhibitor sulindac, in combination with epirubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2007;59:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 143. | Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47-50. [PubMed] |
| 144. | Tiwari AK, Sodani K, Dai CL, Ashby CR Jr, Chen ZS. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 145. | Zhang YK, Wang YJ, Gupta P, Chen ZS. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015;17:802-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 146. | Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 147. | Widmer N, Rumpold H, Untergasser G, Fayet A, Buclin T, Decosterd LA. Resistance reversal by RNAi silencing of MDR1 in CML cells associated with increase in imatinib intracellular levels. Leukemia. 2007;21:1561-1562; author reply 1562-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 148. | Mechetner EB, Roninson IB. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci USA. 1992;89:5824-5828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 149. | Fisher M, Abramov M, Van Aerschot A, Xu D, Juliano RL, Herdewijn P. Inhibition of MDR1 expression with altritol-modified siRNAs. Nucleic Acids Res. 2007;35:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 150. | Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515-1519. [PubMed] |
| 151. | Wang Z, Li Y, Ahmad A, Azmi AS, Kong D, Banerjee S, Sarkar FH. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist Updat. 2010;13:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 152. | Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther. 2010;17:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 153. | Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70:4528-4538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 359] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 154. | Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 365] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 155. | Wang F, Xue X, Wei J, An Y, Yao J, Cai H, Wu J, Dai C, Qian Z, Xu Z. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 156. | Hwang JH, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim YT, Funel N, Park JK, Kim MA, Kang GH. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010;5:e10630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 157. | Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B. Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat. 2005;8:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 158. | Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A. Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia. 2007;21:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 159. | Dai CL, Liang YJ, Wang YS, Tiwari AK, Yan YY, Wang F, Chen ZS, Tong XZ, Fu LW. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 160. | Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905-7914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 161. | Kuang YH, Shen T, Chen X, Sodani K, Hopper-Borge E, Tiwari AK, Lee JW, Fu LW, Chen ZS. Lapatinib and erlotinib are potent reversal agents for MRP7 (ABCC10)-mediated multidrug resistance. Biochem Pharmacol. 2010;79:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 162. | Livney YD, Assaraf YG. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv Drug Deliv Rev. 2013;65:1716-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 163. | Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320-12364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 814] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 164. | Xue X, Liang XJ. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer. 2012;31:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 165. | Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537-3547. [PubMed] |
| 166. | Kaye SB. Reversal of drug resistance in ovarian cancer: where do we go from here? J Clin Oncol. 2008;26:2616-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 167. | Relling MV. Are the major effects of P-glycoprotein modulators due to altered pharmacokinetics of anticancer drugs? Ther Drug Monit. 1996;18:350-356. [PubMed] |
| 168. | Ozols RF, Cunnion RE, Klecker RW Jr, Hamilton TC, Ostchega Y, Parrillo JE, Young RC. Verapamil and adriamycin in the treatment of drug-resistant ovarian cancer patients. J Clin Oncol. 1987;5:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 295] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 169. | Park S, Shimizu C, Shimoyama T, Takeda M, Ando M, Kohno T, Katsumata N, Kang YK, Nishio K, Fujiwara Y. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 170. | Marzac C, Garrido E, Tang R, Fava F, Hirsch P, De Benedictis C, Corre E, Lapusan S, Lallemand JY, Marie JP. ATP Binding Cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica. 2011;96:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 171. | Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, Hu T, Jiang L, Li J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016;370:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 563] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 172. | Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 173. | Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs). Essays Biochem. 2011;50:179-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |