Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1429
Peer-review started: January 22, 2018
First decision: February24, 2018
Revised: March 6, 2018
Accepted: March 10, 2018
Article in press: March 10, 2018
Published online: April 7, 2018
Processing time: 72 Days and 12.2 Hours
To compare prognostic relevance of postoperative tumour/node/metastasis (TMN) stages between patients with and without neoadjuvant treatment.
Data from patients with adenocarcinoma of the gastro-oesophageal junction (AEG) who had undergone surgical resection at a single German university centre were retrospectively analysed. Patients with or without neoadjuvant preoperative treatment were selected by exact matching based on preoperative staging. Standard assessment of preoperative (c)TNM stage was based on endoscopic ultrasound and computed tomography of the thorax and abdomen, according to the American Joint Committee on Cancer/Union for International Cancer Control classification system. Patients with cT1cN0cM0 and cT2cN0cM0 stages were excluded from the study, as these patients are generally not recommended for pretreatment. Long-term survival among the various postoperative TNM stages was compared between the groups of patients with or without neoadjuvant treatment. For statistical assessments, a P-value of ≤ 0.05 was considered significant.
The study included a total of 174 patients. The group of patients who had received preoperative neoadjuvant treatment included more cases of AEG (Siewert) type 1 carcinoma (P < 0.001), and consequently oesophagectomy was performed more frequently among these patients (P < 0.001). The two groups (with or without preoperative neoadjuvant treatment) had comparable preoperative T stages, but the group of patients with preoperative neoadjuvant treatment presented a higher rate of preoperative N-positive disease (P = 0.020). Overall long-term survival was not different between the two groups of patients according to tumours of different AEG classifications, receipt of oesophagectomy or gastrectomy, nor between patients with similar postoperative TNM stage, resection margin and grading. However, an improvement of long-term survival was found for patients with nodal down-staging after neoadjuvant therapy (P = 0.053).
The prognostic relevance of postoperative TNM stages is similar for AEG in patients with or without neoadjuvant preoperative treatment, but treatment-related nodal down-staging prognosticates longer-term survival.
Core tip: Neoadjuvant therapy is the standard treatment for locally advanced adenocarcinoma of the gastro-oesophageal junction (AEG). Prognosis of AEG is based mainly on postoperative tumour/node/metastasis (TNM) stages, using the American Joint Committee on Cancer/Union for International Cancer Control classification system. Yet, whether prognostication based on postoperative TNM stage is affected by preoperative neoadjuvant therapy is unclear. Retrospective analysis of 174 patients showed that the prognostic relevance of postoperative TNM stage is independent of preoperative neoadjuvant therapy. However, nodal down-stage response following neoadjuvant therapy was found to result in improvement of survival.
- Citation: Thomaschewski M, Hummel R, Petrova E, Knief J, Wellner UF, Keck T, Bausch D. Impact of postoperative TNM stages after neoadjuvant therapy on prognosis of adenocarcinoma of the gastro-oesophageal junction tumours. World J Gastroenterol 2018; 24(13): 1429-1439
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1429.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1429
Adenocarcinoma of the gastro-oesophageal junction (AEG) is one of the most common cancers worldwide, with a global incidence of 0.7 per 100000[1,2]. It represents an aggressive disease with poor prognosis, and diagnosis is often delayed due to a lack of early disease-specific symptoms. Moreover, these tumours tend to spread to (local) lymph nodes even in early stages[3,4].
Today, curative treatment options involve multidisciplinary approaches including endoscopy, surgery, chemotherapy and radiotherapy. These treatments have led to improvements in clinical management and patient outcome over the last years[4,5]. In particular, effective neoadjuvant chemotherapy and/or radiotherapy approaches have been established for patients with locally advanced adenocarcinoma of the distal oesophagus and the gastro-oesophageal junction. When applied prior to surgery, these pretreatments provide a survival benefit, improve the potential for down-staging of the primary tumour and/or lymph node metastasis, and yield higher rates of complete tumour resection (R0) in contrast to a surgery-alone approach[6-10]. However, whether a patient benefits from neoadjuvant therapy depends on tumour biology, individual patient-related risk factors and stage of disease[6,8,9].
The American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) tumour/node/metastasis (TNM) system has been established as an international standard of classification of local, regional and distant extension/spread for many solid tumours, including AEG, and proven a powerful tool for prediction of prognosis of cancer patients[10-14].
For the first time, the recently published 8th edition of AJCC staging of cancers of the oesophagus and oesophago-gastric junction introduced the postneoadjuvant (yp)TNM stage groupings in addition to the clinical (c)TNM and pathological (p)TNM stagings[12]. Whereas the separate definitions from the previous 7th AJCC/UICC edition for depth of wall infiltration by the primary tumour (the T staging), lymph node involvement (the N staging) and presence of distant metastases (the M staging) of AEG were not changed, stage grouping for neoadjuvant categories (i.e., ypTNM) was newly classified with separate stage grouping for squamous cell carcinoma and adenocarcinoma, to account for the different prognostic implications between ypTNM (postneoadjuvant) and pTNM cancer categories[12,13].
Based on data derived from the Worldwide Esophageal Cancer Collaboration (WECC), involving 7723 patients from different countries and continents, survival for neoadjuvant groups (ypTNM patients) differed from that for equivalent-stage patients that underwent surgery alone (pTNM patients)[13]. In detail, survival for node-negative (ypN0) patients and early-stage disease (ypTNM groups I and II) patients is significantly lower than for equivalently categorised patients that underwent surgery alone (pTNM)[13,15]. However, two other retrospective analyses showed that the prognostic relevance of postoperative AJCC/UICC TNM staging is similar for patients with or without neoadjuvant treatment[16,17].
In summary, the data available on the actual prognostic relevance of postoperative TNM stages of AEG patients who underwent neoadjuvant treatment are still limited and heterogeneous. The objective of this study, therefore, was to retrospectively analyse data from our University Cancer Center to compare the prognostic relevance of postoperative TMN stages between patients with and without preoperative neoadjuvant treatment, following surgery for tumours of the gastro-oesophageal junction.
Between 1996 and 2014, a total of 254 consecutive patients underwent curative surgery for AEG at the University Medical Center Schleswig-Holstein, Campus Lübeck. Data of all these patients were obtained from the institutional database and selected according to the following inclusion criteria: age > 18 years; histological confirmation of AEG (Siewert types I to III) on the basis of postoperative resection specimen analysis; curative intent of surgery/treatment; and, formal eligibility for neoadjuvant/perioperative treatment based on preoperative cTNM stages (according to AJCC Classification 8th edition[12]; for details, please see the “Neoadjuvant/perioperative treatment” section below). Exclusion criteria were in-hospital death (as we aimed to analyse long-term outcome) and early-stage cancers (cT1cN0cM0 and cT2cN0cM0). After identification of eligible patients, we applied exact matching techniques to select the final retrospective study population of patients for the “neoadjuvant treatment” and “no neoadjuvant treatment” groups. Local ethics board approval was obtained (Ethik-Kommission Universität zu Lübeck/Aktenzeichen: 17-379A).
Study parameters included sex, age, AEG (Siewert) classification[18], surgical procedure (see below), preoperative staging (including cT, cN and cM categories according to the AJCC Cancer Staging Manual 8th edition[12]), postoperative staging [including T, N and M categories according to the AJCC/UICC Cancer Staging Manual 7th edition[11], grade of differentiation (G) and resection margin status (R)], long-term survival (defined as time in months from the day of hospital discharge) and pathologic down-staging/response in T and N stages after neoadjuvant therapy. For this study, we defined any pathologic down-staging/improvement in T and N stages after neoadjuvant therapy as ‘down-staged’, in contrast to ‘unchanged’ or ‘up-staged’ T and N stages.
Standard assessment of diagnosis and preoperative TNM stage (cTNM) was based on findings from endoscopy with biopsy, including endoscopic ultrasound and computed tomography of the thorax and abdomen. The postoperative staging was based on the resection specimen (according to the AJCC/UICC Cancer Staging Manual 7th edition[11] and including G and R parameters). In this context, the resected specimens were re-evaluated by an independent pathologist for the purpose of this study.
From the year 2005 onward, neoadjuvant chemotherapy has been used as standard treatment in the context of a multidisciplinary approach for locally advanced cancers. Our local standard protocol for neoadjuvant/perioperative tretment is based on the German National Guidelines for Diagnostics and Treatment of Adenocarcinomas of the Stomach and the Gastroesophageal Junction (http://www.awmf.org/uploads/tx_szleitlinien/ 032009l_S3_Magenkarzinom_Diagnostik_Therapie_Adenokarzinome_oesophagogastraler_Uebergang_2012abgelaufen.pdf). Generally, patients are deemed eligible for neoadjuvant/perioperative treatment if the tumour is locally advanced. In detail, we recommend neoadjuvant/perioperative treatment for patients with locally advanced tumour stages (cT2 node-positive disease as well as cT3/4), and patients with cT1 cN0 cM0 or cT2 cN0 cM0 are not recommended for pretreatment. Prior to 2005, patients received neoadjuvant/perioperative treatment on an individual basis based on recommendations of the local interdisciplinary tumour board. Neoadjuvant/perioperative therapy mainly consisted of cisplatin and fluorouracil (5-FU)-based regimens and included, over the time, different protocols such as cisplatin/5-FU, ECX, FLOT or ECF. For better presentation of results, neoadjuvant/perioperative treatment is referred to as ‘neoadjuvant treatment’ throughout the rest of the manuscript. In 26 cases, patients without neoadjuvant pretreatment had received adjuvant therapy (if recommended according to the local interdisciplinary tumor board). The decision was based on postoperative tumour stages and individual patient-specific risk factors.
The type of surgical resection for the AEG tumours was selected in accordance with tumour location and extent, and was chosen from among either oesophagectomy techniques (open/hybrid/totally minimally invasive oesophagectomies), gastrectomy techniques (transhiatal extended gastrectomy) or combined oesophagectomy-and-total-gastrectomy techniques. For the purpose of this study, patients treated with the combined oesophagectomy-and-total-gastrectomy technique were included in the oesophagectomy group. The standard surgical procedure in our hospital included two-field lymphadenectomy for oesophagectomies and D2-lymphadenectomy for gastrectomies.
The Department of Surgery includes an Outpatient Cancer Clinic for follow up of cancer patients. Most of the cancer patients in our study are receiving their follow-up care in this outpatient clinic. However, for those patients who requested follow up with their general practitioner (e.g., based on the location of their residence), we obtained their follow-up information via telephone and entered the respective information into our database.
Statistical analyses were performed using SPSS software (version 22; IBM Corp., Armonk, NY, United States). For analysis of categorical variables [sex, age, AEG (Siewert) classification, surgical procedure and preoperative staging (cTNM)], Pearson’s chisquare and Fisher´s exact tests were used. Long-term survival was analysed using the Kaplan-Meier method. Log-rank test was used for statistical comparison. For all statistical analyses, a P-value of ≤ 0.05 was considered significant.
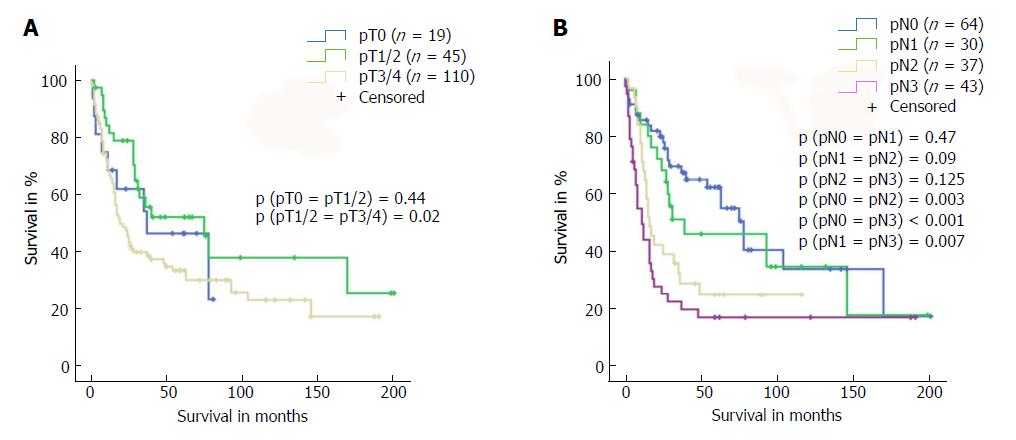
Following the exact matching patient selection, we identified 174 out of the 254 patients for study inclusion. Table 1 presents an overview of the two study groups: “neoadjuvant treatment (tx)” vs “no neoadjuvant tx”. The patients who underwent neoadjuvant treatment were significantly younger than their nontreated counterparts (58 years vs 64 years, P = 0.043) and presented significantly more often with Siewert type 1 AEG tumours (P < 0.001) mandating oesophagectomy rather than gastrectomy (P < 0.001). While patients in both groups presented comparable preoperative T stages, patients in the neoadjuvant treatment group presented higher preoperative rates of N-positive disease (P = 0.02). Rates of N-positive disease were 90% for neoadjuvant tx and 73% for no neoadjuvant tx. Analysis of overall survival of the entire patient population based on postoperative T and N stages confirmed that long-term survival depended on disease stages (Figure 1).
| All, n = 174 | No neoadjuvant tx | Neoadjuvant tx | P value | |
| Male sex | 85.1% | 81.7 | 87.4 | 0.387 |
| Age, median | 61.5 | 64 | 58 | 0.043 |
| Siewert stage | < 0.001 | |||
| I | 35.1% | 16.9 | 47.6 | |
| II | 51.7% | 67.6 | 40.8 | |
| III | 13.2% | 15.5 | 11.7 | |
| cT stage | 0.9 | |||
| T2 | 7.5% | 8.5 | 6.8 | |
| T3 | 74.7% | 74.6 | 74.8 | |
| T4 | 17.8% | 16.9 | 18.4 | |
| cN stage | 0.02 | |||
| Negative | 17.2% | 28.2 | 9.7 | |
| Positive | 82.8% | 72.8 | 90.3 | |
| Surgery | < 0.001 | |||
| Oesophagectomy | 54.0% | 26.8 | 72.8 | |
| Gastrectomy | 46.0% | 73.2 | 27.2 | |
| pT stage | ||||
| T0 | 11% | - | 20.0 | |
| T1/2 | 26% | 21.4 | 30.0 | |
| T3/4 | 63% | 78.6 | 50.0 | |
| pN stage | ||||
| N0 | 37% | 24.3 | 48.8 | |
| N1 | 17% | 18.6 | 15 | |
| N2 | 21% | 20 | 21.2 | |
| N3 | 25% | 37.1 | 15 | |
| pM stage | ||||
| M0 | 94% | 94.3 | 93.8 | |
| M1 | 6% | 5.7 | 6.2 |
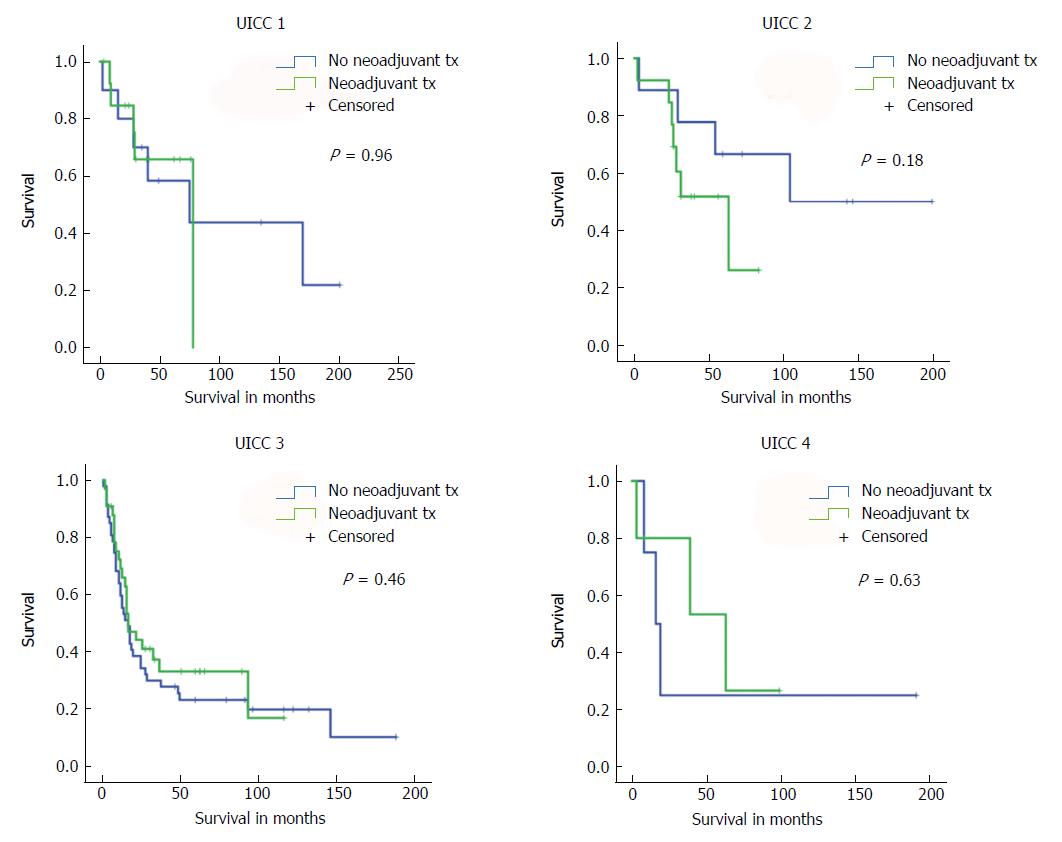
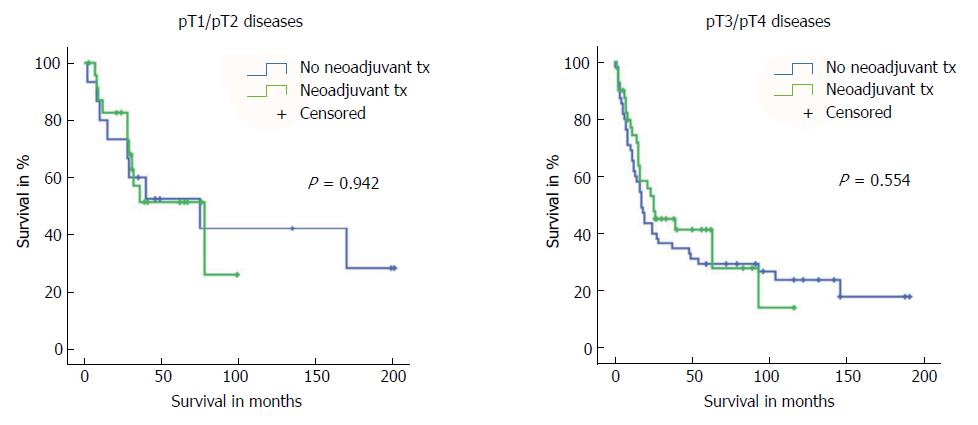
First, we compared long-term survival between groups of patients with or without neoadjuvant treatment who had equivalent postoperative AJCC/UICC TNM stages (stages I-IV according to the 7th edition AJCC/UICC staging). We found no significant differences in long-term survival according to receipt of neoadjuvant treatment for the four AJCC/UICC stage subgroups (Figure 2). Furthermore, analysis of patients with either pT1/pT2 diseases or advanced pT3/pT4 diseases showed no significant difference in long-term survival related to receipt (or no receipt) of neoadjuvant pretreatment (Figure 3).
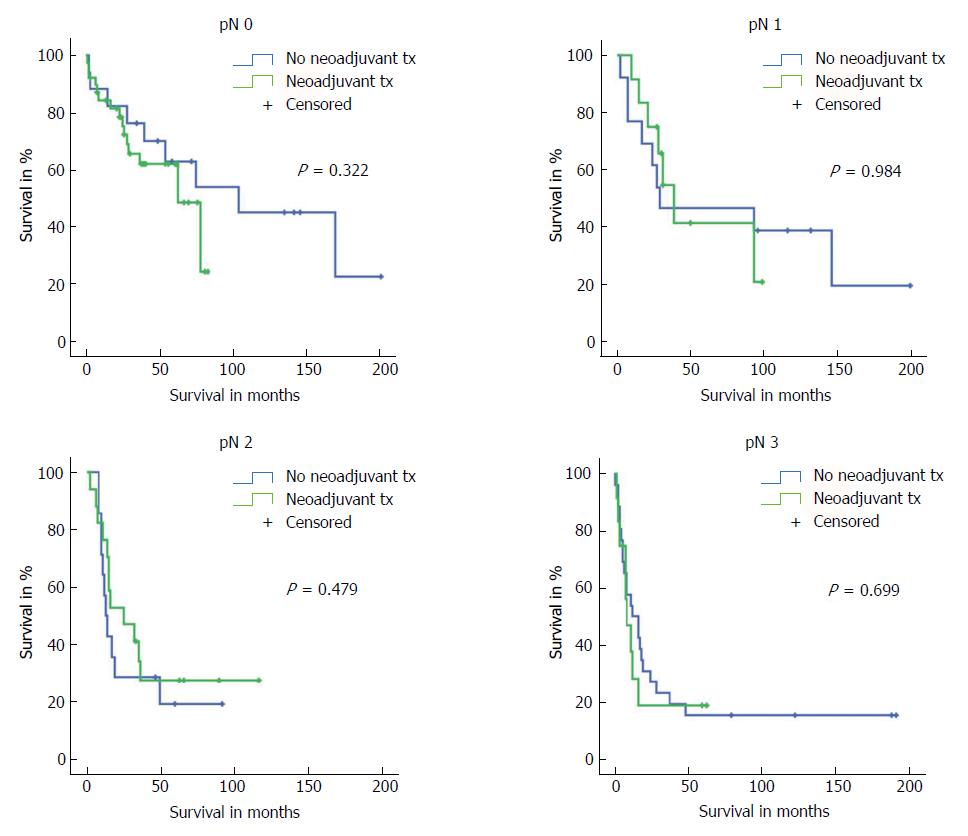
Subgroup analyses on all tumour (pT) stages separately showed that only neoadjuvant-pretreated patients with pT1 stage had slightly better long-term survival (P = 0.046). However, the statistical comparison of these groups included only 8 vs 5 patients. With regards to postoperative N stages (pN), we did not find any differences in outcome between patients with or without neoadjuvant therapy who represented the same postoperative pN stages (Figure 4).
Further subgroup analyses investigating effects of surgical procedure, postoperative G, positive/negative R and Siewert type I/II/III AEG tumours on outcome showed that long-term survival rates were comparable between patients with the same G stage or R stage regardless of neoadjuvant pretreatment (G2: P = 0.580; G3: P = 0.417; R0: P = 0.389; R1: P = 0.825). With regards to the Siewert classification, only patients with pT1 tumours in Siewert type 2 AEG showed a better survival after neoadjuvant therapy (P = 0.017; 7 patients with neoadjuvant tx vs 3 patients with no neoadjuvant tx); otherwise, the location of the tumour classified by Siewert classification did not impact outcome of patients with or without neoadjuvant pretreatment. Similarly, patients with pT1 tumours who received gastrectomy showed a significantly better survival rate after neoadjuvant therapy (P = 0.020; 3 patients with neoadjuvant tx vs 4 patients with no neoadjuvant tx); otherwise, surgical procedures (oesophagectomy vs gastrectomy) did not impact outcome.
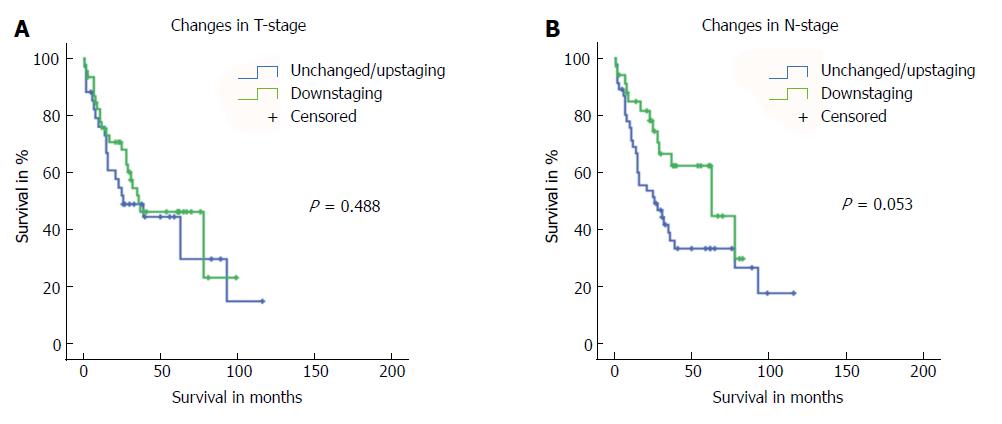
Finally, we analysed if T or N down-staging after neoadjuvant treatment impacted outcome by comparing preoperative and postoperative T and N stages only for those patients that underwent neoadjuvant pretreatment. We found that T down-staging after neoadjuvant therapy did not affect long-term survival (P = 0.488; Figure 5). Subgroup analysis on patients with either unchanged or up-staged disease showed a trend towards worse survival for patients with up-staged T stage (P = 0.628; Supplementary Figure 1). However, these results are limited by the very low number of patients included (n = 30 vs n = 4). In contrast, N down-staging after neoadjuvant treatment resulted in borderline significant improvement in long-term survival (P = 0.053) (Figure 5).
The development of new therapeutic approaches and strategies for AEG within recent years has led to a multidisciplinary approach involving neoadjuvant/perioperative chemotherapy and/or radiotherapy. In contrast to the surgery-alone approaches, the multidisciplinary approaches have resulted in a relevant overall survival benefit to patients[7,19-22] and have become part of standard treatment for AEG tumours. In addition to a survival benefit, neoadjuvant pretreatment further showed potential for down-staging of the primary tumour and/or lymph node metastasis, and finally in improving rates of complete tumour resection (R0)[7,19-22]. While there is a broad consensus that neoadjuvant treatment affects outcome and prognosis of patients with AEG tumours, data are scarce on the exact prognostic relevance of postoperative AJCC/UICC TNM staging in the era of neoadjuvant treatment.
With this current study, we showed that there were no significant differences in the overall long-term survival of patients with or without neoadjuvant treatment, if they presented similar postoperative AJCC/UICC stages (stages I-IV, according to the 7th edition AJCC/UICC), T stages (early pT1/2 and advanced pT3/4 cancers) or N stages (pN0/pN1/pN2/pN3). Furthermore, we could show that surgical procedure, postoperative G, positive/negative R and location (Siewert classification of AEG the tumour) did not affect outcome between patients with or without neoadjuvant treatment, except in some cases of patients with pT1 tumours.
In summary, in our opinion, these results provide an interesting contribution towards answering the question of whether the AJCC TNM staging system can predict or estimate individual prognosis of patients with AEG tumours, regardless of whether they received neoadjuvant pretreatment or not. Oesophageal cancer staging in the 7th edition AJCC/UICC TNM is based on pTNM of patients that had undergone surgery alone[11,14]. Our data provide evidence that this system might also be applicable to patients who receive neoadjuvant treatment, and that prognosis of patients with similar T and N stages might indeed be comparable regardless of neoadjuvant treatment.
However, Rice et al[12,13] recently published the 8th edition of AJCC TNM, which includes, for the first time, neoadjuvant pretreatment stage groupings (i.e. ypTNM). A retrospective comparison of actual WECC survival data of patients with neoadjuvant treatment (ypTNM from the 8th edition) with data of patients who underwent surgery-alone (pTNM from the previous 7th edition) indicated that survival for the neoadjuvant-treated patients (ypTNM data) was lower than that found for patients of equivalent pathological staging that underwent surgery alone (pTNM data)[13,15]. However, these data only partly contradict our results, as Rice et al[13,15] described a worse prognosis of neoadjuvant categories (ypTNM) for adenocarcinoma patients compared to corresponding pTNM data alone for early-stage disease (stages I and II); advanced stage adenocarcinoma patients (stages III-IV) showed no differences in survival[13,15].
We did not find any significant difference in survival for either early or advanced stage disease, apart from limited pT1 cases as discussed below. Our data are further supported by a retrospective analysis published by Davies et al[16] that showed prognostic relevance of postoperative pTNM stage was similar between patients with or without neoadjuvant pretreatment. Similarly, in another series, Swisher et al[17] demonstrated that pTNM-specific survival was similar for patients with down-staged disease but not for those with unchanged disease. We must acknowledge in this context that our study did not include analysis of patients with complete tumour regression (ypT0N0), as this subgroup of patients did not exist among the patients without neoadjuvant treatment. This caveat might impact our results for early-stage cancer patients and might lead to differences in results compared to the data of Rice et al[13]. On the other hand, WECC data represents a fairly heterogeneous patient population as well as of different treatment standards in different countries and continents, which is reflected in the heterogeneous survival rate[13]. In contrast, the patient population in our study might be more homogenous since all data were collected from a single cancer centre. Of note, in our study cohort, patients with ypT0N0 showed long-term survival similar to that of patients with pT1N0 (data not shown).
We found that T down-staging did not affect long-term outcome, whereas N down-staging appeared to improve survival (borderline significance; P = 0.053). This observation is supported by the fact that N involvement is one of the most important and strongest prognostic factors of AEG tumours. Recent data, for example, show that lymph node involvement is more important than regional anatomic location for prognosis[23,24]. The 7th edition AJCC TNM has already heralded the era of data-driven cancer staging and the incorporation of nonanatomic cancer characteristics[25]. And, indeed, factors beyond those included in the AJCC TNM system (e.g., down-staging of the primary tumour and/or lymph node metastasis after neoadjuvant treatment) have been shown to represent independent prognostic factors for overall survival[7,16,17,19-22,26]. However, for prognostication, T or/and N down-staging were still not considered in the currently used 8th AJCC TNM edition[12].
In order to improve prognostication, some authors have suggested modification of the pTNM staging system to incorporate the extent of pathologic response following neoadjuvant treatment, rather than developing separate ypTNM stages[17]. This idea is supported by our data, which indicate that it might be necessary to include information on T or/and N down-staging in the AJCC TNM staging system in order to improve prognostic assessment of patients with AEG tumours.
There are, however, a number of aspects and limitations of the current study that must be considered for proper interpretation of the presented data. First, and most importantly, our study embodies all the known disadvantages of a retrospective study, including potential inhomogeneity of data acquisition and quality, single-centre data, changes of treatment protocols over time, etc. Second, we have to acknowledge that the patients without neoadjuvant treatment had been mainly recruited from the years 1996 to 2004, and patients with neoadjuvant treatment were from the year 2005 onward. This is based on the development and introduction of neoadjuvant treatment protocols into daily clinical practice since 2005. We are fully aware that inclusion of historical cohorts of patients might impact outcome of the respective groups[27]; however, it will be very difficult to recruit a significant number of patients in the current era who qualify for but do not receive any neoadjuvant treatment, as this treatment is part of standard protocols nowadays in most parts of the world.
It is also important to note that our two study groups (neoadjuvant tx vs no neoadjuvant tx) are not completely homogenous. In fact, there are significant differences between the groups in regards to age, location (Siewert classification), N involvement and surgical technique. This fact is based on the use of the method of exact matching that allowed for us to include different numbers of patients into both groups as long as the selected parameters (preoperative TNM stages) were identical. However, the aim of this study was to analyse if similar postoperative T and N stages indicate similar prognosis in patients with or without neoadjuvant pretreatment; such a question might not be highly affected by this selection of patients. We found in our analyses that patients with postoperative T1 stages showed differences in survival between groups in limited cases. This observation, in our opinion, needs very careful interpretation, as the number of patients with pT1 stage in our study cohort was extremely low (n = 24 patients in total). These findings warrant further confirmation, and clinical relevance remains unclear. Furthermore, in our study, 26 patients without neoadjuvant pretreatment received adjuvant therapy; yet, subgroup analysis excluding this patient cohort produced no difference in the results (data not shown).
A number of studies have found that prognosis and tumour biology differs between AEG tumours at different locations according to the Siewert classification (types I to III), supporting the concept that Siewert type III carcinoma represents true gastric adenocarcinoma, having a worse prognosis than Siewert types I and II carcinoma[28,29]. Interestingly, the 7th edition AJCC/UICC TNM classification did not include Siewert classification for prognostication, and instead classified all tumours within 5 cm of the gastro-oesophageal junction as oesophageal carcinoma.
Based on the discrepancy of available data, we performed subgroup analyses with regards to outcome of tumours in different locations according to the Siewert classification. Our data showed that, in general, location of the tumour classified by Siewert classification did not impact outcome of patients with or without neoadjuvant pretreatment. Only patients with pT1 tumours in Siewert type 2 AEG tumours showed a better survival after neoadjuvant therapy (P = 0.017), but this analysis was based on only 7 vs 3 patients, casting suspicion on the final significance of these findings. We can only hypothesize that our study might be underpowered for answering the question of whether location of tumours impacts outcome. Further studies are needed to elucidate this specific and highly relevant question in more detail.
In summary, our retrospective analysis of patients with AEG tumours demonstrated that there were no significant differences in the overall long-term survival of patients with or without neoadjuvant treatment, if they presented similar postoperative AJCC/UICC stages (stages I-IV), T stages (early pT1/2 and advanced pT3/4 cancers) or N stages (pN0/pN1/pN2/pN3). Furthermore, we showed that N down-staging, especially, affected long-term survival of patients undergoing neoadjuvant treatment. Collectively, our data indicate that the pTNM staging system is reliable for assessment of individual prognosis for patients with AEG tumours, regardless of whether neoadjuvant treatment has been received or not. Furthermore, our data support the inclusion of T and/or N down-staging information, rather than separate pTNM and ypTNM stages, as independent risk factors for survival in the next edition of the AJCC TNM staging system.
Adenocarcinoma of the gastro-oesophageal junction (AEG) has a poor prognosis. Neoadjuvant chemotherapy and radiotherapy have significantly improved clinical management and outcome of patients, leading to a major evolution in treatment of oesophageal cancer. Neoadjuvant therapy provides a survival benefit to patients with AEG, through its elimination of micrometastatic disease and potential for down-staging of the primary tumour and/or lymph node metastasis, ultimately leading to higher rates of complete resections (R0). For prediction of prognosis of cancer patients, the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) system has been established. The 8th edition of AJCC staging of cancers of the oesophagus and oesophagogastric junction includes, for the first time, postneoadjuvant tumour/node/metastasis (ypTNM) stage groupings; the previous editions only referred to patients that underwent surgery alone. This raises the question of whether prognosis according to the postoperative pTNM/ypTNM stages is similar between patients that receive neoadjuvant pretreatment (ypTNM) or patients that undergo surgery alone (pTNM). According to the 8th edition AJCC, there are different prognostic implications between postneoadjuvant (ypTNM) and pathologic (pTNM) AEG categories. In detail, prognosis of node-negative (ypN0) and early-stage diseases (ypTNM groups I and II) is worse compared to patients with similar stages who underwent surgery alone. In contrast, for advanced stage AEG, there is no difference of prognosis among patients with identical pTNM/ypTNM stages. Other studies, however, have shown contradictory results. In these studies, the prognostic relevance of postoperative AJCC/UICC TNM staging did not differ between patients with or without neoadjuvant pretreatment.
Due to limited and heterogeneous data, the prognostic relevance of postoperative TNM staging in the era of neoadjuvant therapy of AEG remains unclear. However, due to the generally poor prognosis of AEG and the relevant risk of recurrence, an exact assessment of prognosis according to the TNM staging system is extremely important for the individual patient and for further treatment decision-making.
The main objective of this study was to compare the prognostic relevance of similar postoperative TNM stages between patients with or without neoadjuvant pretreatment. The results were expected to clarify the need of a separate postneoadjuvant stage grouping (ypTNM) for prognostication of AEG patients. Furthermore, in the era of neoadjuvant treatment, other prognostic factors may be relevant for prognostication of survival of patients with AEG.
We conducted a retrospective study analysing 254 patients that underwent curative surgical treatment at our University Medical Center Schleswig-Holstein, Campus Lübeck. After excluding patients with preoperative tumour stages that preclude neoadjuvant pretreatment (cT1cN0cM0 and cT2cN0cM0), we performed exact matching to identify patients with or without neoadjuvant pretreatment who would be eligible for the study. Additionally, in-hospital deaths were excluded since we aimed to analyse long-term survival. Study parameters included sex, age, AEG (Siewert) classification, surgical procedure, preoperative staging (including cT, cN and cM categories according to the AJCC Cancer Staging Manual 8th edition), postoperative staging (including T, N and M categories according to the AJCC/UICC Cancer Staging Manual 7th edition, grade of differentiation (G) and resection margin status (R)), long-term survival (defined as time in months as from the day of hospital discharge) and pathologic down-staging/response in tumour (T) and nodal (N) stages after neoadjuvant therapy. Pearson’s chi-square and Fisher´s exact tests were used for statistical analyses of categorical variables (sex, age, AEG (Siewert) classification, surgical procedure and preoperative staging (cTNM)). Long-term survival was analysed using the Kaplan-Meier method. For statistical comparisons, log-rank test was used. A P-value of ≤ 0.05 was considered significant for all statistical analyses.
After patient selection and exact matching, 174 of the 254 patients were included in the study. Regarding demographics of both groups (no neoadjuvant treatment vs neoadjuvant treatment), patients who received neoadjuvant treatment were significantly younger (58 years vs 64 years, P = 0.043) and presented Siewert type I AEG tumours significantly more often (P < 0.001), resulting in significantly more oesophagectomies than gastrectomies (P < 0.001) for surgical treatment in this group. Patients who received neoadjuvant treatment presented higher preoperative rates of lymph node-positive disease (P = 0.020). Regarding overall survival of the entire study cohort, survival worsened at advanced postoperative AJCC/UICC TNM stages. Comparing long-term survival between patients with or without neoadjuvant pretreatment with identical postoperative TNM stages, no difference could be found. In addition, no difference was found in long-term survival of patients with or without neoadjuvant pretreatment for identical pT, pN or pM stages, G or R. Investigation of other prognostic markers for patients who received neoadjuvant pretreatment involved analysis of the effect of T and N down-staging on long-term survival. Here, we found that T down-staging did not have an impact on long-term survival (P = 0.488), while N down-staging after neoadjuvant treatment provided a significant but borderline improvement in long-term survival (P = 0.053).
Our retrospective study demonstrated that the prognostic relevance of equivalent postoperative AJCC/UICC TNM stages is similar between patients with or without neoadjuvant pretreatment. Our data provide evidence that the pTNM staging system can be applied for assessment of individual prognosis of patients with AEG, regardless of whether or not they received neoadjuvant treatment. Furthermore, our study showed that N down-staging following neoadjuvant treatment positively affects long-term outcome, emphasizing the need of novel markers for prognostication in the era of neoadjuvant therapy.
Our data support the idea of modifying the pTNM staging system by incorporating the extent of pathologic response following neoadjuvant treatment, rather than developing separate ypTNM stages. Prognostic factors or markers that reflect tumour biology, rather than the anatomical extent of growth, are promising for the development of new assessments for prognostication of survival of patients with AEG.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Herbella F, Nishida T, Tsoulfas G S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1027] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11837] [Article Influence: 845.5] [Reference Citation Analysis (4)] |
| 3. | Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048-54; discussion 1054-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1961] [Article Influence: 163.4] [Reference Citation Analysis (5)] |
| 5. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 6. | Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang LY, Law S, Tsao SW, Cheung AL. Suppression of esophageal tumor growth and chemoresistance by directly targeting the PI3K/AKT pathway. Oncotarget. 2014;5:11576-11587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4082] [Article Influence: 314.0] [Reference Citation Analysis (0)] |
| 8. | Forde PM, Kelly RJ. Genomic alterations in advanced esophageal cancer may lead to subtype-specific therapies. Oncologist. 2013;18:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Findlay JM, Middleton MR, Tomlinson I. A systematic review and meta-analysis of somatic and germline DNA sequence biomarkers of esophageal cancer survival, therapy response and stage. Ann Oncol. 2015;26:624-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6465] [Article Influence: 431.0] [Reference Citation Analysis (0)] |
| 11. | Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 639] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 12. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 13. | Rice TW, Lerut TE, Orringer MB, Chen LQ, Hofstetter WL, Smithers BM, Rusch VW, van Lanschot J, Chen KN, Davies AR. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Rice TW, Rusch VW, Ishwaran H, Blackstone EH; Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 357] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 15. | Rice TW, Ishwaran H, Kelsen DP, Hofstetter WL, Apperson-Hansen C, Blackstone EH; Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, Smyth EC, Cunningham D, Allum WH, Mason RC. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983-2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Swisher SG, Hofstetter W, Wu TT, Correa AM, Ajani JA, Komaki RR, Chirieac L, Hunt KK, Liao Z, Phan A. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005;241:810-7; discussion 817-20. [PubMed] |
| 18. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 914] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 19. | Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 950] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 20. | Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 911] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 21. | Arnott SJ, Duncan W, Gignoux M, Hansen HS, Launois B, Nygaard K, Parmar MK, Rousell A, Spilopoulos G, Stewart G. Preoperative radiotherapy for esophageal carcinoma. Cochrane Database Syst Rev. 2005;19:CD001799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4610] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 23. | Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 24. | DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer? World J Gastroenterol. 2017;23:751-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (3)] |
| 25. | Rusch VW, Rice TW, Crowley J, Blackstone EH, Rami-Porta R, Goldstraw P. The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg. 2010;139:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Jensen K; GE Adenocarcinoma Meta‐analysis Group. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013;31:CD008107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Cooke DT, Calhoun RF, Kuderer V, David EA. A Defined Esophagectomy Perioperative Clinical Care Process Can Improve Outcomes and Costs. Am Surg. 2017;83:103-111. [PubMed] |
| 28. | Curtis NJ, Noble F, Bailey IS, Kelly JJ, Byrne JP, Underwood TJ. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J Surg Oncol. 2014;109:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Kulig P, Sierzega M, Pach R, Kolodziejczyk P, Kulig J; Polish Gastric Cancer Study Group. Differences in prognosis of Siewert II and III oesophagogastric junction cancers are determined by the baseline tumour staging but not its anatomical location. Eur J Surg Oncol. 2016;42:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |