Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1120
Peer-review started: December 8, 2017
First decision: December 20, 2017
Revised: December 29, 2017
Accepted: January 24, 2018
Article in press: January 24, 2018
Published online: March 14, 2018
Processing time: 95 Days and 2.9 Hours
To clarify the role of proteinase-activated receptor 2 (PAR2) in hepatocellular carcinoma, especially in the process of metastasis.
PAR2 expression levels were assessed by qRT-PCR and immunohistochemistry (IHC) in patient tissues and in hepatocellular carcinoma cell lines SMMC-7721 and HepG2. Cell proliferation and metastasis were assessed both in vitro and in vitro. Immunoblotting was carried out to monitor the levels of mitogen-activated protein kinase (MAPK) and epithelial-mesenchymal transition markers.
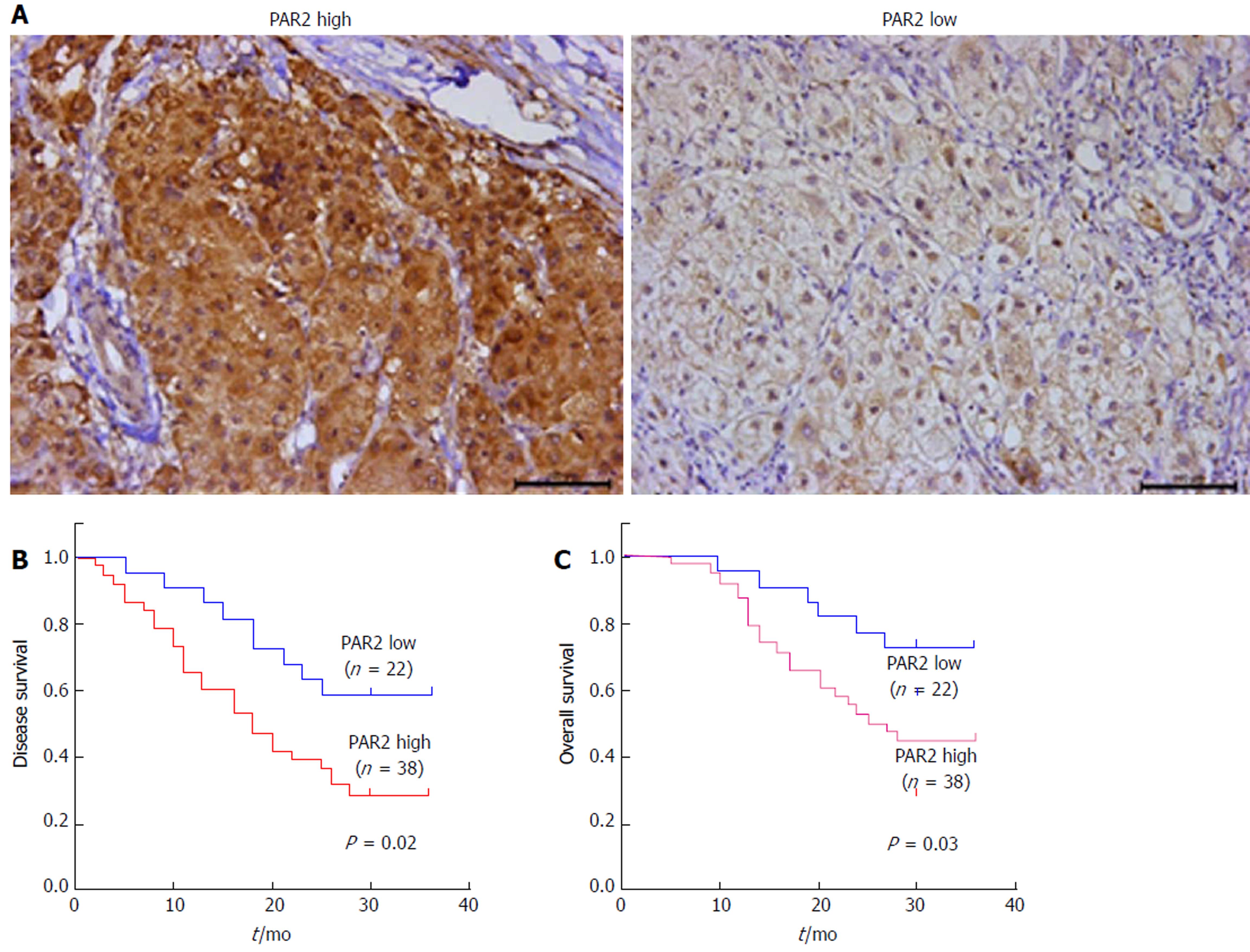
The prognosis was significantly poorer in patients with high PAR2 levels than in those with low PAR2 levels. Patients with high PAR2 levels had advanced tumor stage (P = 0.001, chi-square test), larger tumor size (P = 0.032, chi-square test), and high microvascular invasion rate (P = 0.037, chi-square test). The proliferation and metastasis ability of SMMC-7721 and HepG2 cells was increased after PAR2 overexpression, while knockdown of PAR2 decreased the proliferation and metastasis ability of SMMC-7721 and HepG2 cells. Knockdown of PAR2 also inhibited hepatocellular carcinoma tumor cell growth and liver metastasis in nude mice. Mechanistically, PAR2 increased the proliferation ability of SMMC-7721 and HepG2 cells via ERK activation. Activated ERK further promoted the epithelial-mesenchymal transition of these cells, which endowed them with enhanced migration and invasion ability.
These data suggest that PAR2 plays an important role in the proliferation and metastasis of hepatocellular carcinoma. Therefore, targeting PAR2 may present a favorable target for treatment of this malignancy.
Core tip: The role of proteinase-activated receptor 2 (PAR2) in tumor progression especially metastasis of hepatocellular carcinoma and how it is regulated are still unclear. In this study, we found that PAR2 was upregulated in hepatocellular carcinoma (HCC) tumor tissues and related with poor prognosis in HCC patients. In addition, we proved that PAR2 could not only promote the proliferation and metastasis ability of SMMC-7721 and HepG2 cells in vitro, but also promoted xenograft tumor growth and HCC cell liver metastasis in vivo. These effects were mediated by the activation of ERK, which further induced epithelial-mesenchymal transition of HCC cells.
- Citation: Sun L, Li PB, Yao YF, Xiu AY, Peng Z, Bai YH, Gao YJ. Proteinase-activated receptor 2 promotes tumor cell proliferation and metastasis by inducing epithelial-mesenchymal transition and predicts poor prognosis in hepatocellular carcinoma. World J Gastroenterol 2018; 24(10): 1120-1133
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1120
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide[1,2]. Surgical resection is the main strategy for treating this deadly malignancy[3]. Unfortunately, intrahepatic and extrahepatic metastases often occur in progressive and recurrent patients, thus leading to a poor prognosis[4]. Metastasis is a comprehensive process that facilitates cancer cell transition from a primary lesion to a metastatic focus. Many intrinsic cellular characteristics and extrinsic microenvironmental factors influence the metastatic potential of HCC cells[5]. However, the underlying mechanisms that facilitate this process remain largely unknown, therefore, it is critical to identify new targets for these patients to improve their prognosis.
Proteinase-activated receptor 2 (PAR2) is a member of the G-protein coupled receptor 1 family[6]. PAR2 downstream signaling is mediated through several signaling pathways such as intracellular calcium, phospholipase C (PLC), mitogen-activated protein kinase (MAPK), Rho, and I-kappaB kinase/NF-kappaB[7,8]. It is also transactivated by cleaved F2R/PAR1[9]. PAR2 is known to regulate physiological responses such as vasoregulation, cell growth, inflammation, and nociception[10,11]. However, there is growing evidence that PAR2 also has an important role in tumors, especially in tumors of epithelial origin[12-15]. PAR2 is expressed in HCC tissues and multiple HCC cell lines, where it promotes cancer cell migration and invasion through different signaling pathways including [Ca2+]i mobilization, Src, Met, and p42/p44 MAPK[16,17]. PAR2 also participates in tumor initiation, self-renewal, and metastasis by regulating CD47+ HCC stem cells[18]. Moreover, PAR2 in hepatic stellate cells (HSCs) plays an important role in promoting HCC growth through mediating migration and secretion of pro-angiogenic and pro-mitotic factors[19]. In addition, TF/VIIa/PAR2 signaling is involved in mTOR mediated autophagy in HCC[20]. Collectively, these data suggest an important role for PAR2 in HCC progression. However, the role of PAR2 in HCC metastasis and the underlying mechanism are poorly understood.
Epithelial-mesenchymal transition (EMT) and its intermediate states have been identified as crucial drivers of organ fibrosis and tumor progression[21,22]. EMT is thought to be activated in cancer cells to allow for dissociation from the primary tumor and intravasation into blood vessels[23,24]. However, the impact of PAR2-associated EMT in cancer metastasis is still poorly understood. Based on these findings, we sought to investigate the relationship between PAR2 expression and HCC prognosis and the underlying mechanisms of the EMT in HCC.
Cancer tissue sections were obtained with informed consent from 60 HCC patients undergoing radical resection between 2010 and 2012 at Qilu Hospital of Shandong University. Ethical consent was granted from the Institutional Ethics Committee, Qilu Hospital of Shandong University.
Immunohistochemistry (IHC) was performed using a standard immunoperoxidase staining method. Rabbit anti-PAR2 antibody (#6976) was purchased from Cell Signaling Technology (Danvers, United States). PAR2 expression in HCC tissues was evaluated using a method described previously[25]. The scoring of PAR2 immunohistochemical staining was performed as follows: the degree of staining [yellow (1) and brown (2)] and the percentage of area staining positive [< 50% (1) and > 50% (2)]. The final score was the product of the two parameters, and the samples were grouped as high (2-4 point) or low PAR2 expression (1). Two experienced pathologists evaluated the staining for PAR2.
HepG2 and SMMC-7721 cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. Cells were cultured in DMEM (Gibco, NY, United States) supplemented with 10% fetal bovine serum (FBS; Gibco, NY, United States), 100 U/mL penicillin, and 100 μg/mL streptomycin (Hyclone, UT, United States).
The cell counting kit 8 (CCK8) assay (Tongren Chemical Research Institute, Kyushu, Japan) was used to measure cell proliferation. HepG2 and SMMC-7721 cells in the exponential growth phase were seeded into a 96-well plate at 1000 cells per well with five replicates. DMEM medium containing 10% FBS was used to culture the cells. At 6, 24, 48, 72, 96, or 120 h after plating the cells, 100 μL of DMEM containing 10 μL of CCK-8 solution was added into each well. The wells were then incubated for 2 h at 37 °C, and the absorbance was measured at 450 nm. The absorbance at 6 h of each group was designated as baseline.
Cells were seeded in 6-well plates (500 cells per well) and cultured for 2 wk. The generated colonies were fixed with 4% paraformaldehyde and then stained with 5% crystal violet dye (Sigma, MO, United States). The total number of colonies in each well was manually counted.
Cell invasion and migration assays were carried out using a 24-well plate and 8-μm polyethylene terephthalate membrane filters (Costar, MA, United States). Serum-free medium (200 μL) containing 3 × 104 HepG2 and SMMC-7721 cells was added to the upper chambers, which contained either uncoated (for migration assay) or matrigel coated (for invasion assay) membranes. Each inferior chamber was filled with 600 μL of medium containing 10% FBS. After 16 h of incubation, the filters were taken out, fixed with 4% paraformaldehyde for 30 min, and stained with crystal violet dye for 30 min. Then, the remaining cells on the upper sides of the filters were removed using cotton swabs. Migrated or invaded cells in four randomly chosen fields (× 100 magnification) per well were counted.
HepG2 and SMMC-7721 cells were seeded into 6-well plates and grown to 90% confluence. Then, the confluent cell monolayers were scraped with a 10 μL pipette tip to create wounds. The cellular debris was removed and cells were cultured in FBS-free medium. Pictures were taken under a microscope (Leica) at 0, 24, and 48 h. The difference in wound width represents the migration ability.
The coding region of PAR2 was cloned into a pcDNA3.1(+) plasmid at EcoR I and Xho I sites; this is referred to as pcDNA3.1-PAR2. Empty control and pcDNA3.1-PAR2 vectors were transfected into HepG2 and SMMC-7721 cells using Lipofectamine 2000 (Thermo, United States) according to the manufacturer’s instructions. Primer sequences for vector construction were as follows: forward, 5’-GGAATTCTCGGGGCTTCCAGGAGGA-3’ and reverse, 5’-CCGCTCGAGTTCCCATCTGAGGACCTGG-3’.
pLKO.1 vector encoding shRNA targeting human PAR2 was purchased from Sigma (MISSION shRNA lentivirus-mediated transduction system, SHCLNG-NM_005242). To generate lentivirus that expressed shRNA, HEK293T cells were cultured in DMEM (Gibco, NY, United States) supplemented with 10% FBS (Gibco, NY, United States). Using polyethyleneimine, we transfected cells transiently with pLKO.1-derived plasmids combined with pRev, pEnv-VSV-G, and pMDLg. Retrovirus particles were collected from the media after 12, 24, and 48 h[19]. HepG2 and SMMC-7721 cells were infected three times with the retrovirus particles with 8.0 μg/mL polybrene. At 48 h after the transduction, transduced cells were selected using 2.0 μg/mL puromycin for one week. The efficiency of the shRNA knockdown was measured via quantitative real-time RT-PCR and immunoblot analysis.
Total RNA was extracted from cultured cells using Trizol reagent (Takara, Japan). cDNA was synthesized from at most 1 μg of total RNA (Takara, Japan). RNA expression was measured by qRT-PCR using SYBR-Green (Takara, Japan) according to the manufacturer’s guidelines. Primers for PAR2 were: forward, 5’-GATGGCACATCCCACGTCACT-3’ and reverse, 5’-TTGGCAAACCCACCACAAACAC-3’. GAPDH was used as an endogenous control.
Rabbit anti-PAR2, anti-ERK, anti-phospho-ERK, anti-E-cadherin, anti-N-cadherin, and anti-GAPDH antibodies were obtained from Cell Signaling Technology (Danvers, United States). Cell lysates were prepared in RIPA buffer (Sigma-Aldrich, MO, United States) where equal quantities of cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with skimmed milk, incubated with a primary antibody, washed with TBST three times, and then incubated with a secondary antibody (Cell Signaling Technology, GA, United States). After the secondary antibody incubation, the membranes were washed three more times with TBST, and the proteins were visualized by enhanced chemiluminescence (Millipore, MA, United States). GADPH was used as the internal loading control.
Male Balb/c nude mice (aged 4 wk with an initial body weight of 20 ± 2 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The mice were housed at a temperature of 25 ± 2 °C and a relative humidity of 70% ± 5% under natural light/dark conditions for 1 wk and allowed free access to food and water. The animal experiments were performed in strict accordance with international ethical guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocols were approved by the Institutional Animal Care and Use Committee, Qilu Hospital of Shandong University.
HepG2 or SMMC-7721 cells (2 × 106) suspended in 100 μL of normal saline were subcutaneously injected into the axillae of the nude mice (4 wk). Tumor growth was monitored every week and tumor volume was calculated as follows: tumor volume = 4π/3 × (width/2)2 × (length/2), in which the length and width are the longest and shortest diameters, respectively. Four weeks after injection, the mice were sacrificed and the tumors were dissected and weighed.
HepG2 and SMMC-7721 cells (2 × 106) suspended in 100 μL of normal saline were injected into the spleen of nude mice (4 wk) followed by a splenectomy 3 min later; the mice were monitored every 3 d after the procedure. After the 4th week, the mice were sacrificed and their livers were dissected. The liver tumor was weighed and tumor number on each liver was counted.
All assays were done in triplicate. The data are shown as mean ± SD. To compare the mean values between the two groups, the independent Student’s t-test was used. To compare the mean values among more than two groups, two-way ANOVA was used. Fisher’s exact test was used to compare tumor metastatic rate in the two groups. A Pearson χ2 test was adopted to analyze the clinicopathological variables according to PAR2 expression. For survival analysis, Kaplan-Meier method and log-rank test were used. P < 0.05 was considered statistically significant.
To determine whether PAR2 plays an important role in HCC progression, the expression level of PAR2 in patients’ tumor tissues was detected by IHC. PAR2 was mainly located on the plasma membrane (Figure 1A). The patient distribution of PAR2 IHC score is shown in Table 1. Patients were divided into PAR2 high and PAR2 low groups according to the IHC score (Figure 1A). Patients with high PAR2 levels had advanced tumor stage (P = 0.001, chi-square test), larger tumor size (P = 0.032, chi-square test), and high microvascular invasion rate (P = 0.037, chi-square test; Table 2). Patients with high PAR2 expression levels had significantly shorter overall survival (OS) and disease-free survival (DFS) than those with low PAR2 expression levels (P = 0.02 and P = 0.03, respectively; Figure 1B and C). Multivariate Cox regression analysis further revealed that PAR2 was an independent prognostic marker for the OS of HCC patients (hazard ratio = 1.814; P = 0.041) (Table 3). These data suggest that PAR2 is associated with tumor growth and invasion; it can also predict the prognosis of HCC patients.
| Score | Patient number |
| 1 | 22 |
| 2 | 15 |
| 4 | 23 |
| Clinicopathological variable | PAR2 expression | ||
| Low | High | P value | |
| All cases | 22 | 38 | |
| Gender | |||
| Male | 16 | 28 | 0.832 |
| Female | 6 | 10 | |
| Age (yr) | |||
| < 60 | 10 | 22 | 0.352 |
| ≥ 60 | 12 | 16 | |
| TNM stage | |||
| Early (I-II) | 16 | 12 | 0.001a |
| Late (III-IV) | 6 | 26 | |
| Tumor size (cm) | |||
| Small (≤ 5) | 15 | 15 | 0.032a |
| Large (> 5) | 7 | 23 | |
| Microvascular invasion | |||
| Present | 5 | 21 | 0.037a |
| Absent | 17 | 17 | |
| HBsAg | |||
| Negative | 3 | 7 | 0.632 |
| Positive | 19 | 31 | |
| Serum AFP level (ng/mL) | |||
| < 400 | 18 | 25 | 0.154 |
| ≥ 400 | 4 | 13 | |
| Clinicopathological variable | Hazard ratio | P value |
| Gender (male vs female) | 0.214 | 0.672 |
| Age (< 60 vs ≥ 60) | 0.126 | 0.763 |
| TNM stage (I-II III-IV) | 4.292 | 0.016a |
| Tumor size (≤ 5 cm vs >5 cm) | 2.193 | 0.034a |
| Microvascular invasion (present vs absent) | 2.499 | 0.029a |
| HBsAg (negative vs positive) | 1.072 | 0.092 |
| Serum AFP level (< 400 ng/mL vs ≥ 400 ng/mL) | 3.688 | 0.023a |
| PAR2 expression (high vs low) | 1.814 | 0.041a |
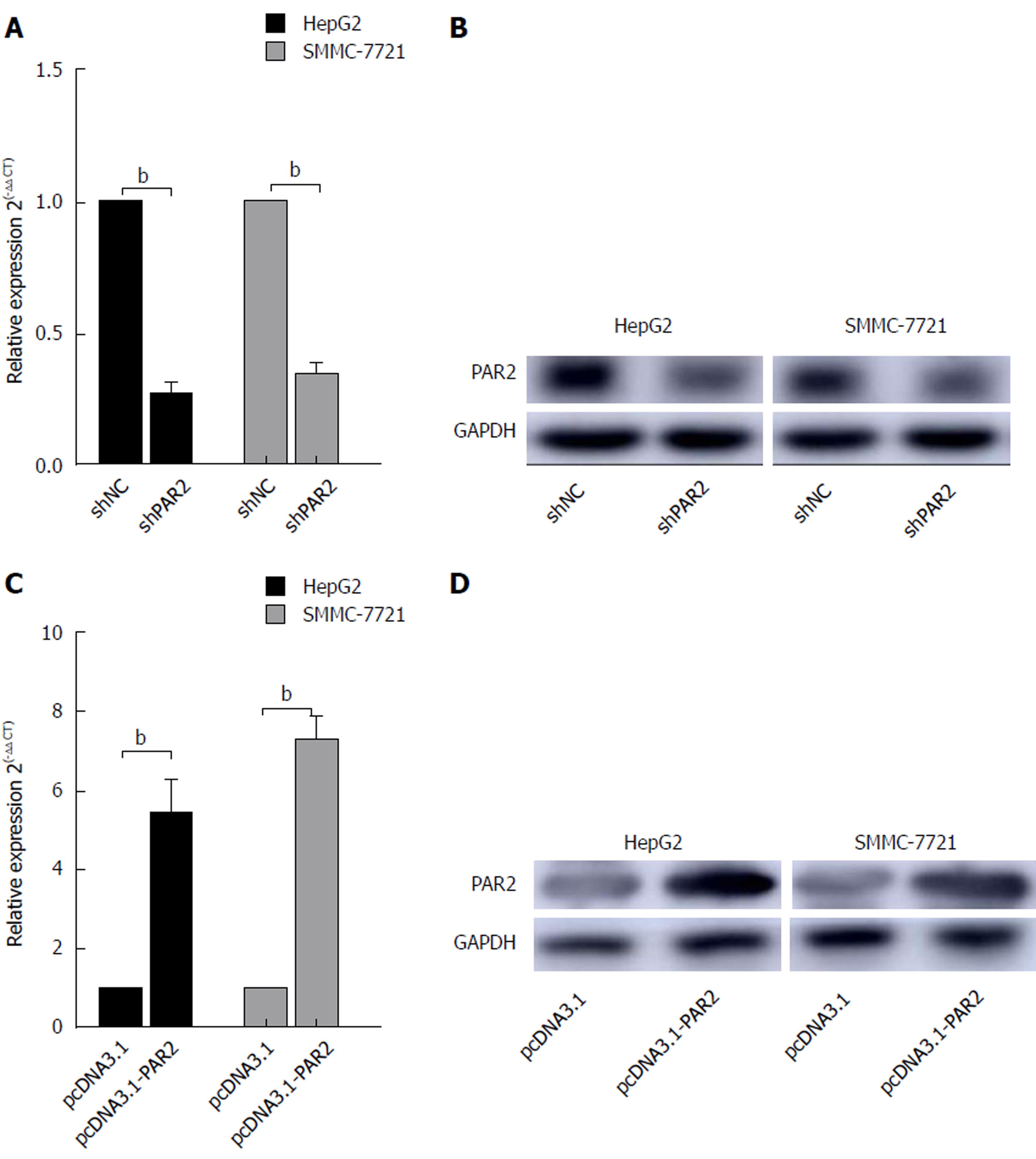
To determine whether PAR2 is essential for HCC carcinogenesis, the expression of PAR2 was stably knocked down using lentiviral-mediated shRNA in HepG2 and SMMC-7721 cells. The PAR2 knockdown is referred to as shPAR2, while the negative control is referred to as shNC. The PAR2 RNA and protein levels both decreased in shPAR2 knockdown cells (Figure 2A and B). To overexpress PAR2, its coding region was amplified and inserted into the pcDNA3.1 (+) plasmid. After transfecting cells with empty pcDNA3.1 or pcDNA3.1-PAR2 using Lipofectamine 2000, PAR2 was transiently overexpressed in pcDNA3.1-PAR2 cells (Figure 2C and D).
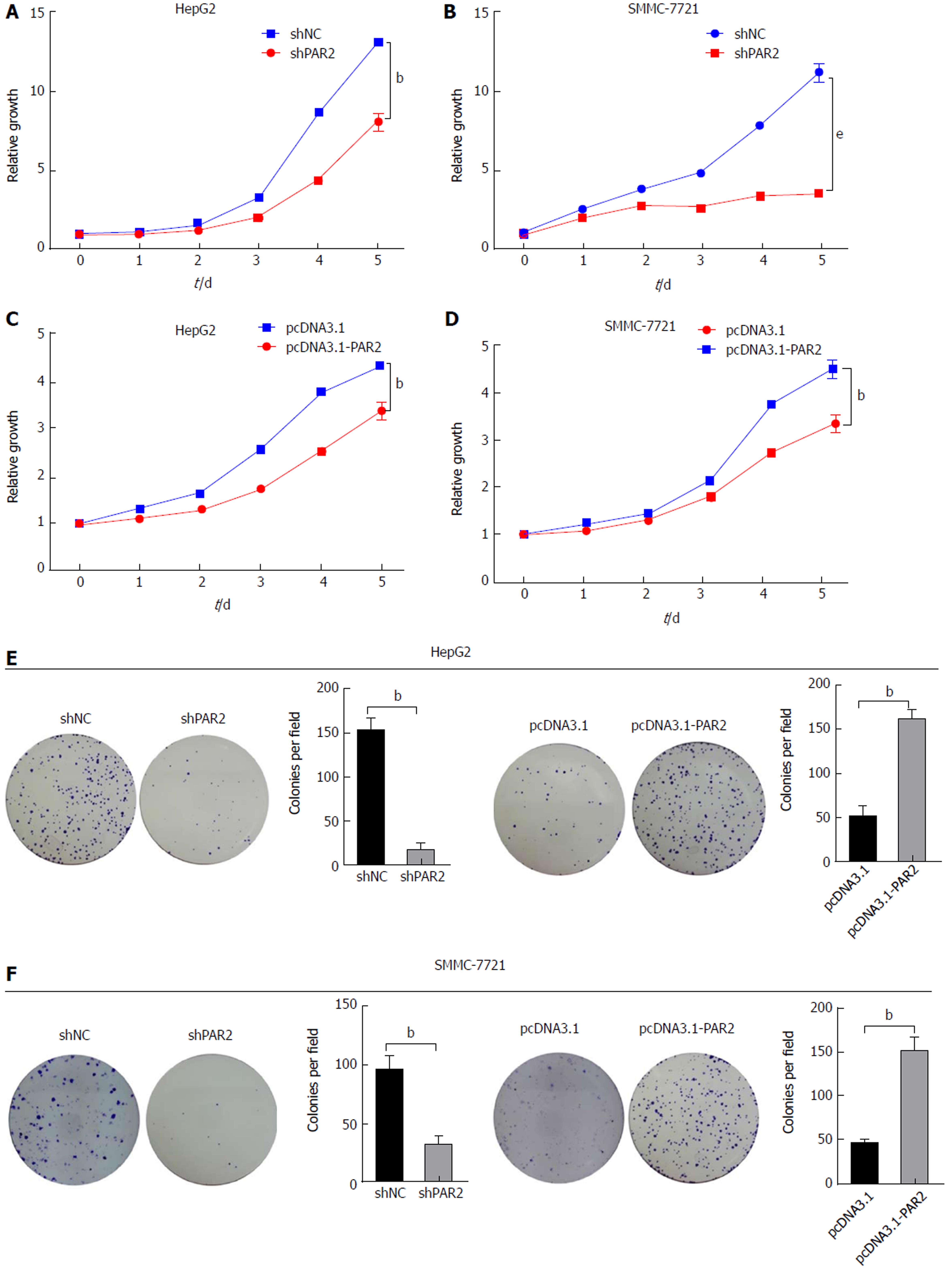
CCK8 and colony formation assays were used to uncover the role of PAR2 in promoting HCC cell proliferation. Cell viability and proliferation were dramatically reduced after PAR2 knockdown (Figure 3A and B), but increased after PAR2 overexpression (Figure 3C and D). The same results were also observed using a colony formation assay (Figure 3E and F). These results demonstrate that PAR2 could promote the proliferation ability of HCC cells.
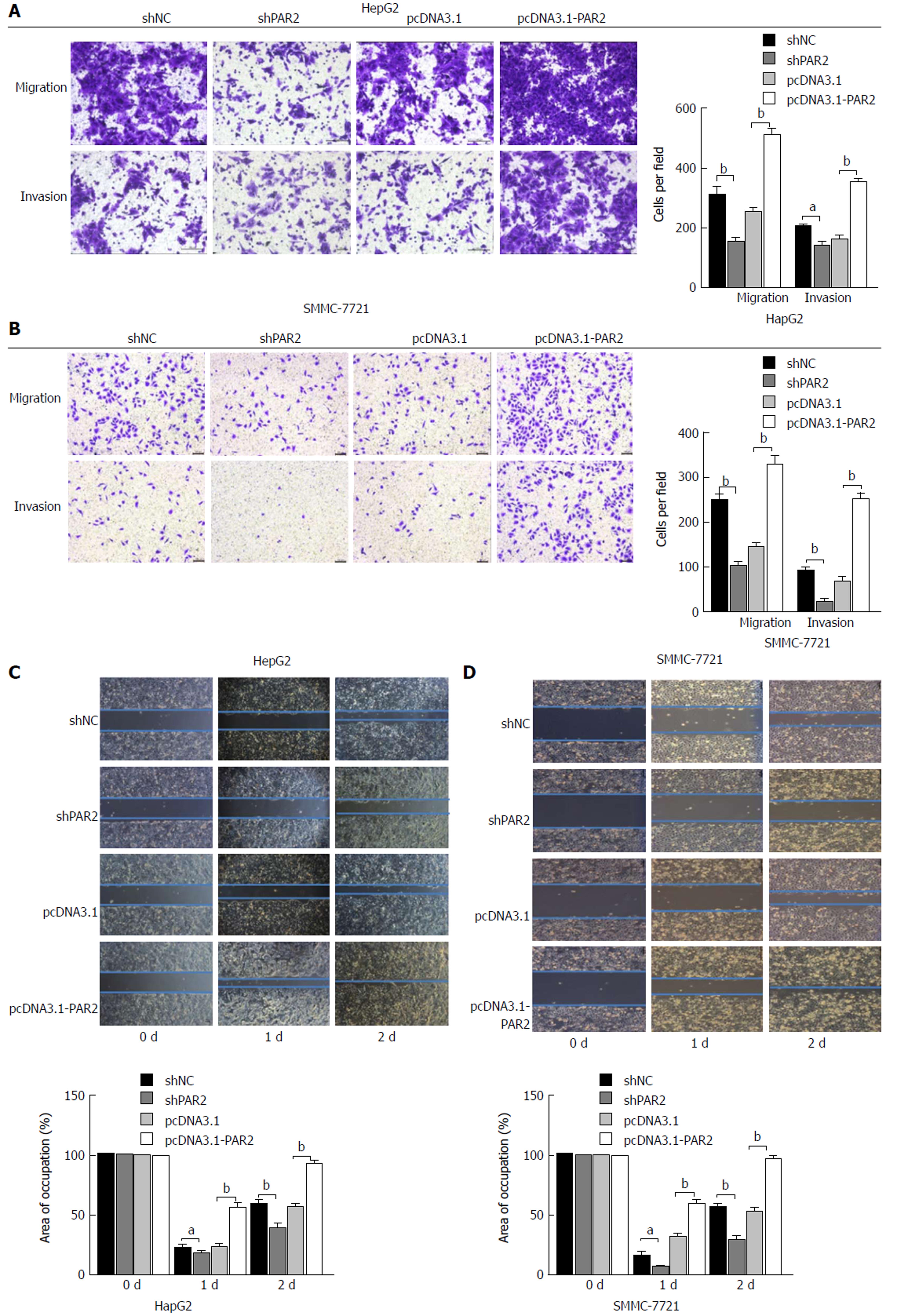
Metastasis often occurs in HCC patients and the results shown in Table 1 suggest that PAR2 is associated with tumor microvascular invasion. To determine whether PAR2 could promote HCC cell migration and invasion, transwells with or without matrigel coating were used to measure cell migration in vitro. As shown in Figure 4A and B, the migration and invasion abilities of HepG2 and SMMC-7721 cells were reduced after PAR2 knockdown, but increased after overexpression. These results were also verified using a wound healing assay (Figure 4C and D). The wound healing assay showed that the migration rate of shPAR2 cells was significantly slower than that of the control shNC cells. Also, the migration rate of pcDNA3.1-PAR2 cells was faster than that of the control pcDNA3.1 cells. These data suggest that PAR2 can influence the process of HCC cell invasion and migration.
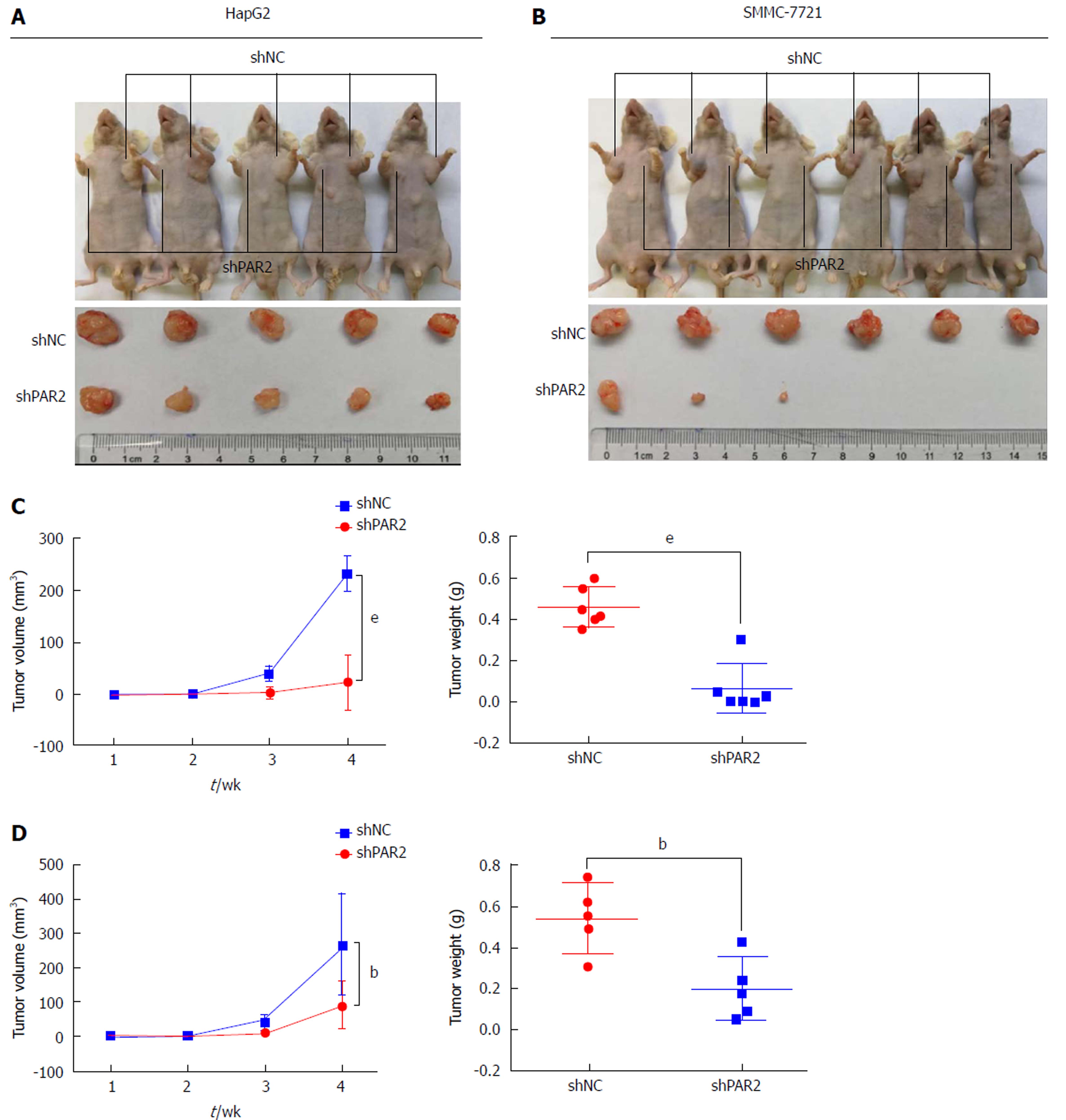
To further confirm the role of PAR2 in promoting cell proliferation in vivo, HepG2 and SMMC-7721 cells with stable knockdown of either PAR2 or NC were subcutaneously injected into nude mice to make tumor xenografts. After 4 wk, tumors from the NC groups were much larger than those from the shPAR2 groups for both HepG2 and SMMC-7721 cells (Figure 5A and B). Tumor weights from the NC groups were much greater than those of the shPAR2 groups (Figure 5C and D). These data suggest that PAR2 knockdown could dramatically inhibit tumor growth in vivo.
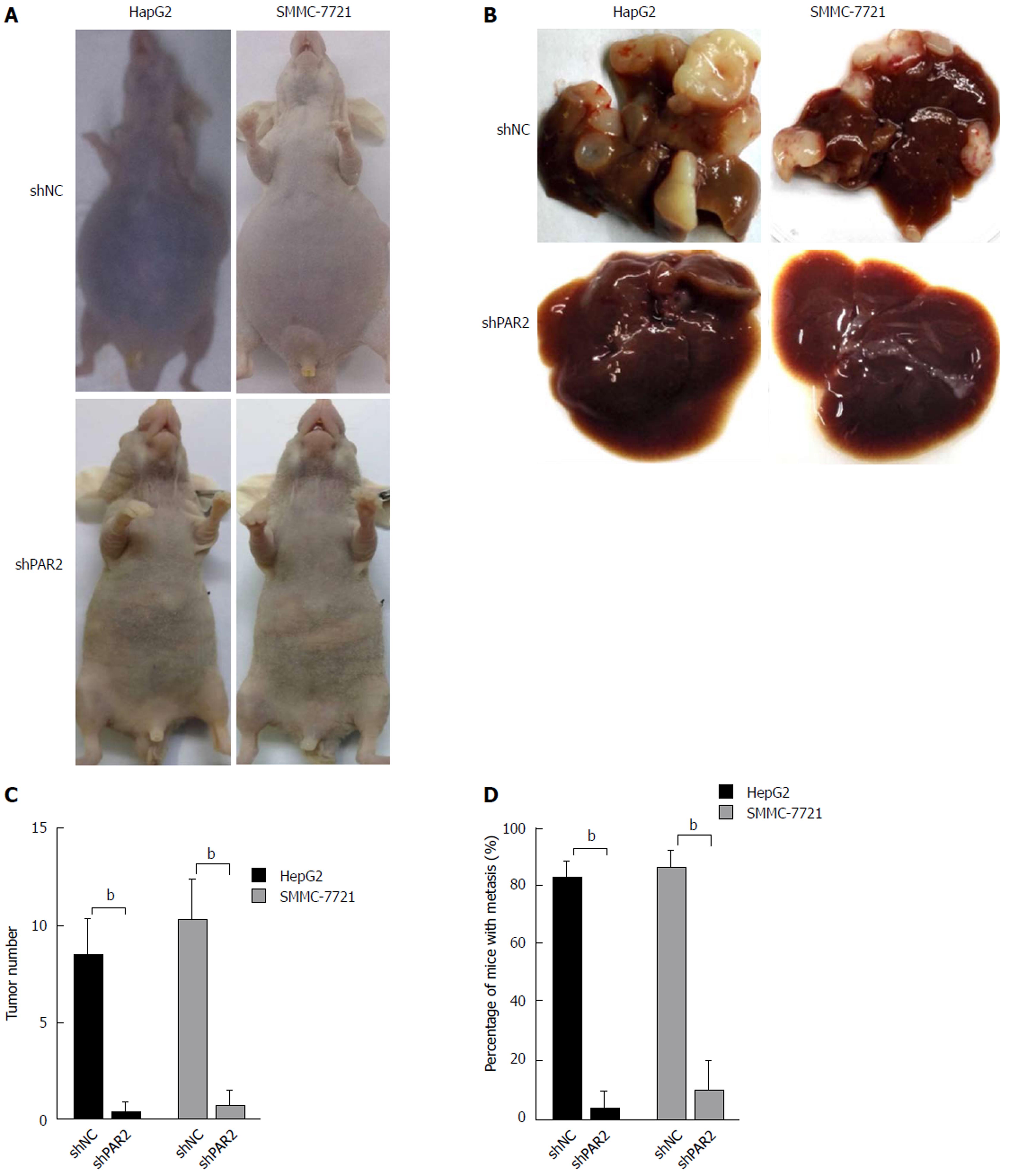
Intrahepatic metastasis is the most common mode of HCC metastasis[26]. To determine whether PAR2 could facilitate tumor metastasis in vivo, a mouse model was established that mimics the process of tumor metastasis to the liver. HepG2 and SMMC-7721 cells stably expressing shPAR2 or shNC were injected into the spleen of nude mice, and then a splenectomy was performed. This allows the tumor cells to circulate into the liver through the portal vein, thus forming liver metastases. After 4 wk, ascites and liver tumors grew in the mice of the shNC groups (Figure 6A), the maximum body weight that increased due to ascites was no more than 10% of the body weight in age-matched shPAR2 group. The mice in both groups were sacrificed and the tumor volumes of the shNC groups were much larger than those in the shPAR2 groups (Figure 6B). Livers in the shNC group also had a higher number of tumors compared to the shPAR2 groups (Figure 6C). There was almost no liver metastasis observed in the shPAR2 groups (Figure 6D). From this metastasis model, it appears that PAR2 is necessary for HCC intrahepatic metastasis. PAR2 knockdown could inhibit the colonization of HCC cells on the liver.
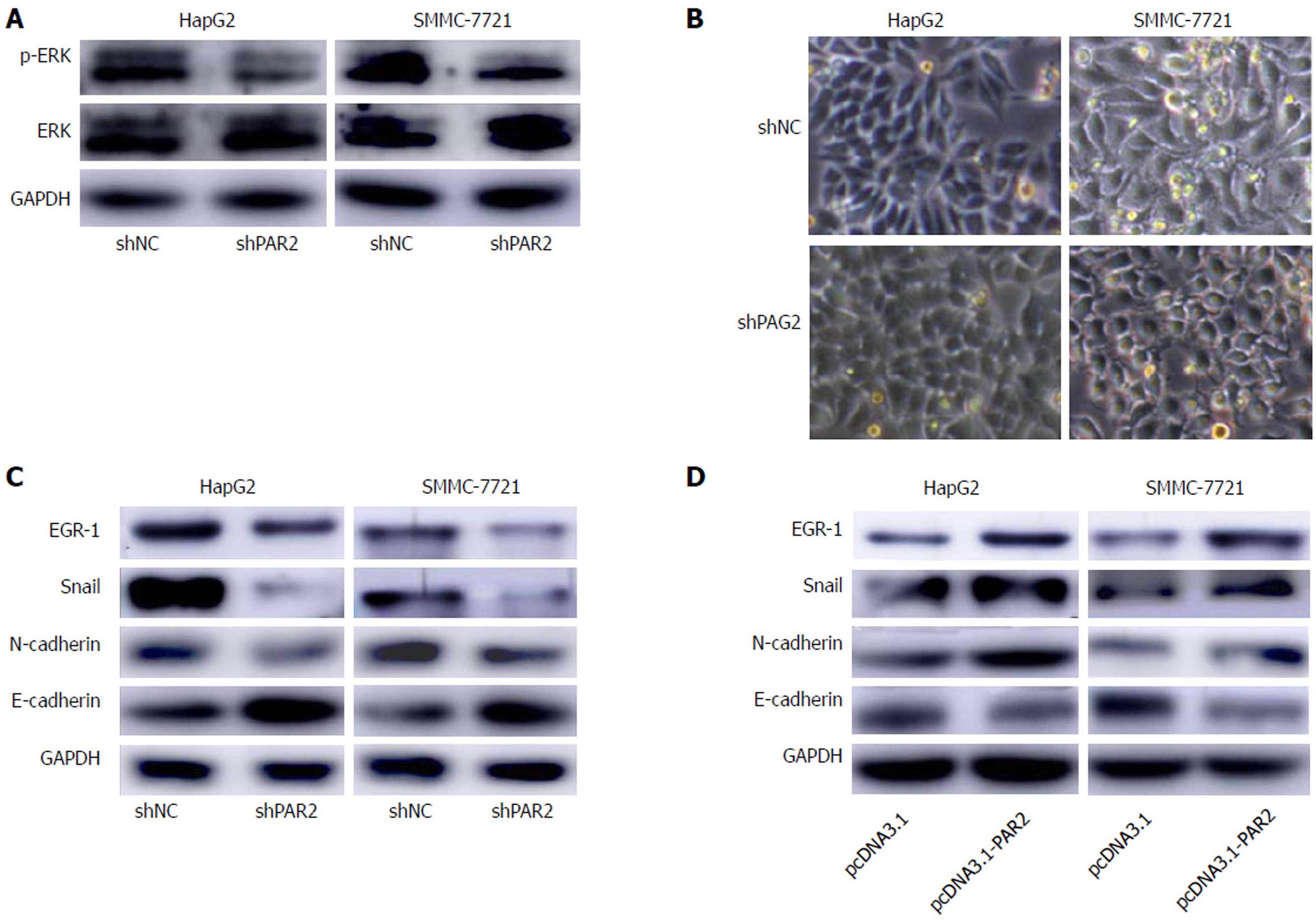
PAR2 is reported to activate ERK to promote HCC cell proliferation[16]; these results were confirmed in HepG2 and SMMC-7721 cells (Figure 7A), but the downstream mechanisms that facilitate metastasis are unclear. EMT is a common mechanism of cancer metastasis, and it is reported that activated ERK can activate EGR-1[27]. As a transcription factor, EGR-1 induces Snail expression to inhibit E-cadherin expression, thus inducing EMT[27-29]. Interestingly, after knockdown of PAR2, HepG2 and SMMC-7721 cells showed obvious morphological changes toward an epithelial phenotype (Figure 7B). Therefore, we measured EGR-1, Snail, and the EMT markers E-cadherin and N-cadherin expression in shNC and shPAR2 cells through immunoblot analysis. As shown in Figure 7C, the epithelial marker E-cadherin was upregulated while EGR-1, Snail, and the mesenchymal marker N-cadherin were downregulated when PAR2 was knocked down. After transient overexpression of PAR2, E-cadherin was downregulated, and EGR-1, Snail, and N-cadherin were upregulated (Figure 7D). These results demonstrate that PAR2 participates in the process of EMT and promotes HCC cell metastasis.
HCC is one of the most lethal malignancies worldwide, especially in China[4]. Although many genes and signaling pathways have been found to participate in HCC carcinogenesis[30-33], the prognosis of the patients is still unsatisfactory, therefore it is urgent to uncover more genes responsible for the development of HCC. The focus of this work was PAR2, which is evident for its important role in the development of tumors, especially of epithelial origin[12-15]. PAR2 is known to promote proliferation, migration, and invasion of HCC cells. Unfortunately, the underlying mechanism of PAR2 activity in HCC metastasis is unclear and few in vivo studies have been carried out elucidating its role in tumor development. Additionally, the association between PAR2 and clinicopathological variables of HCC patients has not been analyzed; this work addressed those questions.
In the clinical association study, expression levels of PAR2 were analyzed in 60 HCC patients by immunohistochemistry. Patients with high PAR2 expression levels tended to have advanced tumor stage, larger tumor size, and high microvascular invasion rate, indicating that PAR2 plays an important role in the development of HCC. Additionally, patients with high PAR2 expression levels had shorter OS and DFS, implying that PAR2 could serve as a biomarker to predict the prognosis of HCC patients.
Because PAR2 is associated with tumor size and microvascular invasion, the role of PAR2 in HCC proliferation and invasion was investigated. PAR2 was stably knocked down using shRNA or transiently overexpressed in HepG2 and SMMC-7721 cells. CCK8 and colony forming assays were used to measure in vitro cell proliferation. These assays showed that PAR2 knockdown inhibited cell proliferation and colony formation ability, while overexpression of PAR2 presented adverse effects. From transwell and wound healing assays, PAR2 was shown to promote tumor cell migration and invasion. These in vitro experiments indicated that PAR2 plays an important role in tumorigenesis and the development of HCC cells.
Two mouse models were used to explore the role of PAR2 in HCC progression in vivo. From a subcutaneous xenograft model, it was determined that knockdown of PAR2 could inhibit tumor growth to a great extent. From the liver metastasis model, PAR2 was shown to help HCC cells plant onto the liver and form metastatic foci. These in vivo data provide solid evidence that PAR2 promotes HCC cell proliferation, invasion, and migration.
After knockdown of PAR2, morphological changes toward an epithelial phenotype were observed in HepG2 and SMMC-7721 cells. EMT is an important step in tumor progression and plays a critical role during cancer invasion and metastasis. During EMT, cells lose their epithelial qualities and acquire mesenchymal features[21] such as increased expression of mesenchymal-related markers (such as N-cadherin), and reduced expression of epithelial-related markers (such as E-cadherin)[28]. It is reported that the function of PAR2 is mediated by activating MAPK proteins such as ERK[16]; ERK activation by PAR2 was verified in this study. Interestingly, it is reported that activated ERK can induce EMT through EGR-1 and Snail[28,29], therefore the expression levels of EGR-1, Snail, E-cadherin, and N-cadherin were monitored through immunoblot analysis in HepG2 and SMMC-7721 cells. PAR2 knockdown resulted in higher levels of E-cadherin and lower levels of N-cadherin, while overexpression of PAR2 reduced the levels of E-cadherin and increased the levels of N-cadherin. This implies that PAR2 can promote EMT in HCC cells through activating ERK, at least in part. As we all know, PAR2 is activated via its N-terminal cleavage by several proteases such as serine proteases[34], but which protease is responsible for PAR2 activation in HCC is still not clear. This is a very interesting direction in the following study. Recently, some growth factors such as HGF were reported to be responsible for EMT in HCC. Whether HGF participates in PAR2-induced EMT could be further investigated based on this study[27,35].
Collectively, this study comprehensively investigated the role of PAR2 in HCC progression in vitro, in vivo, and in clinical samples for the first time. PAR2 could promote HCC cell proliferation, invasion, and migration by activating ERK and thus inducing EMT. These findings highlight the potential role of PAR2 in directing the diagnosis, treatment, and prognosis of HCC.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Metastasis often occurs in HCC patients, thus leading to a poor prognosis. It is urgent for us to find out molecules that are responsible for HCC metastasis to improve the prognosis of HCC patients.
Proteinase-activated receptor 2 (PAR2) is reported to be responsible for HCC development, but the underlying mechanism is unclear. Figuring out the detailed mechanisms for PAR2-induced metastasis of HCC could give us more options for HCC treatment.
Although the role of PAR2 in HCC has been reported, the underlying mechanism for PAR2-induced metastasis is more important. Therefore, we aimed to find out the downstream signaling for PAR2-induced HCC metastasis. This could help us to find targets for HCC treatment.
PAR2 expression levels were assessed by qRT-PCR and immunohistochemistry in patient tissues. Cell proliferation was investigated by CCK8 and colony formation assays in vitro and tumor xenograft in vivo. HCC cell metastasis was assessed by transwell and wound healing assays in vitro and intrasplenic injection of HCC cells in mice in vivo. Immunoblotting was carried out to monitor the levels of mitogen-activated protein kinases and epithelial-mesenchymal transition (EMT) markers to figure out the underlying mechanisms for PAR2. This is the first time to analyze the correlation between PAR2 expression and HCC clinicopathological characteristics. Also, it is the first for us to investigate the underlying mechanism for PAR2-mediated HCC metastasis. What’s more, we proved the role of PAR2 in HCC proliferation and metastasis using an animal model.
The prognosis of patients with high PAR2 levels was poorer than those with low PAR2 levels. Patients with high PAR2 levels had advanced tumor stage, larger tumor size, and high microvascular invasion rate. The proliferation and metastasis ability of SMMC-7721 and HepG2 cells was increased after PAR2 overexpression, while knockdown of PAR2 decreased the proliferation and metastasis ability of SMMC-7721 and HepG2 cells. Knockdown of PAR2 also inhibited HCC tumor cell growth and liver metastasis in nude mice. Mechanistically, PAR2 increased the proliferation ability of SMMC-7721 and HepG2 cells via ERK activation. Activated ERK further promoted the epithelial-mesenchymal transition of these cells with the help of EGR-1 and Snail, which endowed them with enhanced migration and invasion ability.
In this study, we found that PAR2 was upregulated in HCC tumor tissues and related with poor prognosis in HCC patients. In addition, we proved that PAR2 could not only promote the proliferation and metastasis ability of SMMC-7721 and HepG2 cells in vitro, but also promoted xenograft tumor growth and HCC cell liver metastasis in vivo. These effects were mediated by the activation of ERK, which further induced EMT by EGR-1 and Snail of HCC cells. Therefore, targeting PAR2 may present a favorable anticancer target for treatment.
As we all know, PAR2 is activated via its N-terminal cleavage by several proteases such as serine proteases, but which protease is responsible for PAR2 activation in HCC is still not clear. This is a very interesting direction in the following study. Recently, some growth factors such as HGF were reported to be responsible for EMT in HCC. Whether HGF participates in PAR2-induced EMT could be further investigated based on this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Midorikawa Y, Vij M, Ilangumaran S, Sakaguchi T S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1275] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 2. | Ye JZ, Chen JZ, Li ZH, Bai T, Chen J, Zhu SL, Li LQ, Wu FX. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23:7415-7424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Feng LH, Dong H, Lau WY, Yu H, Zhu YY, Zhao Y, Lin YX, Chen J, Wu MC, Cong WM. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143:293-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Chan SL, Wong VW, Qin S, Chan HL. Infection and Cancer: The Case of Hepatitis B. J Clin Oncol. 2016;34:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3231] [Cited by in RCA: 3323] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 6. | Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. 1994;91:9208-9212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 701] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 825] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 8. | Grab DJ, Garcia-Garcia JC, Nikolskaia OV, Kim YV, Brown A, Pardo CA, Zhang Y, Becker KG, Wilson BA, de A Lima AP. Protease activated receptor signaling is required for African trypanosome traversal of human brain microvascular endothelial cells. PLoS Negl Trop Dis. 2009;3:e479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Lin H, Trejo J. Transactivation of the PAR1-PAR2 heterodimer by thrombin elicits β-arrestin-mediated endosomal signaling. J Biol Chem. 2013;288:11203-11215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Weithauser A, Rauch U. Role of protease-activated receptors for the innate immune response of the heart. Trends Cardiovasc Med. 2014;24:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Weithauser A, Bobbert P, Antoniak S, Böhm A, Rauch BH, Klingel K, Savvatis K, Kroemer HK, Tschope C, Stroux A. Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J Am Coll Cardiol. 2013;62:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Kanemaru A, Yamamoto K, Kawaguchi M, Fukushima T, Lin CY, Johnson MD, Camerer E, Kataoka H. Deregulated matriptase activity in oral squamous cell carcinoma promotes the infiltration of cancer-associated fibroblasts by paracrine activation of protease-activated receptor 2. Int J Cancer. 2017;140:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Quanjun D, Qingyu Z, Qiliang Z, Liqun X, Jinmei C, Ziquan L, Shike H. Effect and mechanism of PAR-2 on the proliferation of esophageal cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:4688-4696. [PubMed] |
| 14. | Zeeh F, Witte D, Gädeken T, Rauch BH, Grage-Griebenow E, Leinung N, Fromm SJ, Stölting S, Mihara K, Kaufmann R. Proteinase-activated receptor 2 promotes TGF-β-dependent cell motility in pancreatic cancer cells by sustaining expression of the TGF-β type I receptor ALK5. Oncotarget. 2016;7:41095-41109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Schaffner F, Versteeg HH, Schillert A, Yokota N, Petersen LC, Mueller BM, Ruf W. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood. 2010;116:6106-6113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Kaufmann R, Oettel C, Horn A, Halbhuber KJ, Eitner A, Krieg R, Katenkamp K, Henklein P, Westermann M, Böhmer FD. Met receptor tyrosine kinase transactivation is involved in proteinase-activated receptor-2-mediated hepatocellular carcinoma cell invasion. Carcinogenesis. 2009;30:1487-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Kaufmann R, Mussbach F, Henklein P, Settmacher U. Proteinase-activated receptor 2-mediated calcium signaling in hepatocellular carcinoma cells. J Cancer Res Clin Oncol. 2011;137:965-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Lee TK, Cheung VC, Lu P, Lau EY, Ma S, Tang KH, Tong M, Lo J, Ng IO. Blockade of CD47-mediated cathepsin S/protease-activated receptor 2 signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 19. | Mußbach F, Ungefroren H, Günther B, Katenkamp K, Henklein P, Westermann M, Settmacher U, Lenk L, Sebens S, Müller JP. Proteinase-activated receptor 2 (PAR2) in hepatic stellate cells - evidence for a role in hepatocellular carcinoma growth in vivo. Mol Cancer. 2016;15:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Chen KD, Wang CC, Tsai MC, Wu CH, Yang HJ, Chen LY, Nakano T, Goto S, Huang KT, Hu TH. Interconnections between autophagy and the coagulation cascade in hepatocellular carcinoma. Cell Death Dis. 2014;5:e1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 3385] [Article Influence: 423.1] [Reference Citation Analysis (0)] |
| 22. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6214] [Article Influence: 564.9] [Reference Citation Analysis (0)] |
| 23. | De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1968] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 24. | Chua KN, Poon KL, Lim J, Sim WJ, Huang RY, Thiery JP. Target cell movement in tumor and cardiovascular diseases based on the epithelial-mesenchymal transition concept. Adv Drug Deliv Rev. 2011;63:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | Kudo M. Early detection and curative treatment of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2005;3:S144-S148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534-3545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 278] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M, Beug H. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Cai L, Ye Y, Jiang Q, Chen Y, Lyu X, Li J, Wang S, Liu T, Cai H, Yao K. Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat Commun. 2015;6:7353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Weiler SME, Pinna F, Wolf T, Lutz T, Geldiyev A, Sticht C, Knaub M, Thomann S, Bissinger M, Wan S. Induction of Chromosome Instability by Activation of Yes-Associated Protein and Forkhead Box M1 in Liver Cancer. Gastroenterology. 2017;152:2037-2051.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 31. | Nakagawa H, Mizukoshi E, Kobayashi E, Tamai T, Hamana H, Ozawa T, Kishi H, Kitahara M, Yamashita T, Arai K. Association Between High-Avidity T-Cell Receptors, Induced by α-Fetoprotein-Derived Peptides, and Anti-Tumor Effects in Patients With Hepatocellular Carcinoma. Gastroenterology. 2017;152:1395-1406.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Schneider AT, Gautheron J, Feoktistova M, Roderburg C, Loosen SH, Roy S, Benz F, Schemmer P, Büchler MW, Nachbur U. RIPK1 Suppresses a TRAF2-Dependent Pathway to Liver Cancer. Cancer Cell. 2017;31:94-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Fortin J, Mak TW. Targeting PI3K Signaling in Cancer: A Cautionary Tale of Two AKTs. Cancer Cell. 2016;29:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Cattaruzza F, Amadesi S, Carlsson JF, Murphy JE, Lyo V, Kirkwood K, Cottrell GS, Bogyo M, Knecht W, Bunnett NW. Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants. Br J Pharmacol. 2014;171:3814-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Gui Y, Khan MGM, Bobbala D, Dubois C, Ramanathan S, Saucier C, Ilangumaran S. Attenuation of MET-mediated migration and invasion in hepatocellular carcinoma cells by SOCS1. World J Gastroenterol. 2017;23:6639-6649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |