Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1486
Peer-review started: January 20, 2018
First decision: February 3, 2018
Revised: February 7, 2018
Accepted: February 26, 2018
Article in press: February 26, 2018
Published online: April 7, 2018
Processing time: 75 Days and 0.4 Hours
This case highlights a patient with Gilbert syndrome who underwent endoscopic retrograde cholangiopancreatography (ERCP) with removal of bile duct stones, who then experienced an unexplained increase in bilirubin, with total bilirubin (TBIL) levels increasing from 159.5 μmol/L to 396.2 μmol/L and to a maximum of 502.8 μmol/L after 9 d. Following the decrease in the TBIL level, enhanced magnetic resonance cholangiopancreatography (MRCP) was performed to exclude any possible remaining choledocholithiasis. Nevertheless, the serum bilirubin level increased again, with TBIL levels rising from 455.7 μmol/L to 594.8 μmol/L and a maximum level of 660.3 μmol/L with no remaining bile duct stones. A liver biopsy showed severe bile duct cholestasis with no inflammation. Based on the exclusion of other potential causes of hyperbilirubinemia and the fact that both instances of increased bilirubin occurred after ERCP and MRCP, the contrast agents iopromide and gadoterate meglumine were suspected to be the causes of the hyperbilirubinemia. As of the writing of this report, the patient’s bilirubin levels have spontaneously returned to baseline levels. In summary, ERCP and MRCP utilizing the contrast agents iopromide and gadoterate meglumine may possibly induce prolonged hyperbilirubinemia.
Core tip: Over the past years, only few cases of prolonged postendoscopic retrograde cholangiopancreatography jaundice caused by toxicity of the contrast agent iobitridol have been reported in the world. Up to now, no case of postenhanced magnetic resonance cholangiopancreatography-related jaundice has been reported. Persistent jaundice affects the patient’s quality of life, even seriously to life-threatening. Because of the high rarity, treatment experience is not sufficient and more cases need to be accumulated for further analysis.
- Citation: Qian JD, Hou FQ, Wang TL, Shao C, Wang GQ. Gilbert syndrome combined with prolonged jaundice caused by contrast agent: Case report. World J Gastroenterol 2018; 24(13): 1486-1490
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1486.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1486
Gilbert syndrome is a liver disorder caused by a genetic mutation of the bilirubin UDP-glucuronosyltransferase (UGT1A1) gene, which results in elevated levels of unconjugated bilirubin. The elevation of serum bilirubin is usually mild, typically less than 102.6 μmol/L. Most cases of Gilbert syndrome are detected due to mild jaundice after pubescence or a mild elevation of unconjugated bilirubin found by chance during a blood examination[1].
Both endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) can be used for the diagnosis of cholelithiasis. The major complications related to ERCP involve acute pancreatitis, bleeding, sepsis and perforation. Prolonged postERCP jaundice has been described as a rare postERCP complication due to the known toxicity of the contrast agent iobitridol[2]. However, no cases of postenhanced MRCP-related jaundice have been reported. We report here a case of postERCP and postenhanced-MRCP prolonged hyperbilirubinemia due to toxicity from the contrast agents iopromide and gadoterate meglumine.
A 35-year-old man presented with intermittent upper abdominal pain associated with eating greasy food for more than 4 mo. His eyes became yellow, and he reported dark urine and generalized itching for more than 1 mo. The liver test results at the onset of the disease were as follows: total bilirubin (TBIL) 268.7 μmol/L, direct bilirubin (DBIL) 114.4 μmol/L, alanine aminotransferase (ALT) 155 IU/L, aspartate aminotransferase (AST) 71 IU/L, alkaline phosphatase (ALP) 172 IU/L, and gamma-glutamyl transpeptidase (GGT) 424 IU/L. Abdominal computed tomography showed the presence of cholecystolithiasis with features of cholecystitis and dilation of the common bile duct (CBD) and intrahepatic ducts, due to a distal CBD obstruction. MRCP showed a low signal of the distal CBD and bile duct expansion.
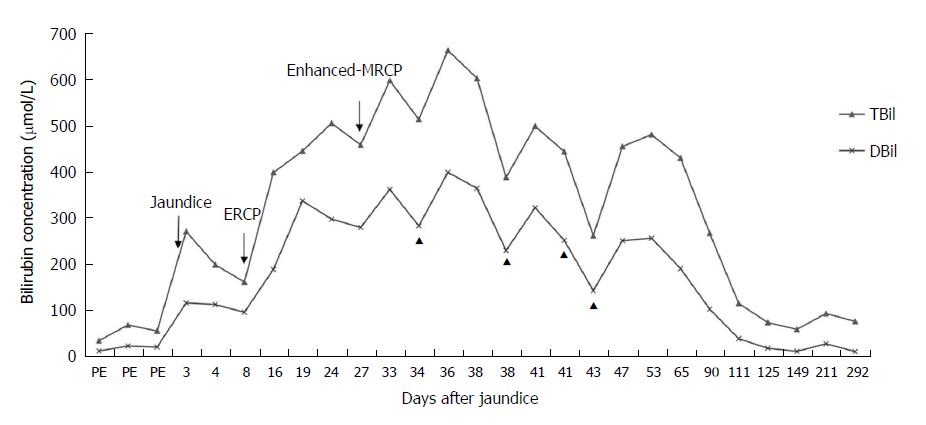
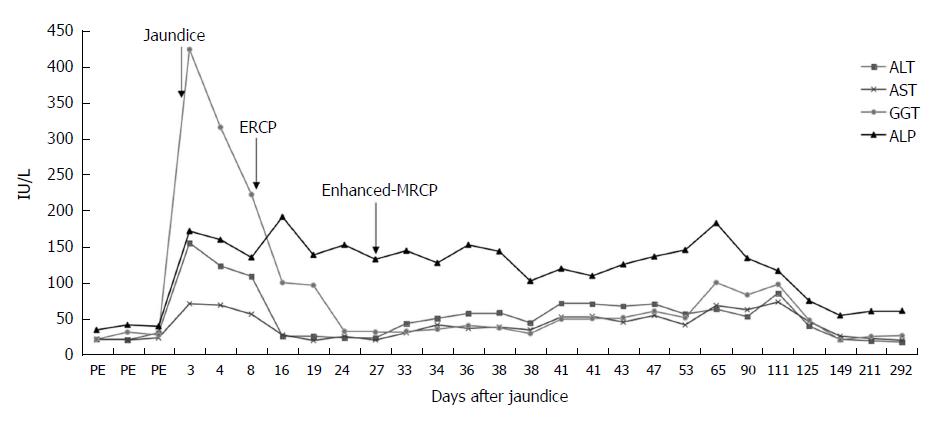
The patient had undergone ERCP with sphincterotomy and extraction of a CBD stone 2 wk before presenting to our hospital, and the contrast agent used was iopromide (370 mg/mL). After ERCP, the patient’s ALT and GGT levels had significantly decreased and quickly returned to normal; although, the bilirubin levels had increased markedly, with the TBIL level increasing from 159.5 μmol/L to 396.2 μmol/L and the DBIL level increasing from 94.2 μmol/L to 186.9 μmol/L, and with the ALP level increasing markedly from 135.6 IU/L to 192 IU/L (Figures 1 and 2). The amylase and lipase levels had been normal. Ursodeoxycholic acid (UDCA) had been prescribed at a dose of 250 mg three times daily, but the bilirubin levels continued to increase, resulting in levels of TBIL up to 442.5 μmol/L and DBIL up to 334.9 μmol/L. Prednisolone (30 mg daily) had been added, although the hyperbilirubinemia did not improve, at which point the patient presented to our hospital for further diagnosis and treatment.
On the day the patient presented to our hospital, his TBIL level was 502.8 μmol/L and his DBIL level was 295.51 μmol/L. Prednisolone was continued at a dose of 30 mg daily. The fourth day after he presented to our hospital, his bilirubin level decreased slightly, with the TBIL level decreasing from 502.8 μmol/L to 455.7 μmol/L and the DBIL level decreasing from 295.51 μmol/L to 277.44 μmol/L. Once his bilirubin levels decreased, he underwent enhanced-MRCP with gadoterate meglumine as the contrast agent to exclude the possibility of any remaining choledocholithiasis. However, after the enhanced-MRCP, the patient’s bilirubin levels increased again, with the TBIL level increasing from 455.7 μmol/L to 594.8 μmol/L and finally to a maximum of 660.3 μmol/L; meanwhile, the patient’s DBIL level increased from 277.44 μmol/L to 359.63 μmol/L and then to a maximum of 396.46 μmol/L (Figure 1). The enhanced-MRCP did not show any positive findings.
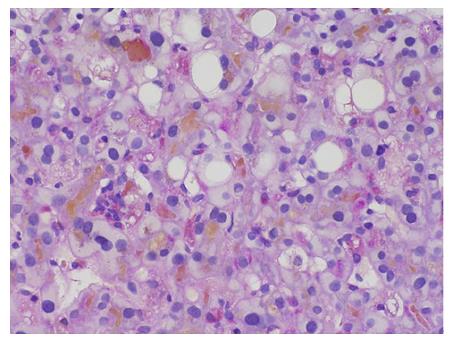
The markers of infection for hepatitis A, B and C, and other viruses (cytomegalovirus, Epstein-Barr virus) were all negative. Immunological tests for antinuclear antibody, smooth muscle antibody, antimitochondrial antibodies, anti-M2 and antibody to liver kidney microsomal antigen were also negative, with the exception of an increase in IgG-4 values (110 mg/dL). The patient’s ferritin level, complete blood count and inflammatory markers were normal. Gilbert gene sequencing was performed, and the analysis revealed the A(TA)7TAA heterozygote [wild type: A(TA)6TAA] in the promoter region (UGT1A1*28) and an UGT1A1.80:-364C>T, UGT1A1.112:-1352A>C heterozygote. A liver biopsy showed marked bilirubinostasis in zones 3 and 2, canaliculi with no evidence of portal tract inflammation or interphase hepatitis, no lesions or paucity of bile ducts, and no bile infarcts or leaks (Figures 3 and 4).
Since the age of 17, the patient had experienced intermittent yellow discoloration of the eyes with no other symptoms. Laboratory examinations revealed normal hemoglobin, reticulocyte, aminotransferases and cholestatic enzymes, while the serum TBIL level fluctuated by 32.8 μmol/L to 66.7 μmol/L, and the ratio of DBIL to TBIL was 32%. The diagnosis of congenital nonhemolytic bilirubin metabolic disorder was confirmed. The patient’s family had no history of similar disorders, but his son had physiologic jaundice at birth. Before onset of the disease, the patient did not take any medications and had no history of alcohol abuse.
Combined with his history of nonhemolytic bilirubin metabolic disorder and genetic test results, the diagnosis of Gilbert syndrome was clear. Because the other potential causes of hyperbilirubinemia were ruled out and the two instances of increased bilirubin occurred postERCP and postMRCP, the use of the contrast agents iopromide and gadoterate meglumine were suspected to be the cause of the hyperbilirubinemia.
The patient was prescribed phenobarbital (60 mg three times daily) and UDCA (500 mg two times daily); prednisolone (30 mg daily) was continued, while cholestyramine (5 g three times daily) was used for symptomatic relief. In addition, with the worsening of cholestasis, bilirubin serum adsorption treatments were performed a total of four times; after each treatment, the serum bilirubin concentration decreased by 11%-23% and clinical symptoms were relieved, but the bilirubin level slightly increased again 1 to 2 d after the bilirubin serum adsorption treatment. Because the bilirubin level declined slowly, the patient was discharged after a 1-mo stay in our hospital.
In the outpatient setting, the oral prednisolone was tapered regularly, and phenobarbital and UDCA were discontinued. At his 11-mo follow-up visit, the serum bilirubin level had slowly decreased, and as of the writing of this report, the patient’s bilirubin levels had spontaneously returned to baseline levels (Figure 1).
Due to the presence of Gilbert syndrome, the patient showed prolonged jaundice postERCP, and enhanced-MRCP accentuated this effect. A liver biopsy revealed marked intrahepatic cholestasis, which may be attributed to large bile obstruction, benign recurrent intrahepatic cholestasis (BRIC) or drug-induced liver toxicity. Enhanced-MRCP had already excluded bile obstruction by stones. BRIC is a rare genetic disorder characterized by intermittent episodes of jaundice and pruritus; each episode can last for several weeks to months. A completely asymptomatic phase occurs in between attacks that can last from months to years. Liver biochemistry is characterized by conjugated hyperbilirubinemia and increased ALP levels. Serum GGT, AST and ALT levels are either normal or mildly elevated. Liver biopsy shows intrahepatic and canalicular cholestasis predominantly involving zone 3[3].
The patient’s liver biopsy features were consistent with BRIC; however, genetic sequencing indicated no mutation in ATP8BI. In addition, the patient had no history of recurrent conjugated hyperbilirubinemia; therefore, the diagnosis of BRIC was not established. Because the two instances of increased bilirubin occurred after ERCP and MRCP, the use of the contrast agents iopromide and gadoterate meglumine was suspected to be the cause of the hyperbilirubinemia.
Prolonged cholestasis is a very rare complication of ERCP, and few cases of this complication are reported in the English literature[2,4-6]. The exact mechanism of this complication, however, remains unknown. Some articles consider that it may be associated with iodine contrast agents (diatrizoate or iobitridol)[2,4], while others posit that it may be caused by cefuroxime, which is one of the cephalosporins used after ERCP to prevent infections[6]. Our patient did not receive cephalosporins postERCP; therefore, the prolonged jaundice was associated with the contrast agents. Additionally, the cause-result time connection between the use of the contrast agents and bilirubin increases supported this possibility.
Gadolinium-based contrast agents (GBCAs) play an important role in enhanced-MRCP examinations. The common side effects of GBCAs include nausea, chest tightness, rash and vascular edema. Gadolinium is mainly renally excreted, although it may also be deposited in the liver, brain, muscle and other organs[7]. No cases of gadolinium-induced cholestasis have been reported in the literature. Although our patient’s bilirubin level increased again after enhanced-MRCP, after his first instance of elevated bilirubin had decreased, we speculated that the gadolinium may have been involved in the cholestasis. The gadolinium used in our patient is ionic, which can lead to high osmotic pressure and dehydration of tissue cells, suggesting that the mechanism of gadolinium-induced hyperbilirubinemia may be due to the high permeability, which further aggravated the damage to the bile duct cells and affected the secretion and excretion of bile.
PostERCP jaundice has been reported to resolve after 2-4 mo, although it took approximately 1 year for this patient’s bilirubin level to return to baseline levels, which may be related to the second incident of damage due to gadolinium.
The general treatment for cholestasis caused by contrast agents is UDCA, and cholestyramine can be used to relieve symptoms. If a trial of these two agents proves unsuccessful, corticosteroids may be added[8] with or without plasmapheresis[9]. In our case, UDCA (500 mg two times daily), cholestyramine (5 g three times daily) and prednisolone (30 mg daily) failed to relieve the patient’s symptoms until bilirubin serum adsorption was performed, which efficiently stabilized the bilirubin level. Therefore, in the case of a poor response to traditional drug treatment methods, providers may try plasma exchange or bilirubin adsorption, which can prevent damage to liver cells due to long-term elevated bilirubin levels.
Our patient’s case illustrates a rare drug-induced liver injury due to contrast agents, which presented as prolonged cholestasis following “successful” therapeutic ERCP for an obstructing distal CBD stone and an enhanced-MRCP that excluded residual stones. Clinicians must be aware that ERCP and MRCP with the contrast agents iopromide and gadoterate meglumine may have the possibility of inducing prolonged hyperbilirubinemia.
A middle-aged male patient presented with abdominal pain, jaundice and dark urine.
The only physical sign of this case was a mild abdominal tenderness.
Viral hepatitis, other viruses infection (cytomegalovirus, Epstein-Barr virus), autoimmune liver disease, IgG-4-related cholangitis, and benign recurrent intrahepatic cholestasis.
The liver test results showed total bilirubin 268.7 μmol/L, direct bilirubin 114.4 μmol/L, alanine aminotransferase 155 IU/L, aspartate aminotransferase 71 IU/L, alkaline phosphatase 172 IU/L, and gamma-glutamyl transpeptidase 424 IU/L.
Abdominal computed tomography showed the presence of cholecystolithiasis, with features of cholecystitis and dilation of the common bile duct (CBD) and intrahepatic ducts due to a distal CBD obstruction.
A liver biopsy showed marked bilirubinostasis in zones 3 and 2, canaliculi with no evidence of portal tract inflammation or interphase hepatitis, no lesions or paucity of bile ducts, and no bile infarcts or leaks.
The patient was prescribed phenobarbital, ursodeoxycholic acid, prednisolone and cholestyramine. In addition, with the worsening of cholestasis, bilirubin serum adsorption treatments were performed a total of four times.
Prolonged cholestasis is a very rare complication of endoscopic retrograde cholangiopancreatography (ERCP), and few cases of this complication are reported in the English literature. The exact mechanism of this complication, however, remains unknown. No cases of post enhanced magnetic resonance cholangiopancreatography (MRCP)-related jaundice have been reported.
ERCP: Endoscopic retrograde cholangiopancreatography, which can be used for the diagnosis of cholelithiasis.
Our patient’s case illustrates a rare drug-induced liver injury due to contrast agents, which presented as prolonged cholestasis following “successful” therapeutic ERCP for an obstructing distal CBD stone and an enhanced-MRCP that excluded residual stones. Clinicians must be aware that ERCP and MRCP with the contrast agents iopromide and gadoterate meglumine may have the possibility of inducing prolonged hyperbilirubinemia.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gkekas I, Isaji S, Kitamura K S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Aiso M, Yagi M, Tanaka A, Miura K, Miura R, Arizumi T, Takamori Y, Nakahara S, Maruo Y, Takikawa H. Gilbert Syndrome with Concomitant Hereditary Spherocytosis Presenting with Moderate Unconjugated Hyperbilirubinemia. Intern Med. 2017;56:661-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Tziatzios G, Gkolfakis P, Papanikolaou IS, Dimitriadis G, Triantafyllou K. An unusual case of prolonged post-endoscopic retrograde cholangiopancreatography jaundice. Hepatobiliary Pancreat Dis Int. 2016;15:220-222. [PubMed] |
| 3. | Luketic VA, Shiffman ML. Benign recurrent intrahepatic cholestasis. Clin Liver Dis. 2004;8:133-149, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Dourakis SP, Mayroyannis C, Alexopoulou A, Hadziyannis SJ. Prolonged cholestatic jaundice after endoscopic retrograde cholangiography. Hepatogastroenterology. 1997;44:677-680. [PubMed] |
| 5. | Chavalitdhamrong D, Donepudi S, Pu L, Draganov PV. Uncommon and rarely reported adverse events of endoscopic retrograde cholangiopancreatography. Dig Endosc. 2014;26:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Niriella MA, Kumarasena RS, Dassanayake AS, Pathirana A, de Silva Hewavisenthi J, de Silva HJ. Worsening cholestasis and possible cefuroxime-induced liver injury following “successful” therapeutic endoscopic retrograde cholangiopancreatography for a distal common bile duct stone: a case report. J Med Case Rep. 2016;10:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Ramalho J, Ramalho M, Jay M, Burke LM, Semelka RC. Gadolinium toxicity and treatment. Magn Reson Imaging. 2016;34:1394-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Lee HM, Bonis PA, Kaplan MM. Persistent cholestatic jaundice after ERCP. Am J Gastroenterol. 2006;101:204-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Saritas U, Aydin B, Ustundag Y. Plasmapheresis and corticosteroid treatment for persistent jaundice after successful drainage of common bile duct stones by endoscopic retrograde cholangiography. World J Gastroenterol. 2007;13:4152-4153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |