INTRODUCTION
Helicobacter pylori (H. pylori) is a Gram negative bacterium that preferentially colonizes the human gastric mucosa[1,2]. Infection due to this organism is usually established during childhood[1,2], which then causes various upper gastrointestinal (GI) disorders, including atrophic gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer. Thus, it is now generally accepted that persistent H. pylori infection in the gastric mucosa is the highest risk factor for the development of the aforementioned diseases[3]. This notion is supported by recent studies indicating that successful eradication of H. pylori prevents the development of gastric cancer[4,5].
Colonization of the human stomach by H. pylori triggers innate and adaptive immune responses. As in the cases of other microbial infections, sensing of H. pylori by pattern recognition receptors (PRRs) expressed in innate immune cells, such as epithelial cells (ECs) and antigen-presenting cells (APCs), is an initial step for eradicating this organism. Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are the prototypical PRRs and represent the first line of defense against H. pylori[6,7]. Indeed, gastric epithelial cells and APCs express functional TLRs and lipopolysaccharide (LPS)-mediated TLR4 activation is involved in the development of gastric mucosal inflammatory responses[8]. However, the ability to stimulate TLRs by H. pylori-derived antigens is much lower than that by other pathogenic bacteria. For example, H. pylori-derived LPS and flagellin exhibit low stimulatory activity toward TLR4 and TLR5[9,10]. Thus, H. pylori might evade the major innate immune system molecules, TLRs, to establish persistent gastric infection. Therefore, it is possible that PRRs other than TLRs might play a major role in mucosal host defense systems against H. pylori although roles played by TLRs need to be determined in future studies.
NOD1 is a prototypical innate immune receptor belonging to the NLR protein family, which detects small molecules derived from Gram-negative bacteria[7,11]. NOD1 activation induced by intestinal microflora is associated with lymphoid tissue genesis[12] and development of pancreatitis[13-15]. In 2004, Viala et al[16]. demonstrated that gastric mucosal host defense against H. pylori depends on the activation of NOD1 in gastric ECs. Many efforts have been made by gastroenterologists, microbiologists, and immunologists to elucidate the molecular mechanisms by which colonization of the human stomach by H. pylori induces the activation of NOD1 and such NOD1 activation mediates antimicrobial immune responses[11]. In this review, we have summarized and discussed how sensing of H. pylori by NOD1 mediates the prevention of chronic gastritis and gastric cancer.
CYTOKINE AND CHEMOKINE RESPONSES IN THE GASTRIC MUCOSA HARBORING H. PYLORI INFECTION
Gastric inflammation caused by chronic H. pylori infection is mediated by gastric mucosal T helper type 1 (Th1) and Th17 cells producing IFN-γ and IL-17, respectively[17]. Initial studies addressing the role of IFN-γ in H. pylori-induced gastritis revealed that lack of chronic gastritis in IFN-γ-deficient mice is associated with higher colonization of the gastric mucosa by this organism than in IFN-γ-intact mice[18]. In addition, gastric mucosal CD4+ T cells isolated from H. pylori-infected patients have been reported to produce a high level of IFN-γ[19]. Thus, gastric mucosa harboring chronic H. pylori infection is characterized by Th1 responses that are involved in both eradication and inflammation[20]. In addition to a well-established role played by Th1 cells, recent studies have highlighted the importance of another type of Th cells, Th17 cells, producing IL-17[20]. The development of chronic gastritis is significantly attenuated in IL-17-deficient mice in long-term H. pylori infection[20]. Moreover, treatment of mice with a neutralizing anti-IL-17 antibody reduced the H. pylori burden and inflammation in the stomach[21]. In line with these experimental studies, Serrano et al[22]. provided evidence that downregulation of Th17 responses is associated with reduced gastritis in H. pylori-infected patients. Therefore, both Th1 and Th17 cells are involved in the development of chronic gastritis caused by persistent H. pylori infection in the gastric mucosa.
Differentiation of Th1 and Th17 cells requires cytokines produced by APCs such as dendritic cells and macrophages[23]. Differentiation of Th1 cells depends on IL-12, whereas that of Th17 cells depends on IL-1β, IL-6, and IL-23. Expression of IFN-γ and IL-17 in the gastric mucosa of mice challenged with H. pylori was accompanied by IL-12 and IL-23 expression, derived from APCs[21]. Furthermore, the levels of APC-derived pro-inflammatory cytokines in the gastric mucosa, including IL-1β, IL-6 and TNF-α were significantly higher in H. pylori-positive patients than in H. pylori-negative patients[24]. Thus, it is likely that pro-inflammatory cytokines produced by APCs contribute to H. pylori-induced gastric pathology through differentiation of Th1 and Th17 cells. Consistent with this idea, the exposure of human APCs to H. pylori results in robust production of IL-6, IL-12, and TNF-α[25,26].
ECs are an important source of chemokines that attract immune cells to the lesions[27,28]. Yamaoka et al[28]. assessed chemokine responses in the gastric mucosa of patients with H. pylori infection and found that H. pylori infection is associated with increased expression of C-X-C motif chemokine ligand 8 (CXCL8) and chemokine (C-C motif) ligand 5 (CCL5). In addition to CXCL8 and CCL5, the gastric mucosa of H. pylori-positive patients exhibited enhanced expression of CXCL9 and CXCL10[29]. Given the fact that CXCL8 is a strong attractant for neutrophils and that CXCL9 and CXCL10 are strong attractants for Th1 cells[28,29], these results suggest that EC-derived chemokines are also involved in the development of chronic gastritis caused by persistent H. pylori infection. Taken together, these findings suggest that cytokines and chemokines produced by immune cells and ECs play a substantial role in the development of H. pylori-induced gastric pathology.
TYPE IV SECRETION SYSTEM OF H. PYLORI AND NOD1 ACTIVATION
NOD1 is expressed in the cytosolic regions of innate immune cells, such as APCs and ECs[7,11]. Peptidoglycan (PGN) is a polymer consisting of sugars and amino acids that constitute the cell wall of both Gram-positive and Gram-negative bacteria[7]. Small peptides derived from the PGN layer of Gram-negative bacteria activate intracellular NOD1[7,11]. γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP) is considered as the minimal motif of the NOD1 ligand, and NOD1-deficient mice exhibit impaired responses to iE-DAP[30]. Two models have been proposed by which H. pylori activates intracellular NOD1.
H. pylori is classified into two types according to the expression of cag pathogenicity island (cagPAI)[1]. cagPAI is a gene locus necessary to assemble type IV secretion system (T4SS), a syringe and needle-like structure[1,31]. The primary function of T4SS, encoded by cagPAI, is the injection of pathogenic factors, such as cytotoxin-associated gene A (CagA) into the host gastric ECs upon attachment to the epithelium[1,31]. Thus, cagPAI-positive H. pylori can cause gastric mucosal injury through injection of CagA mediated by T4SS. Hence, T4SS may enable H. pylori to deliver its cell wall components, such as PGN, into the host ECs. Viala et al[16]. addressed this possibility and demonstrated that intracellular NOD1 expressed in gastric ECs sense H. pylori-derived PGN delivered to the cytosolic region through T4SS. NOD1 activation is not observed in gastric ECs upon exposure to H. pylori harboring non-functional cagPAI, which supports the idea that NOD1 functions as an intracellular innate immune sensor for cagPAI-positive H. pylori. Interestingly, H. pylori burden in the stomach was much higher in NOD1-deficient mice than in the NOD1-intact ones, when they were orally challenged with cagPAI-positive H. pylori[16]. In contrast, H. pylori burden in the stomach was comparable between NOD1-intact and NOD1-deficient mice when mice were orally challenged with cagPAI-mutated H. pylori. Thus, these studies showed that NOD1 is an intracellular receptor for cagPAI-positive H. pylori and that NOD1 activation is necessary for eradication of this organism.
OUTER MEMBRANE VESICLE OF H. PYLORI AND NOD1 ACTIVATION
Outer membrane vesicles (OMVs), which are released by Gram-negative bacteria during normal growth, contain bacterial cell components, including PGN[32]. Kaparakis et al[33]. addressed the possibility that OMVs released from H. pylori activate cytosolic NOD1 through intracellular delivery of PGN. OMVs isolated from H. pylori activate nuclear factor kappa B (NF-κB) in AGS cells, a gastric cancer cell line, in a cagPAI-independent manner. Importantly, knockdown of NOD1 expression by siRNA abrogated CXCL8 production in AGS cells upon exposure to H. pylori-derived OMVs. Furthermore, intragastrically delivered OMVs efficiently induced gastric mucosal expression of CXCL2, a murine chemoattractant for neutrophils, and antibody responses against OMVs. These innate and adaptive responses to OMVs depend on NOD1 activation because NOD1-deficient mice exhibit defective CXCL2 expression and OMV-specific antibody responses. Thus, these data suggest that intracellular delivery of PGN as a form of OMVs activates NOD1 in gastric ECs in a cagPAI-independent manner.
A study has highlighted the role of autophagy to address the molecular mechanisms accounting for OMV-mediated NOD1 activation[34]. Irving et al[34]. first found that H. pylori-derived OMVs induce autophagy in ECs. Consistent with autophagy induction, mouse embryonic fibroblasts (MEFs) deficient in ATG5, a critical molecule for autophagy, exhibited diminished production of CXCL2 compared with ATG5-intact MEFs upon exposure to H. pylori-derived OMVs. Autophagosome formation was diminished in NOD1-knockdown AGS cells stimulated with H. pylori-derived OMVs, suggesting the involvement of NOD1 activation in autophagy induction. Fluorescent labeling studies clearly demonstrated that EEA1 (early endosome antigen 1)-positive early endosomes containing both OMVs and PGN recruit NOD1 and its downstream kinase, receptor interacting protein 2 (RIP2). Such endosomal interactions between H. pylori-derived OMVs, NOD1, and RIP2 are necessary for chemokine production and autophagy induction, as RIP2 inhibitor efficiently blocks these responses. Collectively, these studies provide the evidence that NOD1 recognizes H. pylori-derived PGN within EEA1+ early endosomes and subsequently activates RIP2 to induce autophagy and pro-inflammatory chemokine responses[34]. However, it should be noted that involvement of RIP2 in the induction of NOD1-mediated autophagy requires future studies, as it has been previously observed that NOD1 activation induces RIP2-independent autophagy in case of Shigella flexneri infection[35].
CYTOKINE AND CHEMOKINE RESPONSES AGAINST H. PYLORI BY NOD1 ACTIVATION
NOD1 senses H. pylori-derived PGN that is delivered to the cytosolic region of gastric ECs via T4SS and/or OMV transport. The next question is how NOD1 activation leads to the induction of Th1 and Th17 responses, both of which are characteristics of chronic H. pylori infection (Figure 1).
Figure 1 Nucleotide-binding oligomerization domain 1-mediated mucosal host defense against Helicobacter pylori infection.
Nucleotide-binding oligomerization domain 1 (NOD1) recognizes Helicobacter pylori (H. pylori)-derived peptidoglycan (PGN) or outer membrane vesicles (OMVs). Sensing of H. pylori-derived PGN or OMVs by intracellular NOD1 in the gastric epithelial cells induces production of type I IFN and C-X-C motif chemokine ligand 10 (CXCL10) through the receptor interacting protein 2 (RIP2)-TNF receptor-associated factor 3 (TRAF3)-interferon regulatory factor 7(IRF7)-IFN-stimulated gene factor 3 (ISGF3) pathway, thereby promoting T helper type 1 (Th1) responses. ISGF3 is a heterotrimeric complex composed of signal transduction and activator of transcription 1 (Stat1), Stat2, and IRF9. NOD1 activation also induces production of anti-microbial peptides (AMPs) through nuclear translocation of nuclear factor-kappa B (NF-κB) subunits. IFN-γ and AMPs exert bactericidal effects.
NOD1 activation leads to the physical interaction between NOD1 and RIP2, its downstream effector molecule[7,11]. NOD1-induced RIP2 activation triggers the pro-inflammatory signaling cascade through nuclear translocation of NF-κB subunits[7,11]. In addition to NF-κB, the interaction between NOD1 and RIP2 leads to the activation of mitogen-activated kinases (MAPKs), including extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38[7,11]. Thus, one major outcome of NOD1-mediated signaling pathways is the activation of NF-κB and MAPKs[7,11]. Activation of NF-κB and MAPKs as well as production of CXCL8 is induced in gastric ECs, such as AGS cells, upon exposure to H. pylori[36-38]. However, it remains controversial whether activation of NF-κB/MAPKs and production of CXCL8 are dependent upon the recognition of H. pylori by NOD1. Grubman et al[36] established a stable AGS cell line with diminished expression of NOD1 (NOD1 knockdown, NOD1 KD cells), and found that NF-κB activation and CXCL8 production are markedly reduced in NOD1 KD cells than in AGS cells with intact NOD1 expression. Moreover, H. pylori-induced CXCL8 production by gastric ECs is partially mediated by MAPK activation following the recognition of this organism by NOD1, as knockdown of NOD1 expression by siRNA results in reduced activation of MAPKs and MAPKs inhibitors efficiently blocks CXCL8 production[39]. These reports support the idea that activation of NF-κB/MAPK and production of CXCL8 induced by exposure to H. pylori are dependent on NOD1. On the other hand, Hirata et al[38] reported that knockdown of NOD1 or RIP2 expression by specific siRNAs did not affect H. pylori-induced NF-κB/MAPK activation or CXCL8 production in AGS cells. Future in vitro studies are required to determine the contribution of NOD1 in NF-κB activation in response to H. pylori infection.
Human gastric mucosa with persistent H. pylori infection is characterized by Th1 responses. CXCL9, CXCL10, and CXCL11 are EC-derived chemokines that play a pivotal role in the generation of Th1 responses through the attraction of Th1 cells expressing C-X-C chemokine receptor type 3 (CXCR3)[40]. High expression of CXCL9 and CXCL10 in the human gastric mucosa with chronic H. pylori infection strongly suggests that CXCL9 and CXCL10 contribute to the generation of Th1 responses[29,41]. We discerned from previous studies that stimulation of colon and gastric cancer cell lines (HT-29 and AGS cells) with NOD1 ligands lead to the robust production of CXCL9, CXCL10, and CXCL11[11,27,42]. Surprisingly, NOD1-induced CXCL10 production by colonic and gastric ECs is not dependent on NF-κB or MAPK activation, because blockade of these pathways by specific pharmacological inhibitors or siRNA transfection did not alter the production of CXCL10[11,27,42]. Instead, NOD1-induced CXCL10 production is markedly decreased by the addition of type I IFN receptor antibody, suggesting that type I IFN production is one of the major outcomes following NOD1 activation. Indeed, HT-29 cells produce a large amount of type I IFN upon stimulation with NOD1 ligand.
Next, we focused on identifying the signaling pathways involved in type I IFN production through NOD1 activation. Detailed knockdown and over-expression studies revealed the involvement of TNF receptor-associated factor 3 (TRAF3) in the induction of type I IFN[11,27,42]. The interaction between NOD1 and RIP2 initiates recruitment of TRAF3 to this complex and leads to the activation of downstream signaling molecules, TANK-binding kinase 1 (TBK1) and IκB kinase ε (IKKε), both of which play an indispensable role in the induction of type I IFN responses through nuclear translocation of interferon regulatory factor 3 (IRF3) and IRF7[43,44]. Indeed, the RIP2-TRAF3-TBK1-IKKε-IRF7 axis plays a key role in inducing the production of type I IFN by ECs[27,42]. Furthermore, NOD1-mediated type I IFN production promotes the transcription of CXCL10 through nuclear translocation of the heterotrimeric complex, IFN-stimulated gene factor 3 (ISGF3), composed of signal transduction and activator of transcription 1 (Stat1), Stat2, and IRF9, because gene silencing of Stat1 or Stat2 by siRNA leads to a marked reduction in CXCL10 production. Thus, these data suggest that NOD1 activation induces the production of type I IFN and CXCL10 through activation of the RIP2-TRAF3-TBK1-IKKε-IRF7-ISGF3 pathway[11,27,42].
The relevance of NOD1-mediated type I IFN responses was examined in animal studies in which NOD1-intact and NOD1-deficient mice were challenged with H. pylori. As expected, NOD1-deficient mice exhibited a higher bacterial burden in the stomach two weeks after the infection, and the effects were accompanied by reduced expression of type I IFN-related factors, such as IFN-β, IFN-γ and CXCL10, rather than NF-κB-related factors, such as TNF-α and CXCL2[11,27,42]. Reduced expression of phospho-Stat1 (p-Stat1) and p-Stat2 is observed in the gastric mucosa of NOD1-deficient mice, when compared with that in NOD1-intact mice. However, comparable levels of NF-κB activation are observed in both mice. Finally, the blockade of type I IFN signaling pathways by Stat1 siRNA increased bacterial burden in the stomach upon oral infection with H. pylori in NOD1-intact mice. Its effects were accompanied by reduced expression of IFN-γ and CXCL10 in the stomach. In contrast, blockade of NF-κB signaling pathways by NF-κB decoy oligonucleotide did not alter the bacterial burden or expression of IFN-γ or CXCL10 in the stomach, although these treatments reduced the gastric expression of TNF-α and CXCL2. Collectively, these data suggest that sensing of H. pylori-derived PGN by intracellular NOD1 in gastric ECs induces production of type I IFN and CXCL10 through the RIP2-TRAF3-TBK1-IKKε-IRF7-ISGF3 pathway and thereby promotes Th1 responses. Because IFN-γ produced by Th1 cells enhances the expression of NOD1[27,42,45], we propose that the type I IFN-CXCL10-IFN-γ axis induced by NOD1 activation forms a positive feedback loop for the generation of Th1 responses in the gastric mucosa with persistent H. pylori infection.
Little is known about the molecular mechanisms accounting for NOD1-mediated Th17 responses in H. pylori infection. In this regard, a recent study has highlighted the importance of NOD1 activation in non-hematopoietic cells, i.e. ECs, in the generation of Th17 responses[46]. Therefore, it is possible that the sensing of H. pylori-derived PGN or OMVs by intracellular NOD1 in gastric ECs is involved in Th17 responses.
RESPONSE OF ANTI-MICROBIAL PEPTIDES AGAINST H. PYLORI BY NOD1 ACTIVATION
Antimicrobial peptides (AMPs) constitute a part of the innate host defense system[47]. AMPs released by APCs and ECs rapidly act to eradicate invading microorganisms[47]. Grubman et al[36] reported that AGS cells release β-defensins upon exposure to cagPAI-positive H. pylori. Production of β-defensins by AGS cells is dependent on NOD1 activation, because stable NOD1-knockdown AGS cells exhibit reduced production of AMPs. Moreover, AMPs induced by exposure to H. pylori exhibit potent H. pylori eradicating activity. Consistent with this, the expression of β-defensin 4 in the stomach is markedly decreased in NOD1-deficient mice than in NOD1-intact mice following H. pylori infection[48]. Collectively, these in vitro and in vivo studies suggest the possible involvement of NOD1-dependent production of AMPs in the mucosal host defenses against H. pylori infection (Figure 1).
NOD1 POLYMORPHISMS AND UPPER GASTROINTESTINAL DISEASES
Several reports have suggested an association between NOD1 gene polymorphisms and upper GI diseases[49-51]. Wang et al[51] identified the NOD1 rs7789045 TT genotype as an increased risk for gastric cancer in a Chinese population. Another Chinese cohort study reported that the risk of gastric cancer is high in H. pylori-infected subjects carrying the NOD1 rs 2709800 TT genotype[52]. Moreover, Hofner et al[53] reported that the G796A NOD1 polymorphism is associated with peptic ulcers in H. pylori-infected patients. Although these studies support the correlation between NOD1 polymorphisms and upper GI disorders caused by H. pylori, the mechanisms by which NOD1 polymorphisms lead to the development of H. pylori-associated diseases remain unknown. Because NOD1 deficiency increases gastric H. pylori burden in animals and its expression is lower in the cancerous tissues of the stomach than in the non-cancerous tissues[54], it would be interesting to study whether NOD1 function is impaired or enhanced in the presence of such polymorphisms associated with gastric cancer.
PREVENTION OF GASTRIC CANCER BY NOD1 ACTIVATION
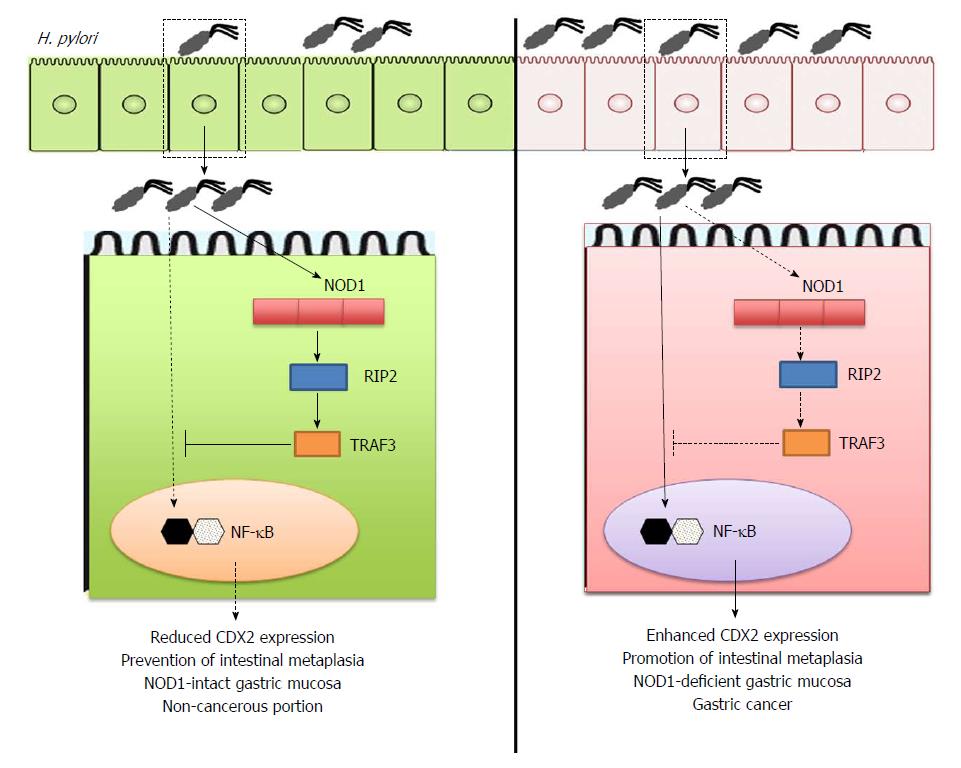
NOD1 activation is required for mucosal host defense against H. pylori infection. This protective effect is partially mediated by the activation of type I IFN signaling pathways following the molecular interaction between NOD1 and TRAF3[11,27]. Suarez et al[54] have addressed the clinical relevance of NOD1-TRAF3 interaction in human H. pylori-associated diseases. They reported that expression levels of NOD1 and TRAF3 are much weaker in gastric cancer tissues than in non-cancerous tissues. Thus, these studies utilizing human gastric cancer samples strongly suggest that impaired activation of NOD1 and TRAF3 is involved in the pathogenesis of gastric cancer and that NOD1-TRAF3 interaction may play a protective role in the development of gastric cancer (Figure 2).
Figure 2 Prevention of gastric cancer development by nucleotide-binding oligomerization domain 1.
Sensing of Helicobacter pylori (H. pylori)-derived peptidoglycan (PGN) by intracellular nucleotide-binding oligomerization domain 1 (NOD1) in gastric epithelial cells induces activation of TNF receptor associated factor 3 (TRAF3) as mentioned in Figure 1. TRAF3 activation by NOD1 negatively regulates expression of caudal-related homeobox 2 (Cdx2) via the inhibition of nuclear factor-kappa B (NF-κB) activation to prevent intestinal metaplasia and gastric cancer (left panel). On the other hand, lack of NOD1-mediated negative regulation on Cdx2 expression promotes the development of gastric cancer (right panel).
Thus, after confirming the possible involvement of NOD1 activation in the development of human gastric cancer, the next question is how NOD1 serves as a protective factor for gastric cancer development. Intestinal metaplasia, wherein the gastric mucosa exhibits an intestinal phenotype, is a pre-neoplastic lesion of gastric cancer[55]. Aberrant expression of caudal-related homeobox 2 (Cdx2)[55], a transdifferentiation factor, in the gastric tissue induces intestinal metaplasia and gastric carcinogenesis. We hypothesized that NOD1 activation inhibits the development of gastric cancer through negative regulation of Cdx2[37]. To address this question, we performed a long-term infection study in which NOD1-intact and NOD1-deficient mice were challenged with cagPAI-positive H. pylori. Interestingly, formation of gastric intestinal metaplasia was observed in NOD1-deficient mice, eight months after initial challenge with H. pylori, but not in NOD1-intact mice. This effect was accompanied with higher expression of Cdx2 in the gastric mucosa of NOD1-deficient mice than in the NOD1-intact mice. On the contrary, expression of TRAF3 was lower in the gastric mucosa of NOD1-deficient mice than in NOD1-intact mice. Furthermore, development of gastric intestinal metaplasia in the absence of intact NOD1 signaling pathways is associated with enhanced activation of NF-κB, because most gastric ECs are positive for nuclear p65 staining. Detection of H. pylori in gastric mucosa exhibiting intestinal metaplasia is difficult in human samples[56]. Consistent with this, H. pylori burden in the gastric mucosa was much lower in NOD1-deficient mice than in the NOD1-intact mice[37]. Thus, the results of our long-term H. pylori infection study support the data[54] obtained from human gastric cancer samples, demonstrating that impaired activation of NOD1-TRAF3 signaling pathways is involved in the development of intestinal metaplasia[37].
Regarding the molecular mechanisms by which NOD1 activation prevents the development of intestinal metaplasia and gastric cancer, we provided evidence that NOD1 activation upon exposure to H. pylori negatively regulates Cdx2 expression through activation of TRAF3[37]. Exposure to H. pylori upregulates Cdx2 expression in gastric cancer cell lines, and the effects are enhanced or diminished by gene silencing of NOD1 by siRNA or over-expression of TRAF3, respectively[37]. Furthermore, promoter gene and gel shift assays revealed that interaction between NOD1 and TRAF3 inhibits the expression of Cdx2 through negative regulation of NF-κB activation. Thus, these in vitro studies have elucidated a part of the molecular mechanisms accounting for the prevention of intestinal metaplasia followed by gastric cancer via H. pylori-induced NOD1 activation. Collectively, these two recent studies strongly suggest that NOD1 activation by H. pylori infection plays a protective role in the development of gastric cancer.
CONCLUSION
NOD1 contributes to mucosal host defense against H. pylori infection through the activation of type I IFN signaling pathways and production of AMPs. In addition, NOD1 activation negatively regulates Cdx2 expression, and thereby inhibits the development of gastric cancer. Molecules involved in NOD1-mediated signaling pathways might be new therapeutic targets for treating chronic gastric diseases and gastric cancer.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Day AS, Ozen H, Slomiany BL S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y