Published online May 7, 2018. doi: 10.3748/wjg.v24.i17.1839
Peer-review started: March 28, 2018
First decision: April 11, 2018
Revised: April 15, 2018
Accepted: April 23, 2018
Article in press: April 23, 2018
Published online: May 7, 2018
Processing time: 40 Days and 8.7 Hours
Tumor immunity proceeds through multiple processes, which consist of antigen presentation by antigen presenting cells (APCs) to educate effector cells and destruction by the effector cytotoxic cells. However, tumor immunity is frequently repressed at tumor sites. Malignantly transformed cells rarely survive the attack by the immune system, but cells that do survive change their phenotypes to reduce their immunogenicity. The resultant cells evade the attack by the immune system and form clinically discernible tumors. Tumor microenvironments simultaneously contain a wide variety of immune suppressive molecules and cells to dampen tumor immunity. Moreover, the liver microenvironment exhibits immune tolerance to reduce aberrant immune responses to massively-exposed antigens via the portal vein, and immune dysfunction is frequently associated with liver cirrhosis, which is widespread in hepatocellular carcinoma (HCC) patients. Immune therapy aims to reduce tumor burden, but it is also expected to prevent non-cancerous liver lesions from progressing to HCC, because HCC develops or recurs from non-cancerous liver lesions with chronic inflammatory states and/or cirrhosis and these lesions cannot be cured and/or eradicated by local and/or systemic therapies. Nevertheless, cancer immune therapy should augment specific tumor immunity by using two distinct measures: enhancing the effector cell functions such as antigen presentation capacity of APCs and tumor cell killing capacity of cytotoxic cells, and reactivating the immune system in immune-suppressive tumor microenvironments. Here, we will summarize the current status and discuss the future perspective on immune therapy for HCC.
Core tip: Hepatocellular carcinoma (HCC) develops or recurs from non-cancerous liver lesions with chronic inflammatory states and/or cirrhosis, and these lesions cannot be cured and/or eradicated by local and/or drug therapies. Immune therapy may be effective for HCC treatment by preventing non-cancerous liver lesions from progressing to HCC as well as reducing tumor burdens. However, tumor immunity is frequently depressed in tumor sites, particularly in liver microenvironment, which is prone to exhibit immune tolerance, to reduce aberrant immune responses to massively-exposed antigens via portal veins. At present, cancer immune therapy employs two distinct strategies; enhancing the effector cell functions and unleashing the immune suppressive tumor microenvironments. Here, we will summarize the current status and discuss the future perspective on immune therapy for HCC.
- Citation: Mukaida N, Nakamoto Y. Emergence of immunotherapy as a novel way to treat hepatocellular carcinoma. World J Gastroenterol 2018; 24(17): 1839-1858
- URL: https://www.wjgnet.com/1007-9327/full/v24/i17/1839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i17.1839
Hepatocellular carcinoma (HCC) is ranked as the sixth most common malignancy and is the third leading cause of cancer-related mortality worldwide[1]. Despite recent progress in prevention and diagnosis, many HCC cases are still diagnosed at an advanced stage, for which there are few effective and/or curative treatment options, and as a consequence, their prognosis remains poor. These circumstances necessitate the development of a novel therapeutic strategy for HCC, particularly for HCC at advanced stages.
HCC ensues from chronic liver diseases, particularly liver cirrhosis, arising from various risk factors including chronic hepatitis B- or C-virus infection, aflatoxin B1 exposure, excessive alcohol consumption, and occurrence of non-alcoholic fatty liver. Other independent risk factors include tobacco use[2], diabetes[3], and obesity[4]. In conjunction with the declining incidence of HBV and HCV infections, non-alcoholic fatty liver disease is becoming an important cause of HCC in the advanced economies, as the number of patients suffering from metabolic syndromes is rapidly increasing in these countries[4].
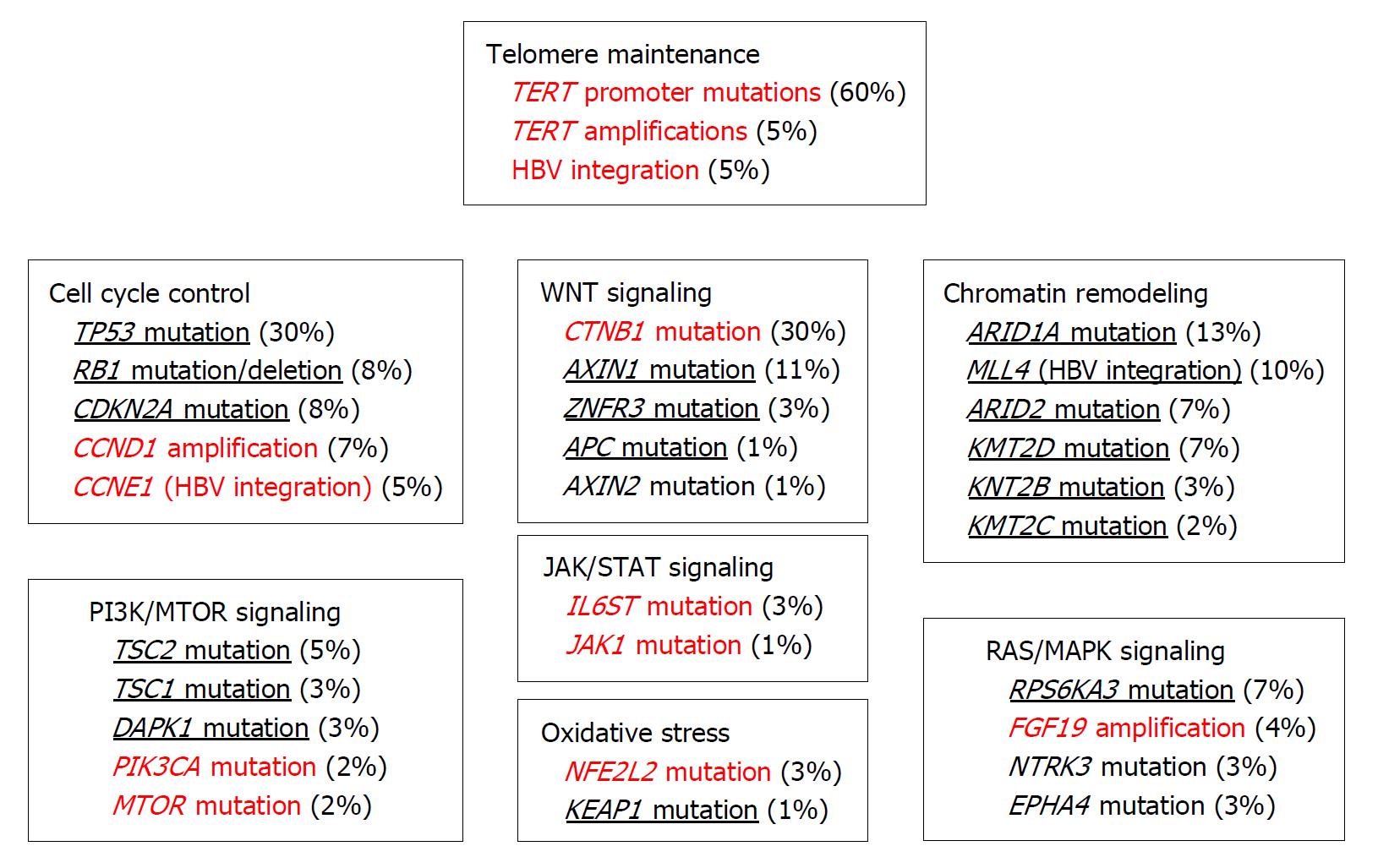
All these etiologic conditions cause sustained inflammatory reactions, consisting of persistent oxidative stress, sustained hepatocyte necrosis and regeneration, and fibrotic changes[5]. These events can lead to HCC development through the accumulation of somatic genetic alterations and epigenetic modifications in various passenger and driver genes, and these changes have been extensively clarified with the advent of next-generation sequencing technology (Figure 1)[6]. Aberrant telomerase reverse transcriptase (TERT) activation is observed in about 70% of HCC cases, arising from its promoter mutation and amplification, and viral genome integration[7]. Thus, TERT activation and subsequent telomerase reactivation can be a key event in malignant transformation, leading to unrestrained proliferation of HCC cells[8]. Inactivating mutations are also frequently observed in CTNB1 (about 30%), which codes for β-catenin[7]. Moreover, inactivating mutations are detected in other members of the WNT pathway, such as AXIN1 (11%), AXIN2 (1%), ZNRF3 (3%), or APC (1%). Inactivating mutations of TP53 are also frequently observed in HCC (~30% of cases) but are rarely detected together with CNTB1 mutations, suggesting that distinct molecular pathways are responsible for HCC evolution. Additional mutations are observed in genes involved in other pathways including chromatin remodeling, PI3K/AKT/mammalian target of rapamycin (mTOR) signaling, Ras/MAPK signaling, JAK/STAT signaling, and oxidative stress pathways[6].
DNA copy number alterations are also frequently observed with broad genomic deletions at 1p, 4p-q, 6q, 8p, 13p-q, 16p-q, 17p, 21p-q, 22q, and gains at 1q, 5p, 6p, 8q, 17q, 20q, Xq[6,7,9]. Recurrent homologous deletions involve various genes including AXIN1, CDKN2A/CDKN2B, CFH, IRF2, MAP2K3, PTEN, RB1, and RPS6KA3[6]. In contrast, broader DNA gains affect JAK3, MET, and MYC[6] while focal amplifications at 11q13 and 6p21 lead to the amplification of FGF3/4/19/CCDN1[10] and VEGFA[11], respectively. Focal amplification of FGF19 is associated with tumor progression[10] and that of VEGFA confers a high sensitivity to sorafenib, the first-line treatment for advanced HCC[11].
A substantial proportion of HBV-infected patients develop HCC even when fibrotic changes are absent in the liver[12], suggesting that HBV can be directly oncogenic. A non-structural HBV protein, HBx protein, is proposed to act as an oncogene based on its in vitro capacity to modulate cell cycle, signaling pathways, and DNA repair in hepatocytes[13], but evidence for direct transforming activity of HBx is scarce. Like other DNA viruses, HBV can cause insertional mutagenesis[12], which can induce DNA deletions at the integration sites, thereby promoting chromosomal instability and inactivation of tumor suppressor genes. Moreover, integration of the HBV genome into loci with enhancer and promoter activities can modulate the expression and function of the genes near the integration sites, and can eventually promote clonal proliferation and malignant transformation[12]. Thus, the differences in integration sites can profoundly impact the types of the affected genes and subsequent molecular pathological changes.
Knowledge of molecular changes in HCC has expanded rapidly with the advent of gene technology, particularly next-generation sequencing technology, but has not been efficiently translated into clinical practice. A major reason is that the types of mutated driver genes and associated pathways differ considerably in each HCC case. These heterogeneities can hinder the identification and/or selection of target molecule(s) to develop molecular target drugs. Immunotherapy can overcome this problem, because it can enhance anti-tumor activity of the host cells, irrespective of the molecules and the signal pathways involved in hepatocarcinogenesis. In this review, we will discuss the present status and future perspectives on immunotherapy for HCC. The other clinical aspects of HCC including drug therapy have been reviewed in several other recent articles[1,14,15].
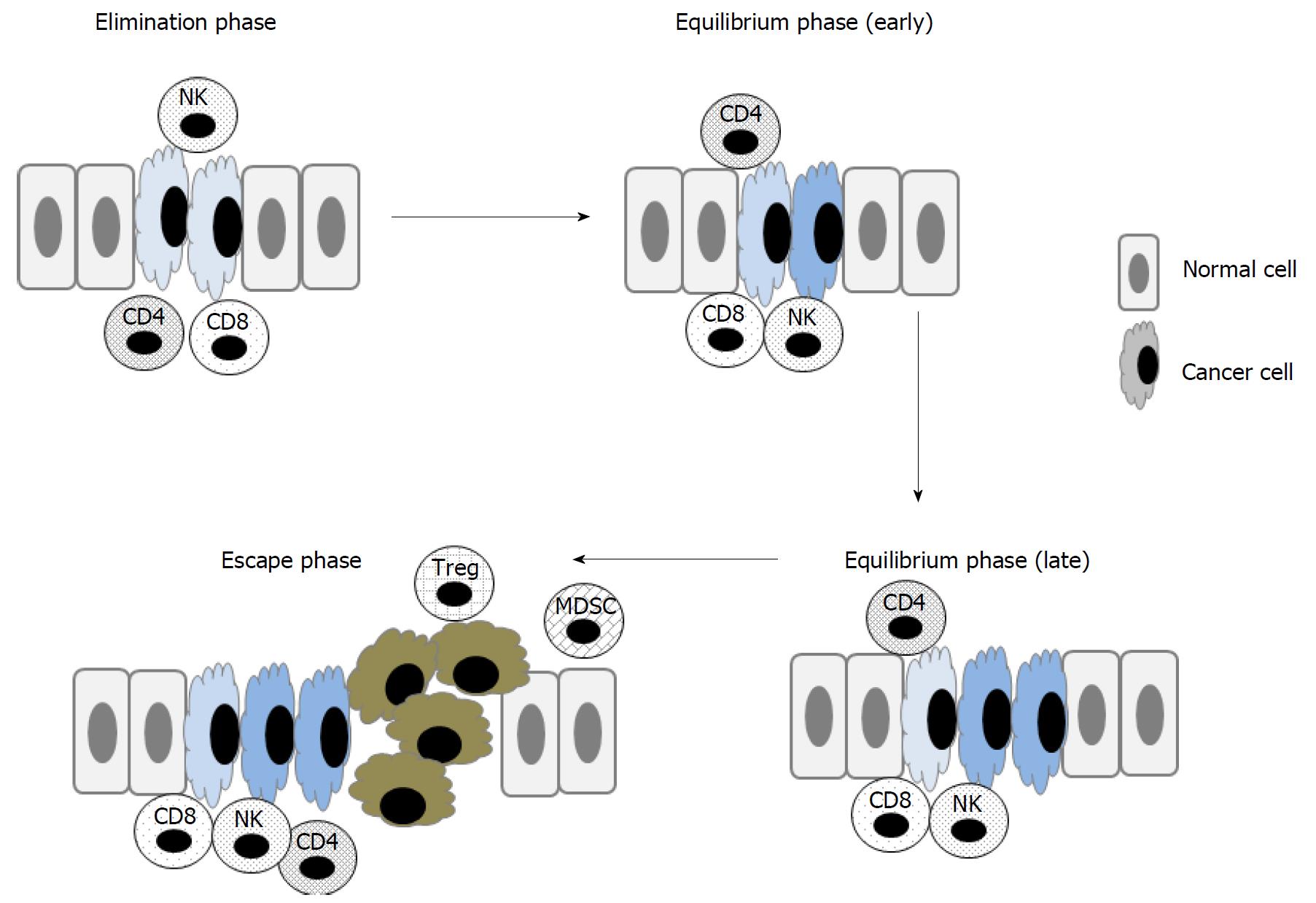
Evasion of the immune system is now acknowledged as the key event necessary for the transformation of normal cells into malignant cells and their subsequent survival[16]. The immune system can sculpt cancer cells through a complicated mechanism called immunoediting (Figure 2)[17]. At the elimination phase, transformed cells are destroyed by immune cells such as cytolytic lymphocytes (CTLs) and natural killer (NK) cells, but resistant tumor cells sporadically appear and constantly change their phenotypes in the presence of the immune system. As a consequence, at the equilibrium phase, tumor cells reduce their immunogenicity and simultaneously escape the immunemediated killing mechanisms, thereby forming clinically appreciable tumor formation at the escape phase. Moreover, immune response can be dampened by immunoregulatory cells including regulatory T cells (Treg) and myeloid-derived suppressive cells (MDSCs) - cells that are abundant at tumor sites. The liver is constantly exposed to high levels of various antigens via the portal vein. Consequently, in order to prevent autoimmune liver injury, the liver microenvironment constantly exhibits potent immunosuppression[18]. Furthermore, immune dysfunction is frequently associated with liver cirrhosis[19], which is widespread in HCC patients. Moreover, cirrhosis can be a basis of HCC but cannot be completely removed, even after curative locoregional therapy with surgery, radiofrequency ablation (RFA), or transarterial chemoembolization (TACE)[1]. Thus, in addition to eradicating tumor mass, immunotherapy should aim to prevent the recurrence of HCC after curative locoregional therapy[20].
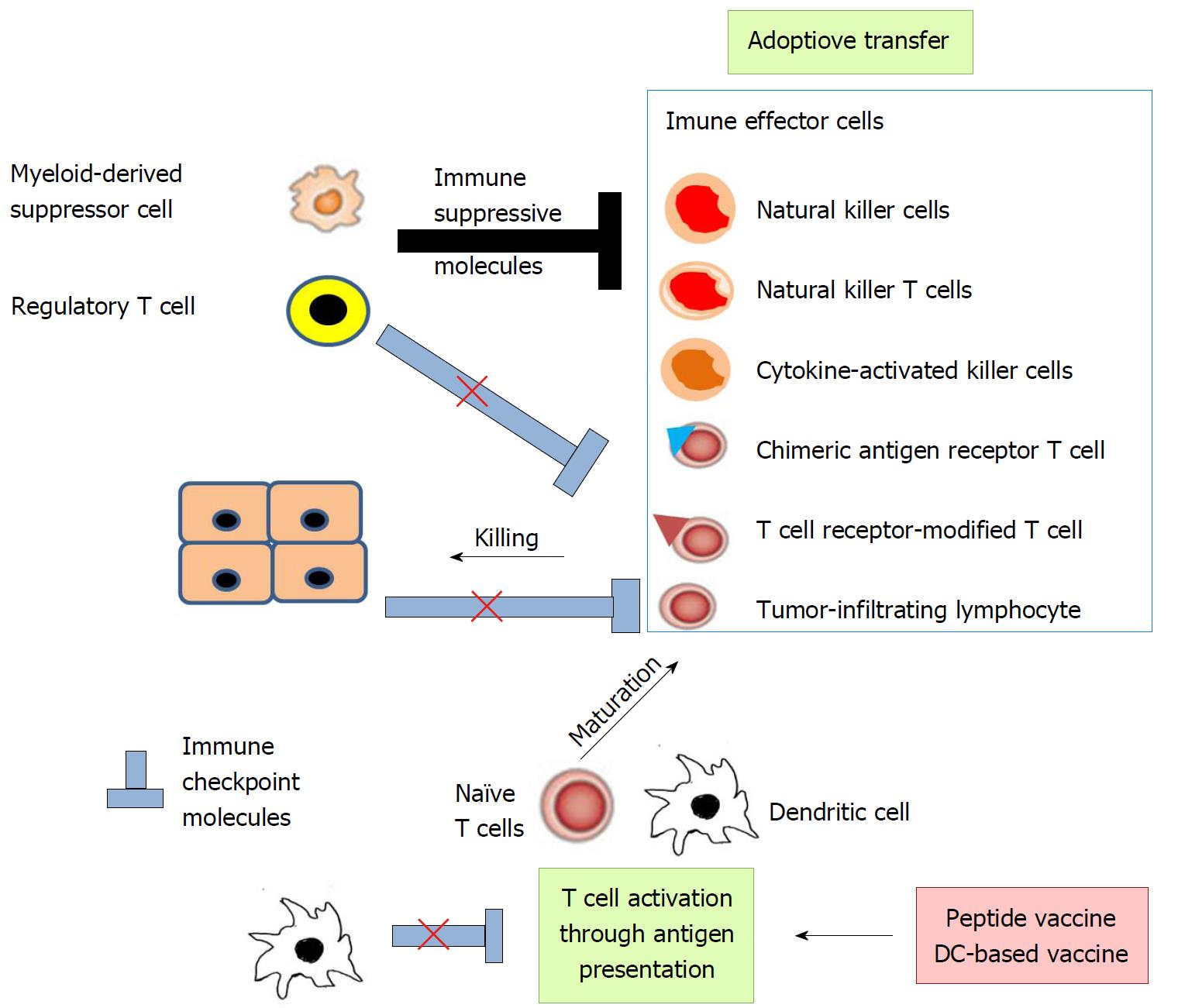
Immunotherapy approaches for HCC can be summarized in two ways: Activation of cytotoxic cell functions and correction of depressed immune functions inherent in HCC (Figure 3)[21]. Among the cytotoxic cells, CD8-positive CTLs are the most effective for specifically detecting and killing tumor-associated antigen (TAA)-expressing cancer cells. Antigen-presenting cells (APCs), particularly DCs, degrade exogenous and endogenous TAAs to be loaded on major histocompatibility complex (MHC) class I and class II, respectively (Figure 4). CD8-positive CTLs and CD4-positive helper T cells recognize the TAA-derived peptide on MHC class I and class II, respectively (Figure 4). In order to promote T cell survival, APCs simultaneously deliver co-stimulatory signals using several pathways including CD80/CD86-CD28 and CD40-CD40 ligand pathways (Figure 4)[22].
Antigen presentation efficiency can be improved by administering TAA-derived peptides, and/or the transfer of APCs, particularly DCs, which are loaded with or without TAA-derived peptides (Figure 3). These measures are named tumor vaccine therapy, as a whole. Adoptive immune therapy consists of transferring a large number of CTLs with T cell receptors recognizing specifically TAAs and/or other cytotoxic cells like NK cells into patients (Figure 3). With these maneuvers, the cells are obtained in most cases from patients and expanded ex vivo. The resultant cells are adoptively transferred to patients, sometimes after genetic modifications.
APCs prime T cells with the help of co-stimulatory molecules: CD80/86 on APCs and CD28 on T cells[22]. Simultaneously, a co-inhibitory molecule, CTL antigen-4 (CTLA-4) on T cells interacts with CD80/86 on APCs to dampen T cell activation (Figure 5A). Following the priming phase, CD8-positive CTLs are activated to exert cytotoxicity against foreign materials including tumor cells by using perforin, granzymes, and Fas ligand[23]. During this effector phase, T cell activation can be negatively regulated by co-inhibitory molecules expressed on APCs and other somatic cells including tumor cells[24]. One representative pathway is the programmed cell death (PD)-1-PD ligand 1(PD-L1)/PD-L2 pathway (Figure 5B), which often works in the tumor microenvironment. Thus, immune checkpoint therapy can restore immune responses to tumors by suppressing these co-inhibitory pathways, leading to the control of tumor growth and/or its regression (Figure 3)[25]. With a main focus on the observations obtained from human clinical trials, we will discuss the immune therapy for HCC in the next chapter.
Peptide vaccine therapy: α-fetoprotein (AFP) is a well-known TAA in HCC and is used as a tumor peptide vaccine. A phase I clinical trial demonstrated that all six tested patients generated CD8-positive T-cell responses to the peptides as measured by direct IFN-γ enzyme-linked immunospot (ELIspot) and MHC class I tetramer assays[26]. Specific CD8-positive T cell response may be augmented by the use of AFP conjugated with heat shock protein (HSP)70[27], HSP72[27], or glycoprotein 96[28], as revealed by studies using mouse AFP-expressing tumors. Butterfield and colleagues further examined the efficacy of AFP-pulsed DC transfer and demonstrated that six out of the ten subjects generated significant AFP-positive T cell responses to the administered peptides, although nine showed progressive disease[26]. The lack of apparent clinical responses can be attributed to the presence of an expanded pool of partially differentiated but non-functional AFP-specific CD8-positive T cells and the absence of CD4-positive T cell responses in AFP-positive HCC patients[29].
The high prevalence of TERT overexpression in HCC (Figure 1)[7] incited the use of TERT-derived peptides as a tumor vaccine for HCC patients. A phase II clinical trial was conducted to examine the efficacy of a TERT-derived peptide vaccine in patients with advanced HCC when it was administered together with cyclophosphamide and GM-CSF[30]. The treatment increased specific T cell responses and decreased Foxp3-positive Tregs. Vaccine administration was well tolerated, and about half of the patients remained in stable condition six months after the treatment but without any complete or partial response to the treatment. Mizukoshi and colleagues also examined the efficacy of subcutaneous injection of TERT-derived peptide emulsified in incomplete Freund’s adjuvant in 14 HCC patients[31]. The vaccination induced an increase in TERT-specific T cells with the effector memory phenotype and the capacity to produce multiple cytokines in ten patients. Moreover, eight out of the ten patients with TERT-specific immunity did not show relapse, whereas all patients without TERT-specific immunity recurred. Thus, vaccination with TERT-derived peptide may be effective to prevent recurrence, which is frequently observed after locoregional therapy.
Another candidate molecule for tumor vaccination is an oncofetal antigen, glypican-3 (GPC3), which is expressed in the embryonic liver but scarcely expressed in the normal adult liver, and is overexpressed in HCC[32]. A phase I/II clinical trial of GPC3-derived peptide vaccination was conducted on 11 patients with advanced HCC[33]. Vaccination induced GPC3-specific CTLs that infiltrated into the tumor. These CTLs were present in the tumor tissues as well as peripheral blood, as revealed by sequencing T cell receptor genes of tumor-infiltrating lymphocytes (TILs). Moreover, the frequency of GPC3-specific CTL after vaccination was correlated with overall survival. These observations imply the efficacy of GPC3-derived peptide vaccination for advanced-stage HCC. Moreover, repeated vaccination with GPC3-derived long peptide (LP) induced LP-specific and HLA class II-restricted CD4+ cell responses in 14 of 20 vaccinated HCC patients[34]. Moreover, the presence of specific helper CD4+ cells was correlated with prolonged overall survival.
Additional molecules have been proposed as candidates for peptide vaccine therapy. Aspartate-β-hydroxylase (ASPH) is also overexpressed in HCC and ASPH-derived peptides induced during T cell activation in vitro in both an HLA class I- and class II-restricted manner when peripheral blood mononuclear cells from HCC patients were used[35]. Administering an adenovirus vector expressing HBx protein was effective at both protective and therapeutic antitumor immunity in hepatoma models in immune-competent mice[36], suggesting its efficacy against HBV-positive HCC. Moreover, the treatment induced infiltration of CD8+ T cells, which mainly mediated its antitumor effects. Annexin A3 (ANXA3) expression is enhanced in the CD133-expressing cancer stem-like/initiating cell (CSC/CIC) population, compared with the non-CSC/CIC population of HCC[37]. Moreover, HCC CSC/CICs were preferentially killed by T cells primed with ANXA3-transfected DCs. Likewise, antigen-specific T cell responses against HCC were generated when T cells were primed with New York esophageal squamous cell carcinoma-1 (NYESO1) protein-loaded DCs[38], suggesting the potential of NYESO1-derived peptides as a tumor vaccine.
To date, vaccination with TAA-derived peptides has yielded a marginal clinical benefit in HCC patients, similar to the results reported in other types of cancer[39]. This may arise mainly from its suboptimal immunogenicity and the tolerogenic tendency of intrahepatic DCs[18]. The former can be overcome by improving antigen selection and vaccine formulation, while the latter may be solved by adoptive transfer of DCs pulsed with TAAs. Peptide vaccination alone may not be able to de-repress immunosuppressive tumor microenvironments, but immune checkpoint therapy can abolish T cell dysfunction in HCC tissues and eventually can enhance specific T cell responses to tumor antigens. Hence, the combination of a peptide vaccine and immune checkpoint therapy will warrant detailed analysis in the future. Moreover, preclinical studies using mouse models demonstrated the potential efficacy of other types of vaccines such as RNA-based adjuvants[40], DC-derived exosomes[41], or an attenuated Listeria vaccine that can express HCC-specific antigens[42].
DC-based vaccine therapy: DCs are a professional APC and can initiate and maintain T cell-mediated immune responses when they are pulsed with antigens[43]. In addition to T cells, DCs can also activate NK cells[44]. However, DC-induced immunity is frequently repressed in tumor sites, arising from multiple mechanisms including a low number of DCs in tumor sites, the low antigen-presenting capacity of DCs, and poor access of DCs to tumor antigens[43]. A low number of DCs can be overcome by administering ex vivo expanded DCs from peripheral blood mononuclear cells (PBMCs), which are stimulated with combinations of various cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Moreover, the additional stimuli such as Toll-like receptor (TLR) agonists, are required for generating mature DCs with a potent antigen-presenting capacity, and several measures are proposed to circumvent poor access of DCs to tumor antigens: Pulsing with tumor lysates, TAAs, or TAA-derived peptide; transfection of DNA constructs encoding TAAs; and fusion with tumor cells[43].
A phase II clinical trial was conducted to investigate the safety and efficacy of intravenous vaccination with autologous DCs pulsed ex vivo with a liver tumor cell line lysate (HepG2) in advanced HCC patients[45]. The treatment was well tolerated, and in the patients who received at least three vaccine infusions ELIspot assay demonstrated the induction of T cell responses to vaccines and/or AFP and about 25% of patients showed a partial response or stable disease condition, as revealed by serological AFP determination or radiological examination.
Several groups reported DC-based vaccination using AFP as a TAA. A phase I/II clinical trial examined the effect of intradermal injection of AFP-derived peptide-pulsed DCs, which were prepared from autologous adherent PBMCs cultured with GM-CSF and IL-4[46]. The same group further reported that six of the ten tested subjects exhibited statistically significantly expanded levels of AFP-specific T cells. In addition to T cells, the transfer of AFP-derived peptide-primed DCs enhanced NK cell activation and decreased Treg frequencies in vaccinated HCC patients[47]. However, the priming of DCs with peptides was not efficient, and therefore, in order to efficiently pulse DCs, AFP gene transduction into DCs was attempted using viral vectors such as lentivirus[48] or adeno-associated virus (AAV) vectors[49]. Adoptive transfer of lentivirus-transduced DCs induced superior anti-tumor Th1 polarization in a preclinical model, compared with peptide-pulsed DCs[48]. MHC class I and class II and co-stimulatory molecules were expressed to a similar extent on recombinant AAV/AFP-pulsed and cancer cell lysate-pulsed DCs. However, recombinant AAV/AFP-pulsed DCs exhibited superiority over cancer cell lysate-pulsed DCs in terms of their capacity to stimulate proliferation of T cells, to induce T cells to secrete IFN-γ, and to generate an AFP-specific MHC class I-restricted CTL response in a preclinical study[49]. Thus, the use of viral vectors may be able to prime DCs more efficiently than TAA-derived peptides to activate CTL.
Fifteen patients with advanced HCC were treated with intradermal vaccination of mature autologous DCs pulsed with cell lysates of a human HCC cell line, HepG2[50]. The treatment increased CD8-positive T cells in peripheral blood and serum IFN-γ levels. Overall survival was improved with partial radiological response in two patients, stable course in nine patients, but progressive disease in four patients. DCs transfected with HepG-2 hepatoma cell-derived RNA could induce CTLs to specifically kill HepG2 cells in vitro, and injection of T lymphocytes from HCC patients and transfected DCs was effective in a preclinical study using severe combined immunodeficiency mice[51]. In another clinical trial, autologous DCs were pulsed with patient-derived irradiated tumor cell lines established from surgically resected tumor tissues[52]. After one course of TACE, tumor cell-primed DCs suspended in GM-CSF were administered subcutaneously three times at one-week intervals. The treatment was well tolerated, without exacerbation of HBV infection[52].
In another clinical trial, DCs were generated from PBMCs in the presence of GM-CSF and IL-4, and pulsed with cytoplasmic transduction of peptide-attached recombinant fusion proteins consisting of three TAAs: AFP, GPC3, and MAGE-1[53]. A phase I/II clinical trial demonstrated that T cell response and clinical benefit were observed when subcutaneous injection of the resultant DCs near the inguinal lymph node was followed by topical application of a TLR-7 agonist. Lee and colleagues reported the results obtained from a similar phase I/II clinical trial using DCs pulsed with AFP, GPC3, and MAGE-1 although they did not administer a TLR-7 agonist[54]. They observed similarly enhanced anti-tumor immune responses after DC vaccination, particularly in recurrence-free patients, as evidenced by lymphocyte proliferation and IFN-γ ELIspot assays. The median time to tumor progression was 36.6 mo in the DC-vaccination group and 11.8 months in the control group. Favorable results prompted the same group to conduct a randomized phase II trial on 156 HCC patients who were treated for HCC with no evidence of residual tumors after standard therapeutic modalities[55]. Tumor-specific immune responses were significantly enhanced in the immunotherapy group, but with a higher frequency of overall adverse events, which are mainly mild to moderate in severity. The recurrence-free survival was not significantly different between the immunotherapy and control groups. However, DC immunotherapy significantly reduced the risk of tumor recurrence in the non-RFA group patients but unexpectedly increased the risk of recurrence in the RFA group. Baseline serum IL-15 was statistically correlated with prolonged recurrence-free survival within the immunotherapy groups[55]. Thus, DC immunotherapy may be effective for HCC patients who are treated with standard treatment modalities but not RFA.
Another TAA, heat-shock protein (HSP) 70, was used to prime DCs, based on its overexpression in HCV-related HCC. DCs transfected with HSP70 mRNA were administered intradermally in a phase I clinical trial on 12 advanced HCC patients[56]. The trial demonstrated that the treatment was well tolerated, with complete response without any recurrence in two patients, stable disease in five, and progression of disease in five.
TACE can induce HCC cells to die and release high levels of TAAs, which can be internalized, degraded, and presented to immune cells by APCs including DCs. As a consequence, following TACE, tumor immunity can be enhanced. Supporting this notion, we observed that AFP-specific T cell frequency was further increased in HCC patients receiving TACE, and that the increment was enhanced by simultaneous transarterial administration of DCs[57]. Our subsequent clinical trial further demonstrated that the co-infusion of mature DCs into tumor sites following TACE, was well tolerated in advanced HCC patients and prolonged recurrence-free survival of patients, compared with the historical controls[58].
Nevertheless, the clinical response to adoptive DC transfer is still not satisfactory, and, as a consequence, several measures have been devised to augment the efficacy of the adoptive DC transfer. Several groups proposed the priming of DC with other antigens, such as hepatocellular carcinoma-associated antigen-519/targeting protein for Xkl-2 (HCA519/TPX2)[59], epithelial cell adhesion molecule (EpCAM)[60], or ANAXA3[37]. Since EpCAM and ANAXA3 are selectively expressed in CSCs/CICs, the priming of DCs with these antigens may be effective to kill CSCs/CICs that are rather resistant to standard therapies such as chemotherapy and/or molecular targeted therapy. Moreover, in order to enhance immunostimulating activities of DCs, other groups have tried to transfect DCs with the genes of immunostimulating cytokines, such as IL-2[61] or IL-12,[62] in preclinical or in vitro studies. The other measure includes the combined administration of effector cells like cytokine-activated killer cells (CIKs) with antigen-pulsed DCs, as we discuss in the following section.
Adoptive transfer of immune effector cells: Several immune effector cells are adoptively transferred cell to enhance tumor immunity; Two types of T cells are commonly used for adoptive cell therapy to enhance tumor immunity: TILs, genetically modified T cells, NK cells, natural killer T (NKT) cells, and CIKs, TILs (Figure 3).
TILs are considered to have a higher specific immunological reactivity against tumor cells than the non-infiltrating lymphocytes, and evidence is accumulating to indicate the potential role of TILs as biomarkers reflecting the immune response to the tumor[63]. TILs are obtained from surgically obtained tumor specimens and are expanded ex vivo with anti-CD3 antibody treatment before being transferred back to patients[64]. Adoptive cell therapy using TILs can be effective for metastatic melanoma[65], but no clinical trials are in progress to evaluate the adoptive transfer of TILs for HCC, probably due to the difficulty in obtaining a sufficient number of TILs during surgical resection of HCC.
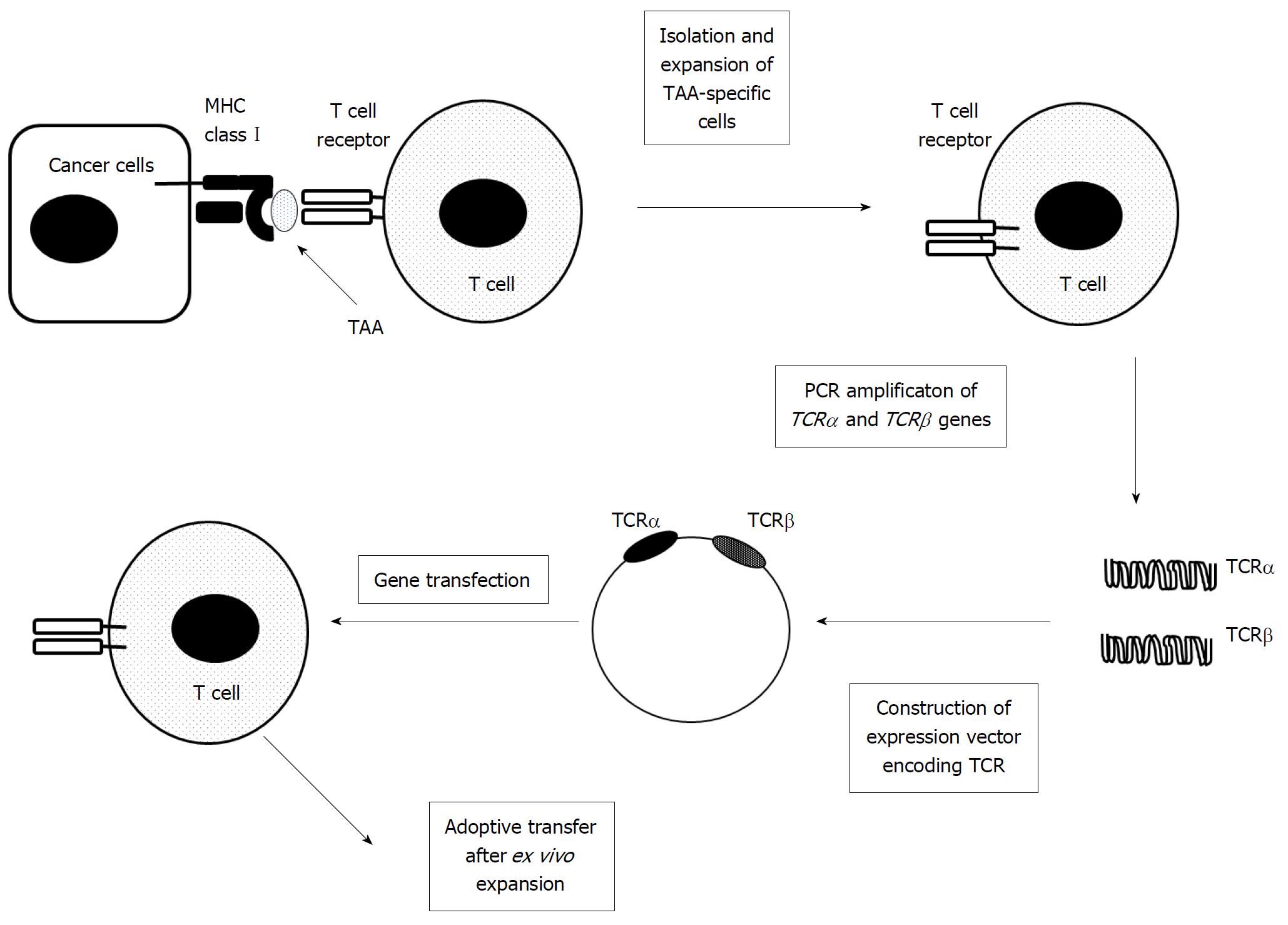
T cells can be genetically engineered to express a T cell receptor (TCR) against a specific TAA (Figure 6)[66]. Metastatic melanoma was treated with adoptive transfer of autologous T cells with a modified TCR recognizing a melanocyte-differentiating antigen (MART-1), and the treatment resulted in long-term persistence of infused cells and tumor regression in two out of 17 patients[67]. The adoptive transfer of T cells expressing a higher-affinity TCR caused a better benefit, with tumor regressing in six out of 20 patients[68]. Subsequently, several phase I clinical trials were conducted to evaluate the efficacy of adoptive transfer of autologous T cells, which are genetically modified to express a TAA-specific T cell receptor, and some favorable results have been reported involving melanoma, colorectal cancer, synovial cell sarcoma, and multiple myeloma to date[66]. With these results, two phase I/II clinical trials are now in progress to evaluate the adoptive transfer of T cells with a modified TCR, which can recognize HBV antigens, in HCC patients with HBV infection (NCT026863712, 02719782). One additional phase I clinical trial is also recruiting participants to evaluate the safety and anti-tumor activity of autologous T cells expressing TCRs specific for AFP in advanced HCC patients (NCI03132792). Nevertheless, further progress in TCR-modified T cell therapy requires the identification of additional TAAs, the comprehensive elucidation of the structure of TCRs that specifically recognize TAAs, and improvements in genetic engineering of TCRs.
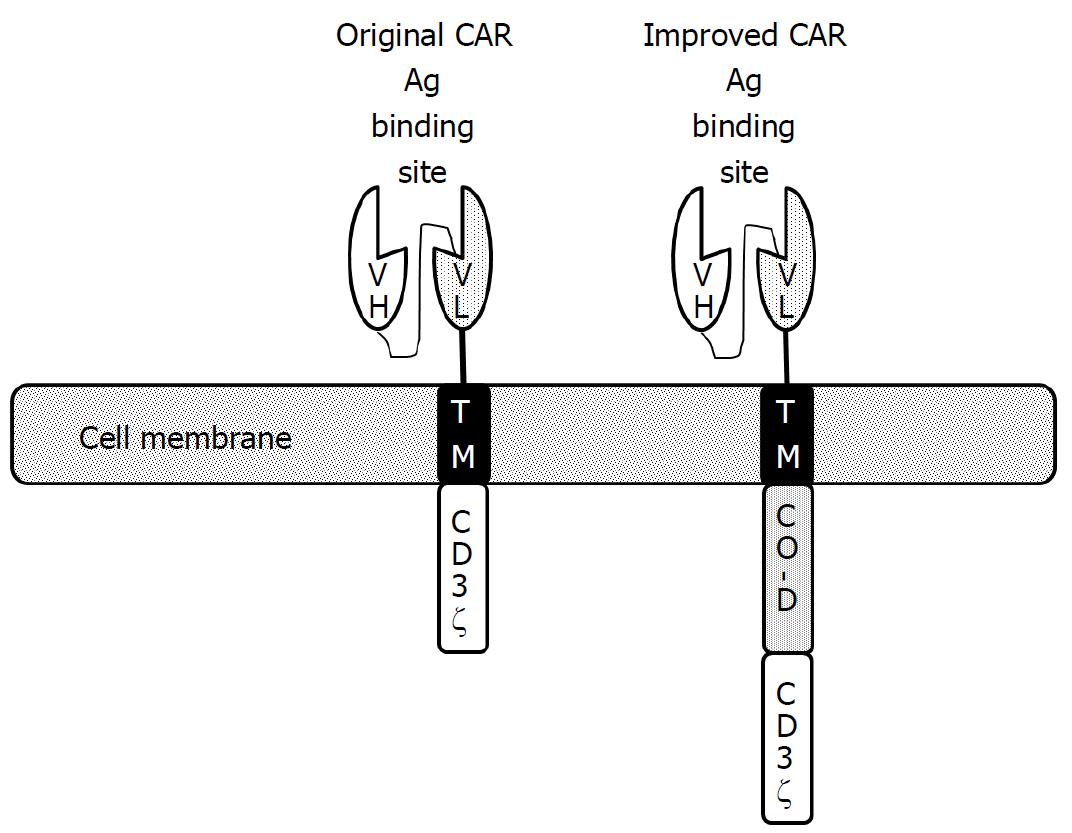
Another type of genetically modified T cell utilizes the chimeric antigen receptor (CAR) gene, which is prepared by fusing the transmembrane and cytoplasmic domains of CD3ζ with the antigen-binding portion of an antibody that can recognize a particular TAA (Figure 7)[69] The generated CAR gene is transduced to T cells, mostly with the help of a lentivirus vector. The resultant CAR T cells can deliver activating signals once they bind with a specific TAA using the antigen-binding domain of their extracellular portions. In order to enhance their in vivo persistence and function, CAR genes were further modified by adding one or two co-stimulator domains derived from co-stimulatory molecules such as CD28, 4-1BB, and OX-40 (Figure 7)[69]. At the end of 2017, the Food and Drug Administration (FDA) had approved two distinct CAR T cell therapies using modified CARs to treat acute lymphoblastic leukemia and large B-cell lymphoma. These groundbreaking successes have spurred research to apply CAR T cell therapy to solid tumors including HCC, beyond hematological malignancy.
GPC3 was frequently used as a target for CAR T cells, since it is expressed abundantly in HCC cells[32]. The CAR gene was generated by fusing the anti-GPC3 single chain variable region (scFv), CD8α hinge, CD28 transmembrane and intracellular signaling domain, 4-1BB, and CD3ζ[70]. The resultant GPC3-targeted CAR T cells could effectively kill GPC3-positive HCC cells, but not GPC3-negative cells, in vitro. Moreover, GPC3-targeted CAR T cells eradicated HCC xenografts with a high level of GPC3 expression, and efficiently suppressed the growth of HCC xenografts with a low GPC3 expression level, in a preclinical mouse model. Similar observations were observed on T cells with GPC3-specific CARs that encoded CD3ζ with costimulatory domains derived from CD28, 4-1BB, or CD28 and 4-1BB[71]. These observations promoted two phase I clinical trials to examine the safety of anti-GPC3 CAR T cell transfer into HCC patients (NCT02395250, NCT02723942). These studies have been completed but the results are not yet available.
In order to reduce off-tumor toxicity, Chen and colleagues prepared dual-targeted CAR T cells coexpressing GPC3 and asialo-glycoprotein receptor 1 (ASGR1) (a liver tissue-specific protein)-targeted CARs containing both CD28 and 4-1BB signaling domains, and proposed that dual-target T cells can reduce the risk of off-tumor toxicity while maintaining relatively potent antitumor activities for GPC3+ASGR1+ HCC[72]. Moreover, CAR T cells were generated to target EpCAM[73] and mucin 1[74], and phase I clinical trials are in progress to evaluate their safety (NCT03013712 and NCT02587689).
Collectively, CAR T therapy for HCC is still in its infancy and requires further progress in many aspects: Selection of appropriate TAAs, enhancement of the binding affinity of CAR to TAAs, improvement of trafficking of CAR T cells to tumor site, and prolongation of in vivo survival of CAR T cells. Advances in these aspects are required for the clinical application of CAR T cells for HCC therapy.
Human NK cells express CD56 but not CD3, and are a major player in innate immunity involved in defense against both cancers and some virus-infected cells[75]. NK cells express germline-encoded activating and inhibitory receptors, and the balance between these two distinct types of receptors determines NK cell function. Activating receptors bind ligands on the target cells and induce cell lysis, whereas inhibitory receptors recognize MHC class I molecules that normal cells abundantly express, and eventually inhibit cytotoxicity exerted by activating receptors[76]. NK cells can kill target cells by releasing cytotoxic granules or utilizing death-inducing receptors including Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)[75]. Moreover, antibody-dependent cell-mediated cytotoxicity (ADCC) is exerted mainly by NK cells[77]. Furthermore, although NK cells were once considered to lack memory capacity, accumulating evidence indicates that NK cells can exert immunological memory[78]. Due to these properties, NK cells can be a potent candidate cell type for immune therapy.
Autologous highly purified NK cells can be an ideal candidate, but their low number in peripheral blood precludes their use. NK cells possess killer inhibitory receptors, which can inhibit NK cell responses to the cells expressing the same MHC class I[76]. Thus, NK cells can kill only the cells that do not express their own MHC class I. As a consequence, allogenic NK cells can kill cancer cells expressing different MHC class I more efficiently than autologous NK cells, which share MHC class I with the cancer cells[79]. One clinical trial has been conducted to examine the efficacy of adoptive NK cell transfer for preventing HCC recurrence after curative therapy, but with no results available (NCT02008929).
Liver NK cells can express TRAIL more abundantly upon activation and can exhibit stronger killing activity against HCC, compared with circulating NK cells[80]. Moreover, evidence is accumulating to indicate few cytotoxic effects of TRAIL on normal cells including hepatocytes[81]. Actually, adoptive transfer of IL-2-stimulated NK cells obtained from donor livers increased an antitumor response against HCC in recipients, who were treated with a liver transplant from a live donor, without causing any injury in normal hepatocytes[82]. These promising results paved the way to initiate a phase I clinical trial to examine the feasibility and safety of IL-2-activated NK cells obtained from cadaveric donor liver grafts when they were adoptively transferred to liver transplant recipients with HCC (NCT01147380). No severe adverse effects were observed in the 18 patients who received liver NK cells, indicating the safety of the treatment.
NKT cells are specialized CD1d-restricted T cells that recognize lipid antigens to stimulate both innate and adaptive immune cells in the tumor microenvironment, once activated[83]. In a mouse preclinical model, adoptive transfer of either NKT cells pulsed with HCC-derived antigens or NKT cells obtained from immunized donors resulted in complete disappearance of tumors within four weeks and attenuated weight loss, together with increased serum IFN-γ, IL-12, and IL-4 levels[84]. These promising results led to the initiation of a phase I clinical trial using autologous NKT cells to treat HCC, but the results are not yet available (NCT010801852).
CIKs are non-MHC-restricted cytotoxic cells, which are expanded ex vivo from PBMCs stimulated with anti-CD3 antibody, IL-2, and IFN-γ, and can even exhibit potent in vivo anti-tumor effects[85]. CIKs are T cells that have acquired the natural cytotoxic potential of NK cells[86]. Thus, the cells can recognize tumor cells by using mainly the natural killer group 2 member D (NKG2D) receptor, and eventually kill them without a prior exposure or priming[87,88]. CIKs have typical phenotypes, characteristic of terminally differentiated CD8+ effector memory cells, and simultaneously recognize target cells in a MHC class I-restricted manner[86]. A meta-analysis was conducted on 11 clinical trials with CIK cells for solid tumors including HCC and gastric cancer[89]. The treatment was well tolerated, with a low incidence of severe adverse effects. Of the 384 patients where a clinical response was reported, 24 patients showed a complete response, 27 patients showed a partial response, 40 patients showed a minor response, 161 patients had stable disease, and 129 patients had progressive disease. Disease-free survival rates were significantly higher in patients treated with CIK cells than those in the control group without CIK treatment. A decrease in tumor volume was only described in three patients. Interestingly, a reduction of hepatitis B virus load was described in patients undergoing treatment with CIK cells. These promising results spurred the application of CIK-based immunotherapy to HCC treatment. To date, eight randomized clinical trials (RCTs), six prospective studies, and three retrospective studies have been reported[90]. A meta-analysis of these studies revealed that CIK treatment increased survival rate as a whole, but without any significant prolongation of progression free-survival. Moreover, patients in the CIK cell-treatment group had lower rates of relapse even in RCTs. To date, two phase III clinical trials using CIKs have been completed (NCI00769106, 01749865) but the results are not yet deposited in the database. In order to enhance the efficacy of adoptive transfer of CIKs, patients with HCC were treated with RFA and three courses of immunotherapy, which consisted of the co-injection of CIKs with immature or tumor cell lysate-pulsed DCs[91]. The treatment was well-tolerated, while CD4+CD25high Tregs decreased with a reciprocal increase in CD8+CD28- effector cells one month after the treatment, but no differences were observed six months after treatment. A phase I/II clinical trial is now in progress to evaluate the combination of CIKs, DCs, and anti-PD-1 antibody for HCC treatment (NCT02886897).
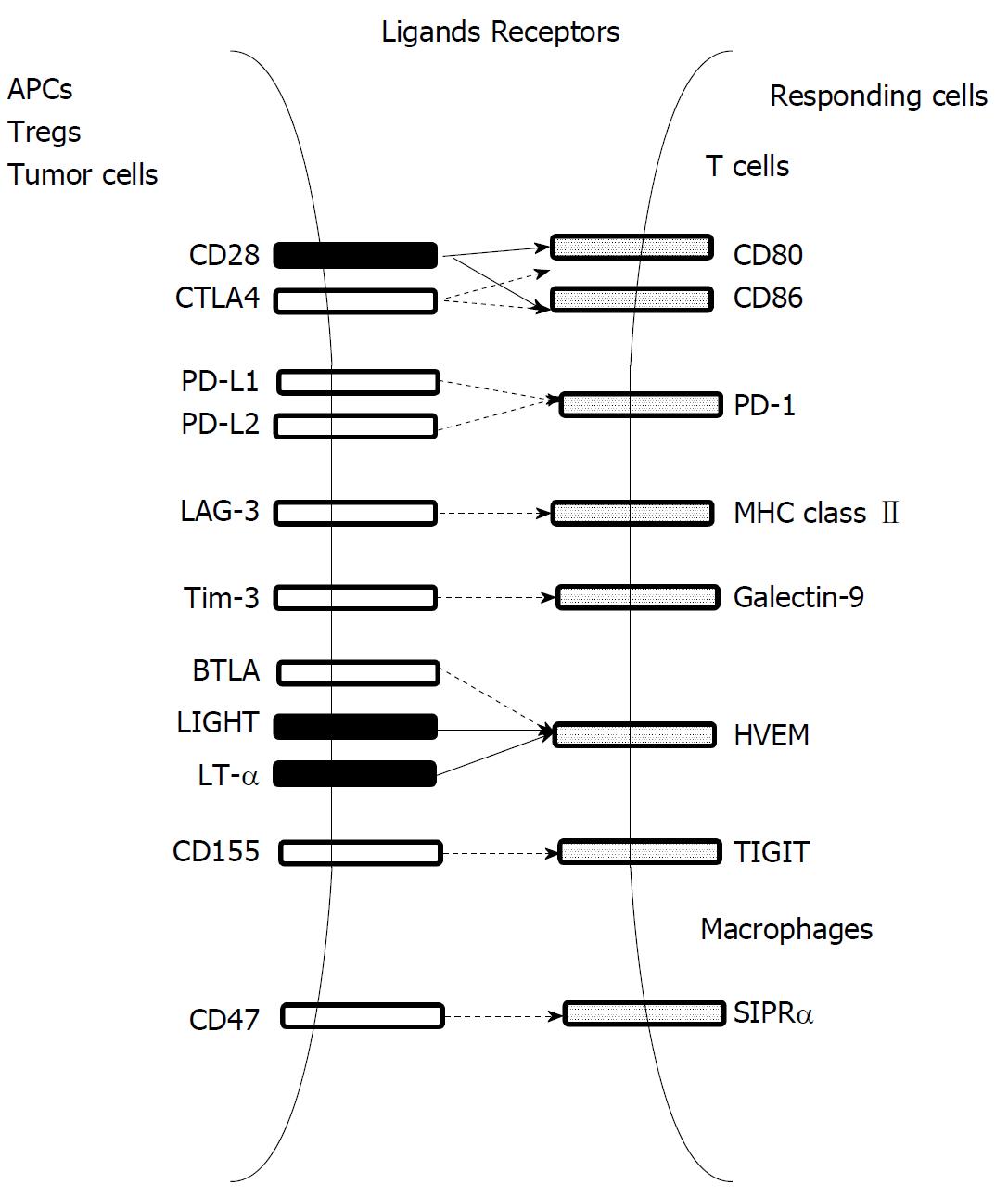
T cells can induce tumor regression upon recognizing TAAs expressed by tumor cells[92], but tumors frequently progress even in the presence of abundant TAA-specific CTLs in tumor tissues[93]. This paradoxical tumor growth can arise from multiple immune suppressive pathways that impair the function of CTLs present in tumor tissues[94]. The most notable immune suppressive mechanisms are immune checkpoint pathways, which include CTLA-4, PD-1-PD-L1/PD-L2, CD47-singal regulatory protein-α (SIRPα), lymphocyte activation gene 3 (LAG-3), T-cell immunoglobulin mucin-3 (Tim-3), T-cell tyrosine-based B and T lymphocyte attenuator (BTLA), and inhibitory motif domain (TIGHT) (Figure 8)[24]. These pathways can dampen T cell activation through ligand-receptor interactions. Moreover, T cell response can also be negatively regulated by several types of resident cells present in the tumor microenvironment, such as Tregs and myeloid-derived suppressor cells (MDSCs)[94].
The concept of tumor immunotherapy has been drastically changed by the clinical success of CTLA-4 and/or PD-1-PD-L1/PD-L2 blockade in treating several types of advanced solid tumors. As a consequence, unleashing the immunosuppressive tumor microenvironment becomes a potential therapeutic measure to enhance tumor immunity. In the next sections, we discuss immune checkpoint therapy and the potential of immune suppressive cell blockade as a novel type of immunotherapy.
Immune checkpoint therapy: Cancer cells or other resident cells in the tumor microenvironment express various ligands that inhibit or stimulate immune activity, and these ligands bind their corresponding receptors on immune cells, thereby modulating immune responses[94]. The ligand-receptor pairs are denoted as immune checkpoints (Figure 8), which control effector T cell- and NK cell-responses at multiple steps from priming by APCs to activation[24]. Based on accumulating evidence to indicate the presence of T cell dysfunction in the tumor microenvironment, a novel type of immunotherapy, immune checkpoint therapy, has been proposed to reverse T cell dysfunction through unleashing immune suppression mediated by inhibitory immune checkpoint pathways. Due to their remarkable effectiveness observed on several types of cancers, the FDA has already approved the antagonistic antibodies targeting two immune checkpoint pathways - CTLA-4 and PD-1-PD-L1/PD-L2 - for cancer treatment[95].
CTLA-4 is expressed on T cells and has greater affinity for CD80 and CD86, the molecules that are expressed on APCs and can bind the co-stimulatory molecule CD28 (Figure 5A)[96]. The interaction between CD28 and CD80/86 is indispensable for T cell activation, particularly at the priming phase. CTLA-4 can interfere with the interaction between CD80/CD86 and CD28, thereby rendering T cells unresponsive to an antigen. Moreover, Tregs can inhibit immune responses using CTLA-4 expressed on their surface[97]. Thus, an antagonistic anti-CTLA-4 antibody was clinically evaluated in advanced melanoma patients, and it elicited enhanced immune responses, with a clinical response in a substantial proportion of patients[95]. This promising observation spurred a phase I clinical trial using an anti-CTLA-4 monoclonal antibody (tremelimumab) for HCC patients with chronic HCV infection (NCT01008358)[98]. Tremelimumab was well tolerated, without any severe adverse effects except an intense, but transient, elevation of transaminases after the first dose in some patients. Specific anti-HCV immunity was enhanced with a significant drop in viral load, but new emerging variants of the hypervariable region 1 of HCV replaced the predominant variants present before therapy. The partial response rate was 17.6% and the disease control rate was 76.4% with time to progression of 6.48 mo. Another phase I clinical trial evaluated the efficacy of tremelimumab for advanced HCC patients when combined with TACE or RFA (NCT01853618)[99]. No dose-limiting toxicities were reported. Of the 19 evaluable patients, five achieved a confirmed partial response. After the treatment, viral load was reduced markedly in 12 of 14 patients with HCV infection. Moreover, at six months after the treatment, tumor biopsies showed an apparent increase in CD8+ T cells restricted to the patients showing a clinical benefit. Six and 12-mo probabilities of tumor progression-free survival were estimated to be 57.1% and 33.1%, respectively, with median time to tumor progression of 7.4 mo and median overall survival of 12.3 mo. Additionally, one phase I/II clinical trial is in the process of recruitment to examine the efficacy of anti-CTLA-4 antibody in combination with ablative therapy (NCT02821754). Nevertheless, a large-scale phase III clinical trial is required to validate these observations.
In contrast to CTLA-4, the PD-1-PD-L1/PD-L2 pathway dampens T cell activation mainly at its effector phase (Figure 5B)[100]. PD-1 is expressed on a wide variety of immune cells, including activated CD4+ and CD8+ T cells, B cells, NK cells, monocytes, and DCs. PD-L1 is expressed on a wide variety of cells, including non-hematopoietic cells such as endothelial cells, mesenchymal stem cells, and corneal cells, as well as hematopoietic cells such as T and B cells, DCs, macrophages, and mast cells. On the contrary, PD-L2 expression is restricted to activated DCs, macrophages, and mast cells. Moreover, PD-L1, as well as PD-L2, is expressed on various tumor cells. As a consequence, in the tumor microenvironment, the interaction between PD-1 and PD-L1/PD-L2 can dampen T cell receptor-mediated signaling pathways to inhibit T cell activation and subsequent antitumor immunity[100].
Immunohistochemical analysis demonstrated an increased expression of PD-1 and PD-Ls in HCC tissues, with PD-1 expression in liver-infiltration lymphocytes and PD-L1 and PD-L2 expression in non-parenchymal liver cells and tumor cells[101]. Moreover, PD-L1 expression was significantly correlated with hepatitis B virus infection and with HCC stage. Consistently, the expression of PD-Ls positively correlates with FoxP3+ Treg infiltration but not granzyme B-expressing CTL infiltration, suggesting that PD-L expression contributes to immunosuppression in HCC tissues[102]. Moreover, a higher expression of PD-L1 and PD-L2 in HCC tissues has been associated with poorer prognosis. Together with a good safety and substantial clinical responses to the treatment with anti-PD-1 or anti-PD-L antibodies in patients with several types of solid tumors, particularly non-small cell lung carcinoma[103,104], these observations provide a rationale for initiating a clinical trial using anti-PD-1 or anti-PD-L1/PD-L2 antibody for HCC treatment.
A Phase I/II clinical trial was conducted with the support from Bristol-Myers Squibb to evaluate the safety and efficacy of anti-PD-1 monoclonal antibody (nivolumab) for histologically confirmed advanced HCC patients, who were included regardless of complicated HCV or HBV infection, and previous sorafenib treatment[105]. A total of 262 eligible patients were treated with 48 patients in the dose-escalation phase and 214 in the dose-expansion phase, and 202 (77%) of 262 patients have completed treatment. During dose escalation, nivolumab showed a manageable safety profile, including acceptable tolerability and 3 mg/kg every two weeks was chosen as a dosage for dose expansion. The objective response rate in the dose-expansion phase was 20%, at similar levels when a single administration of nivolumab was given for other types of solid tumors[100]. A phase I/II clinical trial has just started to evaluate another anti-PD-1 monoclonal antibody, pembrolizumab, for HCC (NCT 02702414).
The promising results have encouraged the initiation of several phase III clinical trials for HCC patients (Table 1). A phase III clinical trial was conducted to compare the efficacy of nivolumab with that of sorafenib as a first-line therapy (NCT 02576509), but the results are not yet available. Recently, another phase III clinical trial was started to investigate if nivolumab would improve recurrence-free survival, compared with placebo in HCC patients who have undergone complete resection or have achieved a complete response after local ablation, and who are at high risk of recurrence (NCT03383458). Additionally, a phase III trial of pembrolizumab (MK-3475) was conducted in patients with advanced HCC who were systemically treated previously (NCT02702401). The primary objectives of this study were to determine progression-free survival and overall survival of pembrolizumab plus best supportive care (BSC) compared with placebo plus BSC. The following phase III trial was planned to determine the efficacy and safety of pembrolizumab or placebo given with BSC in Asian patients with HCC (NCT03062358). In Japan, a phase III, randomized, open-label, multicenter, global study was designed to compare the efficacy and safety of tislelizumab (BGB-A317) versus sorafenib as a first-line systemic treatment in patients with unresectable HCC (NCT03412773). This study also includes a substudy investigating the safety, tolerability, pharmacokinetics, and preliminary efficacy in HCC in Japanese patients.
| NCT number | Targets | Experimental arm | Comparator arm | Outcome measures | Enrollment | Start date | Completion date | Locations |
| NCT02576509 | PD-1 | Nivolumab | Sorafenib | OS;PFS/PD-L1 expression/ORR | 726 | 25-Nov-15 | 22-Jun-19 | Australia, Austria, Belgium, Canada, China, Czechia, France, Germany, Hong Kong, Israel, Italy, Japan, South Korea, Poland, Russian Federation, Singapore, Spain, Sweden, Switzerland, Taiwan, United Kingdom, United States |
| NCT02702401 | PD-1 | Pembrolizumab + BSC | Placebo + BSC | PFS/OS; ORR/ DCR/TTP/DOR | 408 | 26-May-16 | 1-Feb-19 | |
| NCT03062358 | PD-1 | Pembrolizumab + BSC | Placebo + BSC | OS; PFS/ORR/DOR/ DCR/TTP/AE/Discontinuation | 330 | 27-Apr-17 | 23-Dec-19 | China, Hong Kong, South Korea, Malaysia, Taiwan |
| NCT03298451 | PDL-1 | Durvalumab + tremelimumab | Sorafenib | OS; PFS/ORR/DOR/DCR/TTP/PK | 1200 | 11-Oct-17 | 27-Mar-20 | United States, Brazil, Canada, China, France, Germany, Hong Kong, India, Italy, Japan, Russia, Spain, Taiwan, Thailand, Ukraine, Vietnam |
| NCT03383458 | PD-1 | Nivolumab | Placebo | RFS; OS/TTR | 530 | 18-Dec-17 | 2-May-25 | Japan, South Korea, Taiwan, United States |
| NCT03412773 | PD-1 | Tislelizumab (BGB-A317) | Sorafenib | OS; Safety/AE/DLT/Cmax/Cmin/AUC/ADA/Vital signs/physical examination/clinical laboratory results/electrocardiogram/ORR/PFS/DOR/TTP/HRQoL DCR/CBR/Anti-BGB-A317 antibody | 660 | 28-Dec-17 | May-22 | United States |
Other immune checkpoint pathways are proposed to be candidates for immune checkpoint therapy (Figure 8). SIPRα is a unique immune checkpoint molecule expressed on myeloid cells, particularly on macrophages but not lymphoid cells, and binds CD47, which is expressed abundantly on various types of cancer cells[106]. The CD47-SIRPα interaction can inhibit macrophage function, including its phagocytosis capacity, and therefore, CD47 blockade promotes macrophage phagocytosis of cancer cells[107]. Moreover, several preclinical studies demonstrated that CD47 blockade reduces tumor growth by enhancing macrophage phagocytosis and inducing macrophage phenotype change from pro-tumorigenic M2 to pro-inflammatory and anti-tumorigenic M1 states[108-110]. These promising results spurred the development of various agents targeting the CD47-SIRPα axis, including humanized anti-CD47 monoclonal antibody, SIRPα fused with and human IgG1 Fc portion, and SIRPα variant protein, and the clinical trials using these agents have been initiated[106]. However, these clinical trials are still in the process of patient enrollment.
Treg and anergic T cells abundantly express LAG-3, which binds a nonholomorphic region of MHC class II with greater affinity than CD4 and thereby can negatively regulate CD4+ cell proliferation and cytokine production[111]. Phase I clinical trials were conducted to examine an LAG-3 antagonist or anti-LAG-3 antibody for treating several solid tumors but not HCC[112]. Tim-3 is expressed on IFN-γ-producing T cells, Tregs, DCs, and macrophages, and can suppress their function upon binding its ligand, galectin-9[113]. Tumor outgrowth can be linked to the exhaustion of TAA-specific CD8+ T cells, which frequently express Tim-3 and PD-1 simultaneously[114]. Moreover, the combined targeting of the Tim-3 and PD-1 pathways is more effective in controlling tumor growth in mouse preclinical models than targeting either pathway alone[115]. This observation incited the initiation of several phase I/II clinical trials on the combined administration of anti-Tim-3 and anti-PD-1 antibodies to patients with various solid tumors (NCT02608268, 02817633, 03099109), but the results are not yet available. Another immune checkpoint molecule, BTLA, is expressed on T cells, resting B cells, macrophages, and DCs, and binds herpesvirus entry mediator (HVEM), a member of the tumor necrosis factor (TNF) receptor family, which binds with LIGHT and lymphotoxin-α, members of TNF family[116]. The BTLA-HVEM interaction delivers co-inhibitory signals, whereas the LIGHT-HVEM interaction delivers co-stimulatory signals[116]. Aberrant expression of the BTLA-HVEM axis in tumor tissues[117] and BTLA-mediated inhibition of human CD8+ tumor-specific T cell functions[118] suggest that this axis may be able to be used for cancer immunotherapy. Additionally, TIGIT is expressed on activated T cells, memory T cells, Tregs, and NK cells and can dampen T and NK cell functions through interacting with CD155 expressed on APCs and tumor cells[119]. However, their roles in tumor immunity still remain enigmatic.
Immune checkpoint therapy can confer cancer patients with a remarkable clinical efficacy and durable response, even at advanced disease stages, but many patients do not respond to the therapy. Several measures have been proposed to increase the efficacy of the treatment. One is the identification of a biomarker to select patients who are sensitive to checkpoint blockades[120,121]. PD-L1 overexpression was proposed to be a predictive biomarker for the response to PD-1/PD-L1 antibodies, but PD-L1 staining has low prediction accuracy. Other candidate biomarkers include intratumoral lymphocyte infiltrates and genetic markers such as oncogenic mutations, mismatch repair deficiency, and mutation loads[120,121]. Most HCC cases develop in the presence of chronic inflammation, which can cause innumerable genetic mutations (Figure 1). Thus, genome-wide analysis on HCC genetics may be helpful to determine which patients can respond well to immune checkpoint therapy. Furthermore, recent clinical trials revealed that patient HLA class I genotype influences the response to the treatment with anti-CTLA-4 and ani-PD-1 antibodies in melanoma and lung cancer patients[122]. Maximal heterozygosity at HLA class I and the HLA-B44 supertype was associated with a favorable response, whereas the HLA-B62 supertype or somatic loss of HLA class I heterozygosity was associated with poor outcome. A good response in patients with the HLA-B44 supertype suggests the possibility of improving the efficacy of immune checkpoint therapy by introducing a neoantigen-based therapeutic vaccine.
Another way to enhance the efficacy of immune checkpoint therapy is the combined administration with other treatment modalities, such as radiotherapy, chemotherapy, or molecular targeted therapies[123]. Especially, radiotherapy can cause the abscopal effect, where localized radiation-induced tumor cell death can induce anti-tumor responses against tumors at other sites[124]. Immune checkpoint therapy may be able to augment radiotherapy-induced abscopal effects, and several clinical trials were initiated to evaluate the combined treatment of anti-PD-1 antibody with βirradiation in HCC patients(NCT03033446, 02837029, 03099564). Moreover, phase I/II clinical trials are now evaluating the combined treatment of the anti-PD-1 antibody with anti-angiogenic agents (NCT02572687, 03006926, 02856425, 02942329, 02988440) or molecular targeted therapies (NCT02423343, 02859324, 03095781, 02474537, 02325739) in HCC patients. However, the results are not yet available.
Each immune checkpoint therapy acts at a distinct phase of the immune response to the tumor[123] and therefore, the combination of different immune checkpoint therapies are proposed or being evaluated to treat various types of cancers. However, at present, four phase I/II (NCT01658878, 02519348, 02821754, 03222076) and one phase III clinical trial (NCT03298451) are in progress to evaluate the combined administration of anti-CTLA-4 with either anti-PD-1 or anti-PD-L1 antibody in patients with HCC.
Immune checkpoint therapy can reverse tumor-induced T cell exhaustion, but impaired DC function can depress T cell priming and activation, thereby reducing T cell trafficking to tumor cells[125]. Thus, the supplementation of DC vaccine therapy may be able to enhance the effectiveness of immune checkpoint therapy.
Collectively, immune checkpoint therapy can be a promising therapeutic modality for HCC treatment and/or prevention of its recurrence after curative local and regional therapy, but its clinical application may require an additional thorough analysis to select optimal patients and determine efficient co-administration methods.
Blockade of immune suppressor cells: Tregs and MDSCs are two distinct types of hematopoietic cell-derived immunosuppressive cells present in tumor tissues. Tregs express a transcription factor, FoxP3, and can suppress aberrant T cell-mediated immune responses against TAAs as well as self-antigens through several mechanisms[126]. Tregs display abundantly high-affinity IL-2 receptor α chain (CD25), which can bind IL-2 to limit its amount available to effector T cells, thereby attenuating effector T cell activation and proliferation. Tregs constitutively express CTLA-4 to depress CD80/CD86-mediated co-stimulatory signals and secrete immune suppressive mediators including IL-10 and transforming growth factor (TGF)-β. A detrimental role of Tregs was suggested by an inverse correlation of intratumoral Tregs with overall survival in patients with various types of cancers including HCC[127,128]. Thus, reducing the number of intratumoral Tregs and/or dampening their function may be effective to enhance tumor immunity.
The reduction of intratumoral Tregs was achieved in several mouse models by treating with an anti-CD25 antibody, and this reduction was associated with depressed tumor growth[129]. Moreover, anti-tumor effects were synergistically enhanced by co-administration with an anti-PD-1 antibody. However, the efficacy of the anti-CD25 antibody awaits validation in clinical trials. In other mouse models, intratumoral Tregs and tumor growth were reduced also by treating with an antibody for the chemokine receptor CCR4, which is abundantly expressed on Tregs[130]. The observations were translated into a phase I clinical trial which is in progress to evaluate the combination of anti-CCR4 antibody and anti-PD-1 antibody for various solid tumors except HCC (NCT02946671). Tregs and CD8+ effector cells express glucocorticoid-induced TNF receptor (GITR), and its triggering can abrogate the suppressive activity of Treg cells but co-stimulate responder T cells[131]. Consistently, GITR activation can eradicate established tumors in several mouse preclinical models[132,133]. Consequently, several phase I/II clinical trials are now in progress to evaluate the combined treatment of an agonistic anti-GITR antibody or a GITR agonist with other immune checkpoint inhibitors, such as an anti-PD-1 antibody, for several types of solid tumors, but not HCC[134].
Another immunosuppressive cell type present abundantly in tumor tissues is MDSCs, which are a heterogeneous population of myeloid cells with potent immune regulatory activity that are generated during cancer and chronic inflammation[135]. MDSCs consist of two large groups of cells: polymorphonuclear (PMN)-MDSCs and monocytic (M)-MDSCs, which represent immature neutrophils and a pathological state of activation of monocytes, respectively. In humans, PMN-MDSCs share many surface phenotypes with neutrophils, but exhibit a lower density than neutrophils. M-MDSCs exhibit similar surface phenotypes as monocytes do, but do not express MHC class II and CD11c, in contrast with monocytes[135]. Evidence is accumulating to indicate the association of a high frequency of intratumoral MDSCs with poor clinical outcomes in patients with various types of cancers[136,137]. We also observed that the frequency of MDSCs in HCC patients was significantly increased, and was correlated with tumor progression, but not with the degree of liver fibrosis and inflammation[138]. Moreover, the frequency of MDSCs after treatment was inversely correlated with recurrence-free survival time in HCC patients who received curative RFA therapy. These observations promoted the evaluation of treatments targeting MDSCs.
Indeed, treatment with several chemotherapeutic drugs including gemcitabine[139], 5-fluorouracil[140], and anthracyclines[141], decreased intratumoral MDSCs and attenuated tumor growth in several preclinical mouse models. Similar observations were obtained from preclinical models when administered with selective PI3-kinase δ/γ inhibitors[142] or a JAK2/STAT3 inhibitor[143]. Moreover, an antibody against the chemokine receptor CXCR2 inhibited MDSC trafficking to tumors and enhanced anti-PD-1-mediated anti-tumor effects, also in a mouse preclinical model[144]. These promising results spurred the initiation of more than 40 phase I/II clinical trials to evaluate therapies targeting MDSCs in various types of cancers, including one trial on HCC (NCT03203005).
To date, various maneuvers have been proposed to target Tregs and MDSCs as tumor immunotherapies, but their efficacy requires validation through human clinical trials.
Various immune therapeutic modalities have been proposed to eradicate or reduce tumor burden and/or to prevent recurrence after successfully removing a primary tumor in HCC patients. Promising results have been obtained from preclinical and/or phase I clinical trials to evaluate various types of immune therapies for HCC patients, as discussed here. However, to date, only immune checkpoint therapy using an anti-PD-1 antibody has produced favorable outcomes in phase II clinical trials, and these outcomes need validation in large-scale RCTs.
Adoptive immune cell therapy has several hurdles to overcome before its clinical application to HCC treatment. The first one deals with the preparation of cell populations used for adoptive transfer. At present, cell preparation has not been standardized, and, therefore, it is difficult to compare the results reported by different research teams. Moreover, several papers described the results obtained from cell populations prepared under conditions not in compliance with the good manufacturing practice (GMP) conditions. Thus, the cells should be prepared in a standardized manner and under GMP conditions to be used in large-scale RCTs.
The problem inherent in immune therapy is that it can stabilize disease status for a long period without reducing tumor burden, in contrast with the effects exerted by chemotherapy and/or radiotherapy. Moreover, one object of immune therapy for HCC is the prevention of tumor recurrence after a successful local and regional therapy. Thus, it is absolutely necessary to contrive a measure to evaluate immune therapy for HCC from a standpoint distinct from that used to assess chemotherapeutics.
Immune dysfunction can arise in cancer patients at multiple levels including depressed antigen presentation, reduced effector T cell function, and immunosuppressive tumor microenvironments, and therefore, these results suggest distinct mechanisms responsible for immune suppression present in individual cancer patients. These heterogeneities may account for the efficacy of a single type of immune therapy in a limited proportion of patients. Thus, the combination of several distinct modalities may synergistically augment the effectiveness of immune therapy and future studies should explore this. Alternatively, this finding may arise from the presence of several different patient cohorts who respond differentially to a specific immune therapy. If so, it is necessary to detect the good-responder cohort by identifying a biomarker to predict the responsiveness to each immune therapeutic modality.
Collectively, immune therapy for HCC is still in its infancy. However, most HCC can develop repetitively from chronic inflammatory lesions and/or cirrhosis in non-cancerous liver portions, and recurrence has a great impact on the long-term prognosis of patients with HCC[1]. However, these lesions cannot be eliminated by other therapies at all, and only immune therapy can prevent these non-cancerous tissues from progressing into HCC. Thus, it is absolutely necessary to expand immune therapies for HCC to prevent HCC recurrence, and to eventually improve prognosis in patients with HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kamimura K, Tabll AA, Tavakolpour S S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4110] [Article Influence: 587.1] [Reference Citation Analysis (6)] |
| 2. | Koh WP, Robien K, Wang R, Govindarajan S, Yuan JM, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer. 2011;105:1430-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 4. | Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med. 2016;67:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 499] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 5. | Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 2015;15:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J Hepatol. 2016;65:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 611] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 8. | Satyanarayana A, Manns MP, Rudolph KL. Telomeres and telomerase: a dual role in hepatocarcinogenesis. Hepatology. 2004;40:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1345] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 10. | Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell. 2011;19:347-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 11. | Horwitz E, Stein I , Andreozzi M, Nemeth J, Shoham A, Pappo O, Schweitzer N, Tornillo L, Kanarek N, Quagliata L. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 2014;4:730-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 13. | Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus x protein. Cancer Sci. 2006;97:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 15. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1276] [Article Influence: 141.8] [Reference Citation Analysis (2)] |
| 16. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47161] [Article Influence: 3368.6] [Reference Citation Analysis (5)] |
| 17. | Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases - elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1069] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 18. | Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 599] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 19. | Noor MT, Manoria P. Immune Dysfunction in Cirrhosis. J Clin Transl Hepatol. 2017;5:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 736] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 21. | Obeid JM, Kunk PR, Zaydfudim VM, Bullock TN, Slingluff CL Jr. Rahma OE. Immunotherapy for hepatocellular carcinoma patients: is it ready for prime time? Cancer Immunol Immunother. 2018;67:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Vignali DA. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity. 2016;44:1034-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 23. | Chávez-Galán L, Arenas-Del Angel MC, Zenteno E, Chávez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009;6:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 404] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 25. | Elsegood CL, Tirnitz-Parker JE, Olynyk JK, Yeoh GC. Immune checkpoint inhibition: prospects for prevention and therapy of hepatocellular carcinoma. Clin Transl Immunology. 2017;6:e161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902-5908. [PubMed] |
| 27. | Wang XP, Wang QX, Lin HP, Xu B, Zhao Q, Chen K. Recombinant heat shock protein 70 functional peptide and alpha-fetoprotein epitope peptide vaccine elicits specific anti-tumor immunity. Oncotarget. 2016;7:71274-71284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Wang XP, Wang QX, Lin HP, Wang YL, Yang Y. Glycoprotein 96 and α-fetoprotein cross-linking complexes elicited specific antitumor immunity. Cancer Biother Radiopharm. 2013;28:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Butterfield LH, Ribas A, Potter DM, Economou JS. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol Immunother. 2007;56:1931-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Mizukoshi E, Nakagawa H, Kitahara M, Yamashita T, Arai K, Sunagozaka H, Fushimi K, Kobayashi E, Kishi H, Muraguchi A. Immunological features of T cells induced by human telomerase reverse transcriptase-derived peptides in patients with hepatocellular carcinoma. Cancer Lett. 2015;364:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2016;22:275-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Tsuchiya N, Yoshikawa T, Fujinami N, Saito K, Mizuno S, Sawada Y, Endo I , Nakatsura T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;6:e1346764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Sayem MA, Tomita Y, Yuno A, Hirayama M, Irie A, Tsukamoto H, Senju S, Yuba E, Yoshikawa T, Kono K. Identification of glypican-3-derived long peptides activating both CD8+ and CD4+ T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology. 2015;5:e1062209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Tomimaru Y, Mishra S, Safran H, Charpentier KP, Martin W, De Groot AS, Gregory SH, Wands JR. Aspartate-β-hydroxylase induces epitope-specific T cell responses in hepatocellular carcinoma. Vaccine. 2015;33:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Li Y, Cheng P, Wen Y, Chen P, Yang L, Zhao X, Lv H, Quan Q, Wu Y, Yang H. T lymphocyte responses against hepatitis B virus-related hepatocellular carcinoma induced by adenovirus vaccine encoding HBx. Int J Mol Med. 2010;26:869-876. [PubMed] |
| 37. | Pan QZ, Pan K, Wang QJ, Weng DS, Zhao JJ, Zheng HX, Zhang XF, Jiang SS, Lv L, Tang Y. Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells. Stem Cells. 2015;33:354-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Chen Y, Huang A, Gao M, Yan Y, Zhang W. Potential therapeutic value of dendritic cells loaded with NYESO1 protein for the immunotherapy of advanced hepatocellular carcinoma. Int J Mol Med. 2013;32:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Kumai T, Fan A, Harabuchi Y, Celis E. Cancer immunotherapy: moving forward with peptide T cell vaccines. Curr Opin Immunol. 2017;47:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Circelli L, Petrizzo A, Tagliamonte M, Heidenreich R, Tornesello ML, Buonaguro FM, Buonaguro L. Immunological effects of a novel RNA-based adjuvant in liver cancer patients. Cancer Immunol Immunother. 2017;66:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, Qi H, Guo H, Yin H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 42. | Wan X, Cheng C, Lin Z, Jiang R, Zhao W, Yan X, Tang J, Yao K, Sun B, Chen Y. The attenuated hepatocellular carcinoma-specific Listeria vaccine Lmdd-MPFG prevents tumor occurrence through immune regulation of dendritic cells. Oncotarget. 2015;6:8822-8838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Shang N, Figini M, Shangguan J, Wang B, Sun C, Pan L, Ma Q, Zhang Z. Dendritic cells based immunotherapy. Am J Cancer Res. 2017;7:2091-2102. [PubMed] |
| 44. | Osada T, Clay T, Hobeika A, Lyerly HK, Morse MA. NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity. Cancer Immunol Immunother. 2006;55:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 46. | Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Bray SM, Vujanovic L, Butterfield LH. Dendritic cell-based vaccines positively impact natural killer and regulatory T cells in hepatocellular carcinoma patients. Clin Dev Immunol. 2011;2011:249281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Liu Y, Butterfield LH, Fu X, Song Z, Zhang X, Lu C, Ding G, Wu M. Lentivirally engineered dendritic cells activate AFP-specific T cells which inhibit hepatocellular carcinoma growth in vitro and in vivo. Int J Oncol. 2011;39:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Zhou J, Ma P, Li J, Song W. Comparative analysis of cytotoxic T lymphocyte response induced by dendritic cells pulsed with recombinant adeno-associated virus carrying α-fetoprotein gene or cancer cell lysate. Mol Med Rep. 2015;11:3174-3180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | El Ansary M, Mogawer S, Elhamid SA, Alwakil S, Aboelkasem F, Sabaawy HE, Abdelhalim O. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J Cancer Res Clin Oncol. 2013;139:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Xie BH, Yang JY, Li HP, Zhang B, Chen W, Zhou B, Peng BG, Liang LJ, He Q. Dendritic cells transfected with hepatocellular carcinoma (HCC) total RNA induce specific immune responses against HCC in vitro and in vivo. Clin Transl Oncol. 2014;16:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Wang X, Bayer ME, Chen X, Fredrickson C, Cornforth AN, Liang G, Cannon J, He J, Fu Q, Liu J. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J Surg Oncol. 2015;111:862-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Lee JH, Lee Y, Lee M, Heo MK, Song JS, Kim KH, Lee H, Yi NJ, Lee KW, Suh KS. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer. 2015;113:1666-1676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 55. | Lee JH, Tak WY, Lee Y, Heo MK, Song JS, Kim HY, Park SY, Bae SH, Lee JH, Heo J. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6:e1328335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Maeda Y, Yoshimura K, Matsui H, Shindo Y, Tamesa T, Tokumitsu Y, Hashimoto N, Tokuhisa Y, Sakamoto K, Sakai K. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother. 2015;64:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Mukaida N, Matsushima K, Matsui O, Kaneko S. Enhancement of tumor-specific T-cell responses by transcatheter arterial embolization with dendritic cell infusion for hepatocellular carcinoma. Int J Cancer. 2010;126:2164-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Nakamoto Y, Mizukoshi E, Kitahara M, Arihara F, Sakai Y, Kakinoki K, Fujita Y, Marukawa Y, Arai K, Yamashita T. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol. 2011;163:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Aref AM, Hoa NT, Ge L, Agrawal A, Dacosta-Iyer M, Lambrecht N, Ouyang Y, Cornforth AN, Jadus MR. HCA519/TPX2: a potential T-cell tumor-associated antigen for human hepatocellular carcinoma. Onco Targets Ther. 2014;7:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Choi YJ, Park SJ, Park YS, Park HS, Yang KM, Heo K. EpCAM peptide-primed dendritic cell vaccination confers significant anti-tumor immunity in hepatocellular carcinoma cells. PLoS One. 2018;13:e0190638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Yang JY, Li X, Gao L, Teng ZH, Liu WC. Co-transfection of dendritic cells with AFP and IL-2 genes enhances the induction of tumor antigen-specific antitumor immunity. Exp Ther Med. 2012;4:655-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Vogt A, Sievers E, Lukacs-Kornek V, Decker G, Raskopf E, Meumann N, Büning H, Sauerbruch T, Strassburg CP, Schmidt-Wolf IG. Improving immunotherapy of hepatocellular carcinoma (HCC) using dendritic cells (DC) engineered to express IL-12 in vivo. Liver Int. 2014;34:447-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Badalamenti G, Fanale D, Incorvaia L, Barraco N, Listì A, Maragliano R, Vincenzi B, Calò V, Iovanna JL, Bazan V. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2018;pii:S0008-8749(18)30014-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 64. | Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev. 2014;257:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 65. | Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550-4557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1743] [Cited by in RCA: 1632] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 66. | Debets R, Donnadieu E, Chouaib S, Coukos G. TCR-engineered T cells to treat tumors: Seeing but not touching? Semin Immunol. 2016;28:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 67. | Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2122] [Cited by in RCA: 1991] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 68. | Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1091] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 69. | Hoseini SS, Cheung NV. Immunotherapy of hepatocellular carcinoma using chimeric antigen receptors and bispecific antibodies. Cancer Lett. 2017;399:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res. 2014;20:6418-6428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 71. | Li W, Guo L, Rathi P, Marinova E, Gao X, Wu MF, Liu H, Dotti G, Gottschalk S, Metelitsa LS. Redirecting T Cells to Glypican-3 with 4-1BB Zeta Chimeric Antigen Receptors Results in Th1 Polarization and Potent Antitumor Activity. Hum Gene Ther. 2017;28:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Chen C, Li K, Jiang H, Song F, Gao H, Pan X, Shi B, Bi Y, Wang H, Wang H. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol Immunother. 2017;66:475-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 73. | Deng Z, Wu Y, Ma W, Zhang S, Zhang YQ. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 2015;16:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 74. | Qin L, Lai Y, Zhao R, Wei X, Weng J, Lai P, Li B, Lin S, Wang S, Wu Q. Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells. J Hematol Oncol. 2017;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 75. | Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2338] [Cited by in RCA: 2882] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 76. | Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2096] [Cited by in RCA: 2177] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 77. | Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front Immunol. 2015;6:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 400] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 78. | Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 414] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 79. | Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2598] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 80. | Male V. Liver-Resident NK Cells: The Human Factor. Trends Immunol. 2017;38:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17:352-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 82. | Nishida S, Levi DM, Tzakis AG. Liver natural killer cell inoculum for liver transplantation with hepatocellular carcinoma. Curr Opin Organ Transplant. 2013;18:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Nair S, Dhodapkar MV. Natural Killer T Cells in Cancer Immunotherapy. Front Immunol. 2017;8:1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 84. | Margalit M, Shibolet O, Klein A, Elinav E, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma by transplantation of ex-vivo immune-modulated NKT lymphocytes. Int J Cancer. 2005;115:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 86. | Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, Introna M. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol. 2009;37:616-628.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Morisaki T, Onishi H, Koya N, Kiyota A, Tanaka H, Umebayashi M, Ogino T, Nagamatsu I , Katano M. Combinatorial cytotoxicity of gemcitabine and cytokine-activated killer cells in hepatocellular carcinoma via the NKG2D-MICA/B system. Anticancer Res. 2011;31:2505-2510. [PubMed] |
| 88. | Introna M, Correnti F. Innovative Clinical Perspectives for CIK Cells in Cancer Patients. Int J Mol Sci. 2018;19:pii: E358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2011;137:305-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 90. | Yu R, Yang B, Chi X, Cai L, Liu C, Yang L, Wang X, He P, Lu X. Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: a systematic review and meta-analysis. Drug Des Devel Ther. 2017;11:851-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Zhou P, Liang P, Dong B, Yu X, Han Z, Xu Y. Phase I clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol Ther. 2011;11:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 850] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 93. | Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169-6176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 358] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 94. | Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res. 2016;22:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 95. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3582] [Article Influence: 358.2] [Reference Citation Analysis (0)] |
| 96. | Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 838] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 97. | Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 652] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 98. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 751] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 99. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 100. | Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol. 2016;7:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 101. | Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Gao Q, Wang XY, Qiu SJ, Yamato I , Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 658] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 103. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9912] [Article Influence: 762.5] [Reference Citation Analysis (0)] |
| 104. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6290] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 105. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3314] [Article Influence: 414.3] [Reference Citation Analysis (1)] |
| 106. | Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 107. | Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 890] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 108. | Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, Liu J, Majeti R, West RB, Fletcher JA. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA. 2012;109:6656-6661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 109. | Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126:2610-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 110. | Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, Liu J, Achrol AS, Richard C, Sommerkamp P. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS One. 2016;11:e0153550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 111. | Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 112. | He Y, Rivard CJ, Rozeboom L, Yu H, Ellison K, Kowalewski A, Zhou C, Hirsch FR. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016;107:1193-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 113. | Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 638] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 114. | Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 1052] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 115. | Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187-2194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1641] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 116. | Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 117. | Pasero C, Olive D. Interfering with coinhibitory molecules: BTLA/HVEM as new targets to enhance anti-tumor immunity. Immunol Lett. 2013;151:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 118. | Derré L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 119. | Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 349] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 120. | Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 308] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 121. | Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 2103] [Article Influence: 233.7] [Reference Citation Analysis (0)] |
| 122. | Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 819] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 123. | Wilson RAM, Evans TRJ, Fraser AR, Nibbs RJB. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin Exp Immunol. 2018;191:133-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 124. | Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. 2017;280:249-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 125. | Saxena M, Bhardwaj N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer. 2018;4:119-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 126. | Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1359] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 127. | Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 735] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 128. | Mathai AM, Kapadia MJ, Alexander J, Kernochan LE, Swanson PE, Yeh MM. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Arce Vargas F, Furness AJS, Solomon I , Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity. 2017;46:577-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 345] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 130. | Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I , Ezoe S, Kanakura Y, Sato E, Fukumori Y. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945-17950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 532] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 131. | Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 132. | Hu P, Arias RS, Sadun RE, Nien YC, Zhang N, Sabzevari H, Lutsiak ME, Khawli LA, Epstein AL. Construction and preclinical characterization of Fc-mGITRL for the immunotherapy of cancer. Clin Cancer Res. 2008;14:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 134. | Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 135. | Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1359] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 136. | Sun HL, Zhou X, Xue YF, Wang K, Shen YF, Mao JJ, Guo HF, Miao ZN. Increased frequency and clinical significance of myeloid-derived suppressor cells in human colorectal carcinoma. World J Gastroenterol. 2012;18:3303-3309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 137. | Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 138. | Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 139. | Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713-6721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 830] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 140. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 964] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 141. | Zhang Z, Yu X, Wang Z, Wu P, Huang J. Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy. Cancer Lett. 2015;369:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 142. | Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, Silvin C, Van Waes C, Allen C. Anti-PD-L1 Efficacy Can Be Enhanced by Inhibition of Myeloid-Derived Suppressor Cells with a Selective Inhibitor of PI3Kδ/γ. Cancer Res. 2017;77:2607-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 143. | Liu JF, Deng WW, Chen L, Li YC, Wu L, Ma SR, Zhang WF, Bu LL, Sun ZJ. Inhibition of JAK2/STAT3 reduces tumor-induced angiogenesis and myeloid-derived suppressor cells in head and neck cancer. Mol Carcinog. 2018;57:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 144. | Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 602] [Article Influence: 54.7] [Reference Citation Analysis (0)] |