Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1107
Peer-review started: December 29, 2017
First decision: January 17, 2018
Revised: February 4, 2018
Accepted: February 9, 2018
Article in press: February 9, 2018
Published online: March 14, 2018
Processing time: 75 Days and 2.9 Hours
To establish a rat model for evaluating the maturity of liver regeneration derived from associating liver partition and portal vein ligation for staged hepatectomy (ALPPS).
In the present study, ALPPS, partial hepatecotmy (PHx), and sham rat models were established initially, which were validated by significant increase of proliferative markers including Ki-67, proliferating cell nuclear antigen, and cyclin D1. In the setting of accelerated proliferation in volume at the second and fifth day after ALPPS, the characteristics of newborn hepatocytes, as well as specific markers of progenitor hepatic cell, were identified. Afterwards, the detection of liver function followed by cluster analysis of functional gene expression were performed to evaluate the maturity.
Compared with PHx and sham groups, the proliferation of FLR was significantly higher in ALPPS group (P = 0.023 and 0.001 at second day, P = 0.034 and P < 0.001 at fifth day after stage I). Meanwhile, the increased expression of proliferative markers including Ki-67, proliferating cell nuclear antigen, and cyclin D1 verified the accelerated liver regeneration derived from ALPPS procedure. However, ALPPS-induced Sox9 positive hepatocytes significantly increased beyond the portal triad, which indicated the progenitor hepatic cell was potentially involved. And the characteristics of ALPPS-induced hepatocytes indicated the lower expression of hepatocyte nuclear factor 4 and anti-tryptase in early proliferative stage. Both suggested the immaturity of ALPPS-derived liver regeneration. Additionally, the detection of liver function and functional genes expression confirmed the immaturity of renascent hepatocytes derived in early stage of ALPPS-derived liver regeneration.
Our study revealed the immaturity of ALPPS-derived proliferation in early regenerative response, which indicated that the volumetric assessment overestimated the functional proliferation. This could be convincing evidence that the stage II of ALPPS should be performed prudently in patients with marginally adequate FLR, as the ALPPS-derived proliferation in volume lags behind the functional regeneration.
Core tip: Despite the rapid proliferation of future liver remnant induced by associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), the high mortality and morbidity rates have remained alarming. A plausible reason was the functional proliferation lagged behind the increase in volume. In this study, a rat model was established to evaluate the maturity of ALPPS-derived hepatocytes. Through the identification of hepatic characteristics, detection of liver function, and analysis of functional gene expression, we revealed the immaturity of ALPPS-derived proliferation in early regenerative response, which indicated that the volumetric assessment overestimated the functional proliferation. And clinically, the stage II of ALPPS should be performed prudently in patients with marginally adequate FLR, as the ALPPS-derived proliferation in volume lags behind the functional regeneration.
- Citation: Tong YF, Meng N, Chen MQ, Ying HN, Xu M, Lu B, Hong JJ, Wang YF, Cai XJ. Maturity of associating liver partition and portal vein ligation for staged hepatectomy-derived liver regeneration in a rat model. World J Gastroenterol 2018; 24(10): 1107-1119
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1107.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1107
Given its increasing incidence, liver tumor is one of the most life-threatening diseases worldwide[1]. Despite the development in a variety of therapies based on the property of tumor (e.g., transcatheter arterial chemoembolization, chemotherapy, molecular targeting therapy, etc.) in recent decades, surgery remains the only curative treatment for patients with primary or metastatic hepatic malignancies[2,3]. Although the remarkable regenerative capacity of the liver permits the extended hepatectomy in clinic, postoperative liver failure caused by small-for-size syndrome (SFSS) represents the most common cause of death after hepatectomy[4]. To address this issue, a novel innovation called associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been invented. This technique induces the accelerated growth of future liver remnant (FLR) within transient period through integrating the portal vein occlusion and parenchymal transection[5-8].
However, some experts caution the feasibility and safety of such procedure, and the initial enthusiasm for ALPPS also was tempered because of high morbidity and mortality[9-12]. The improvement of technology and accumulation of experience has decreased the mortality rate to less than 10%, but this remains too high[8]. A rational possibility is that volumetric assessment overestimates the functional proliferation. Given the development of induced pluripotent stem cell technique and the illustration of the roadmap determining the fate of diverse cells, the mechanism of hepatocyte differentiation has been elucidated gradually through the lineage tracing method[13]. Basic research has elucidated that it takes about 8 to 10 d for a hepatoblast to mature into a hepatocyte[14]. In this setting, within a short interval period of ALPPS procedure, the maturity of induced hepatocytes has to be queried. Clinically, the interval time between two stages of ALPPS is usual one or two weeks. Additionally, several studies also have shown that there is a distinct delay in functional gain compared to volumetric increase in ALPPS[15,16]. Therefore, the functional quality of hypertrophic response derived from ALPPS procedure, not just volumetric assessment of the FLR, should be performed to time the stage II.
Despite the establishment of several ALPPS animal studies with remarkable growth in volume, none of models were dedicated to evaluating functional proliferation. Thus, the aim of study was to establish a rat model mimicking ALPPS procedure to assess the maturity of ALPPS-derived liver regeneration functionally and volumetrically. This might be of great value in timing the stage II of ALPPS and improving its safety clinically.
The protocol of this study was reviewed and approved by the animal ethics committee of the Zhejiang University, Hangzhou, China. All experiments were performed in accordance with relevant approved guidelines and regulations. In the present study, male Sprague-Dawley rats, weighing 180 to 230 g from experimental animal center of Zhejiang province, Hangzhou, China, were used. All the rats were housed in a restricted access room with controlled temperature (23 °C) and a light/dark (12 h:12 h) cycle, and had free access to food and water before and after treatment. Initially, a preliminary study was simply performed to screen the feasible models (n = 5, each group). The sham group was adopted as negative control and the appropriate PHx model was regarded as a positive control. Then, the volumetric and functional liver regeneration of three groups, ALPPS group, PHx group, and sham group were compared in this study.
According to the results of preliminary study, the ALPPS, PHx, and sham groups were defined as experimental, positive, and negative control groups in this study. ALPPS group: ligation of the portal vein belonging to left lateral, right, caudate lobes, and transection of parenchyma of middle lobe. PHx group: removal of left lateral, right and caudate lobes. Sham group: Open and close the abdominal cavity.
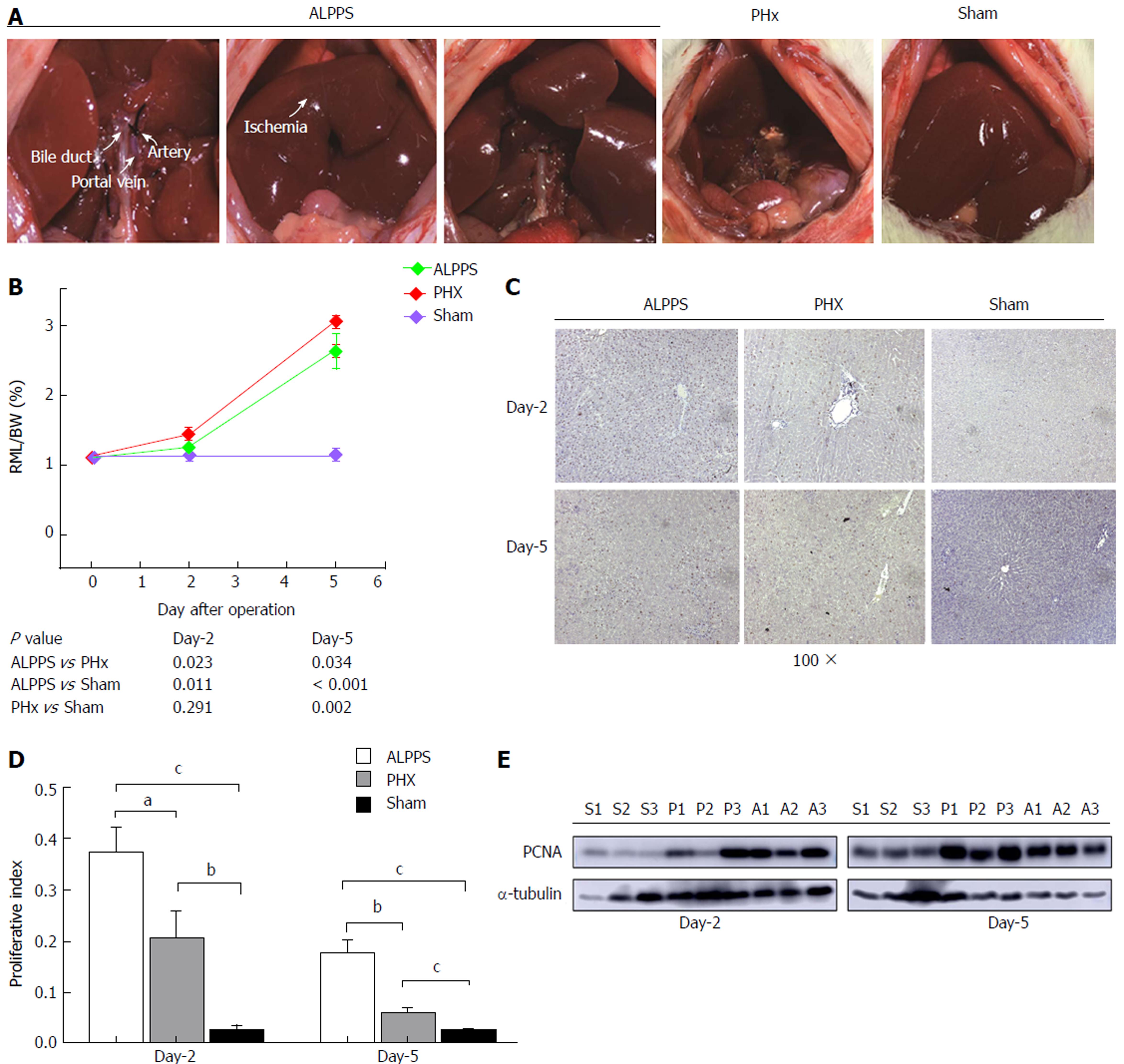
All rats were fasted 8 h before operation. Under the general anesthesia with 8% of chloral hydrate (5.0 mL/kg) by intra-abdominal injection, the abdominal transverse incision was adopted. For the ALPPS procedure, dissection of the left lateral lobe followed by ligation of the portal vein supplying the corresponding lobe with 5-0 silk were performed while artery and biliary duct branches were maintained. Then, the same procedure was conducted in the portal branches of the right and caudate lobes, respectively. The parenchyma was partitioned by 5-0 silks along with the ischemic demarcation line of the middle lobe. Five days after stage I, the stage II was performed, in which the deportalization lobes were removed (n = 5). For the PHx model, the left lateral, right, and caudate lobes were removed after corresponding hepatic pedicle were ligated with 3-0 silks. And for sham group, opening followed by closing the abdominal cavity was performed (Figure 1A).
Previously, several studies indicated the ALPPS procedure was divided into early (1-3 d after stage I) and later stage (4-7 d after stage I) generally[17-21]. Therefore in our study, the rats were sacrificed on the second and fifth day after operation. The specimen was collected for subsequent research. Each group at different time points contained six rats. Half of them were used for evaluating the efficiency of proliferation, and the other three rats were used for primary hepatocyte isolation and subsequent detection of hepatic function.
For RNA extraction, total RNA was extracted from 50 mg of liver specimen by TRIzol reagent (CWBIO, China). 5 μg of RNA were reverse-transcribed by the HiFiScript cDNA Synthesis Kit (CWBIO, China), yielding the complementary DNA template. The quantitative real-time PCR amplification was performed by the ROCHE Light Cycler 480 II. The expression of mRNA was shown as fold induction. The primer sequences were listed in Supplement Table 1.
The detection of proteins was performed by standard western blot assays according to the steps below[22]. Total proteins from liver tissues were extracted with RIPA buffer containing protease inhibitors (Beyotime, China) and quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific, United States). About 40 μg of total protein was separated by 10% SDS-PAGE. Samples were transferred to PVDF membranes (Millipore, United States) and incubated overnight at 4 degrees with primary antibodies. The blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized using the ECL system (Thermo Fisher Scientific, Rochester, NY, United States).
The liver tissues were immersion fixed in 4% formaldehyde overnight. Then, they were embedded, sectioned, dehydrated in ethanol and xylene before being stained with hematoxylin-eosin (HE) immunohistochemistry (IHC). Through the IHC stain, the number of Ki-67 positive hepatocytes were calculated randomly in four visual fields (× 400) and analyzed by Image Pro plus 5.1 (Media Cybernetics, United States), which was presented as the proliferation index. With respect to immunofluorescence, the primary antibody to Sox9 (ab185230) and Albumin (ab106582) were produced from Abcam. The experimental procedure was according to the standard protocol.
The protocol for isolation of primary hepatocytes was according to three-step collagenase perfusion. Briefly, the liver was perfused through the suprahepatic inferior vena cava with calcium-free buffer (0.5 mmol/L EGTA, 1 × EBSS without Ca2+ and Mg2+), followed by the calcium-bearing buffer (10 mmol/L HEPES, 1 × EBSS with Ca2+ and Mg2+), and then irrigated with collagenase [0.2 mg/mL collagenase type IV (Yeasen, China)]. Subsequently, parenchymal cells were purified by 90% Percoll buffer (Sigma) at low speed centrifugation (1000 rpm, 10 min). After the cells were cultured in DMEM medium with 10% FBS for 8 h, PAS stain, OilRed stain, and detection of urea nitrogen were performed following the manufacturer’s instructions (Solarbio, China). In the indocyanine green (ICG) uptake assay, hepatocytes were cultured with 1 mg/mL ICG (Tianyi, China) at 37 °C for 90 min and washed with PBS three times.
The data were expressed as a mean with standard deviation or a percentage. Correspondingly, Student t-test or χ2 test was used to analyze the difference. Significance was considered when a two-tailed P value was less than 0.05. Statistical analysis was performed using SPSS, version 22.0 for Windows (IBM Corporation, Armonk, NY, United States).
To establish a feasible positive control model, various PHx models with different extensions of hepatectomy were compared (Supplement Figure 1). The mortality of extended PHx group (removal of left lateral, left middle, right and caudate lobes, n = 5), which presented the same extension of stage II of ALPPS, was 80%. Compared with extended PHx model (removal left lateral, left middle, right and caudate lobes) and minor PHx model (only removal left lateral), the medium PHx group (removal of left lateral, right and caudate lobes) presented an acceptable mortality (20%) and triggered a remarkable proliferation of FLR. It was therefore determined as positive control group in this study.
Compared with extended PHx group, no rat in ALPPS group which induces rapid hepatic proliferation (removal of left lateral, left middle, right and caudate lobes) died both in stage IV and II. (0% vs 80%, P = 0.053). As the preservation of portal vein of right middle lobe (RLM) in each group, the liver regeneration was assessed by the ratio of RLM weight to body weight (BW). The mean RML/BW of ALPPS, PHx, sham groups were 1.44% ± 0.04%, 1.24% ± 0.09%, 1.14% ± 0.11% on the second day after operation, and 3.06% ± 0.11%, 2.63% ± 0.39%, 1.13% ± 0.10% on the fifth day after operation, respectively (Figure 1B). Compared with sham group, the proliferation of PHx group was remarkably induced in later stage (P = 0.002). However, in the ALPPS group, the hypertrophic response was more active than that of the PHx groups in whole course (P = 0.023 and P = 0.034). To further confirm the regenerative response, the staining of Ki-67 followed by the calculation of proliferation index of different groups were compared (Figure 1C and D). The expression of PCNA, a classical marker for cell proliferation, was detected by western blotting. Apparently, up-regulated expression of PCNA in the ALPPS group appeared earlier than that in PHx group (Figure 1E).
Thus, these results indicated PHx procedure could trigger liver regeneration, but a stronger regenerative response was activated by ALPPS procedure.
As suggested by previous studies showing that the progenitor hepatic cell (HPC) differentiated into mature hepatocyte by the regulation of hepatic transcription factors (Figure 2A), we found that the hepatocytes in ALPPS and PHx presented a larger nucleus around the portal triad in both early and late stages (Figure 2B). These special hepatoctyes, we suspected, might be immature HPC. To verify this hypothesis, classical markers of hepatic progenitor cell were detected (Figure 2C). Compared with sham group, several markers, including Sox9 and Epcam were significantly increased at mRNA level (P < 0.001 and P = 0.002), especially in early proliferative stage. Furthermore, we checked the expression of Sox9 at protein level, and found it remarkable that the Sox9-positive hepatocytes were widespread beyond the portal traid on second day after ALPPS procedure (Figure 2D). These might imply the activation of HPC in early regenerative response, which was in accordance with the powerful regenerative capacity of HPC from literature reports[23]. As suggested by expression of HPC special markers, the maturity of induced liver hypertrophy either by ALPPS or conventional PHx procedure seemed comparable in later stage of proliferation. And thereby, we inferred the ALPPS-derived liver regeneration might be not completely mature in early phase. Meanwhile, a delay of functional proliferation was indicated in comparison of PHx-derived liver regeneration.
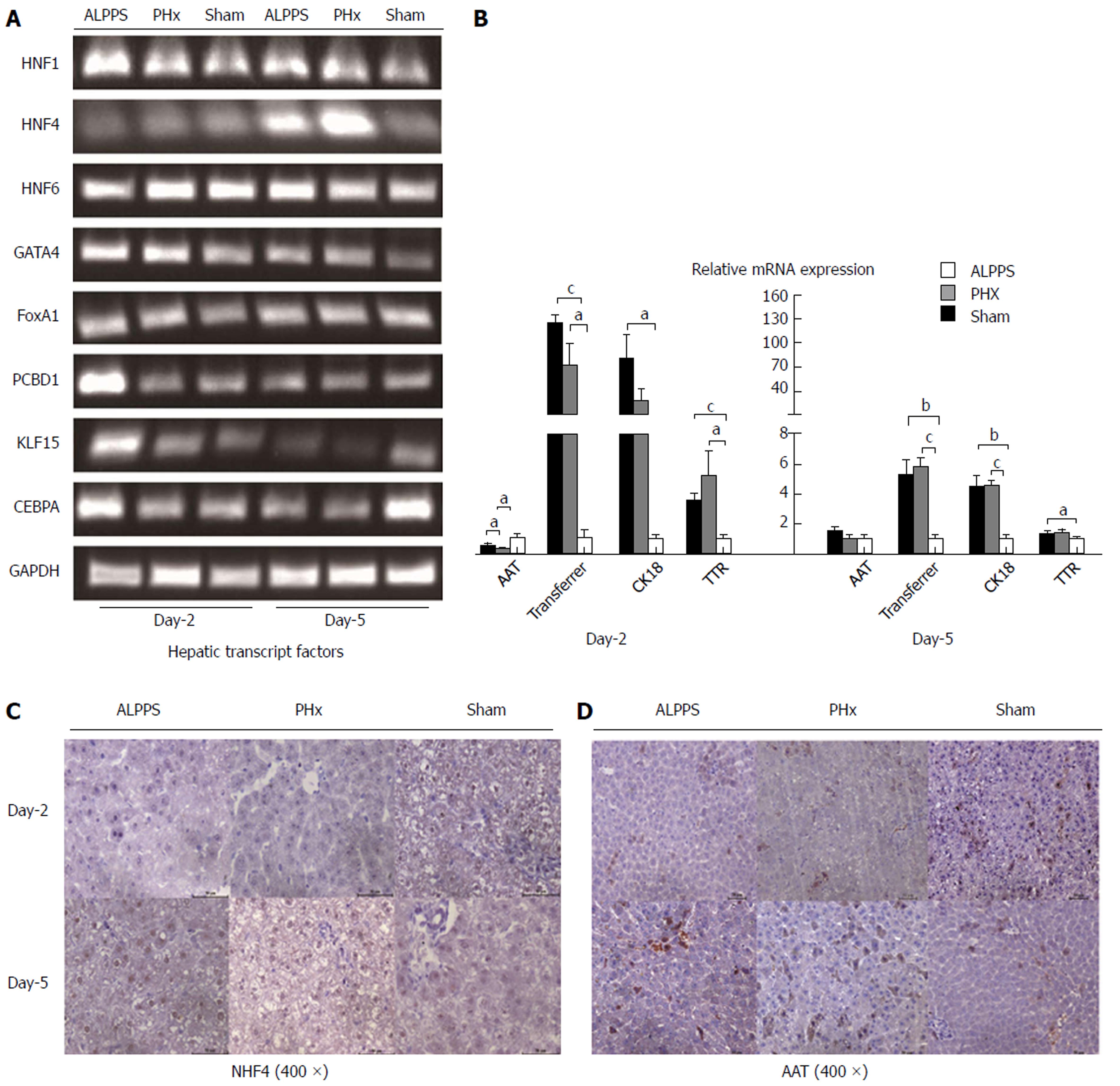
To clarify the above-mentioned inference from the other side, we detected the expression of well-known markers of mature hepatocytes (Figure 3A). Several transcription factors (e.g., HNF1, HNF4, GATA4, FoxA1, etc) had been demonstrated to regulate the lineage reprogramming of fibroblasts into hepatocytes in vitro[24]. In this study, the HNF4 was delayed up-regulation on the fifth day of ALPPS and PHx groups, suggesting the process of differentiation of hepatocyte remained activated even in later phase. To measure the expression of HNF4 at protein level, the IHC stain was performed, which presented a negative result in early stage but a positive result in later stage of hypertrophic response (Figure 3C). Similarly, functional proteins in the cytoplasm were detected. We found that in the initial process of regeneration, the expression of anti-tryptase (AAT) was relatively reduced even in the setting of the increase in amount of hepatocytes (Figure 3B and D).
Taken together, compared with intrinsic hepatocyte in sham group, the immaturity of newborn hepatocytes in early proliferative response of ALPPS and PHx group were elucidated. Among, the differentiation of hepatocyte in ALPPS group appeared to be more prolonged than that in PHx group through the analysis of characteristics of induced hepatocyte.
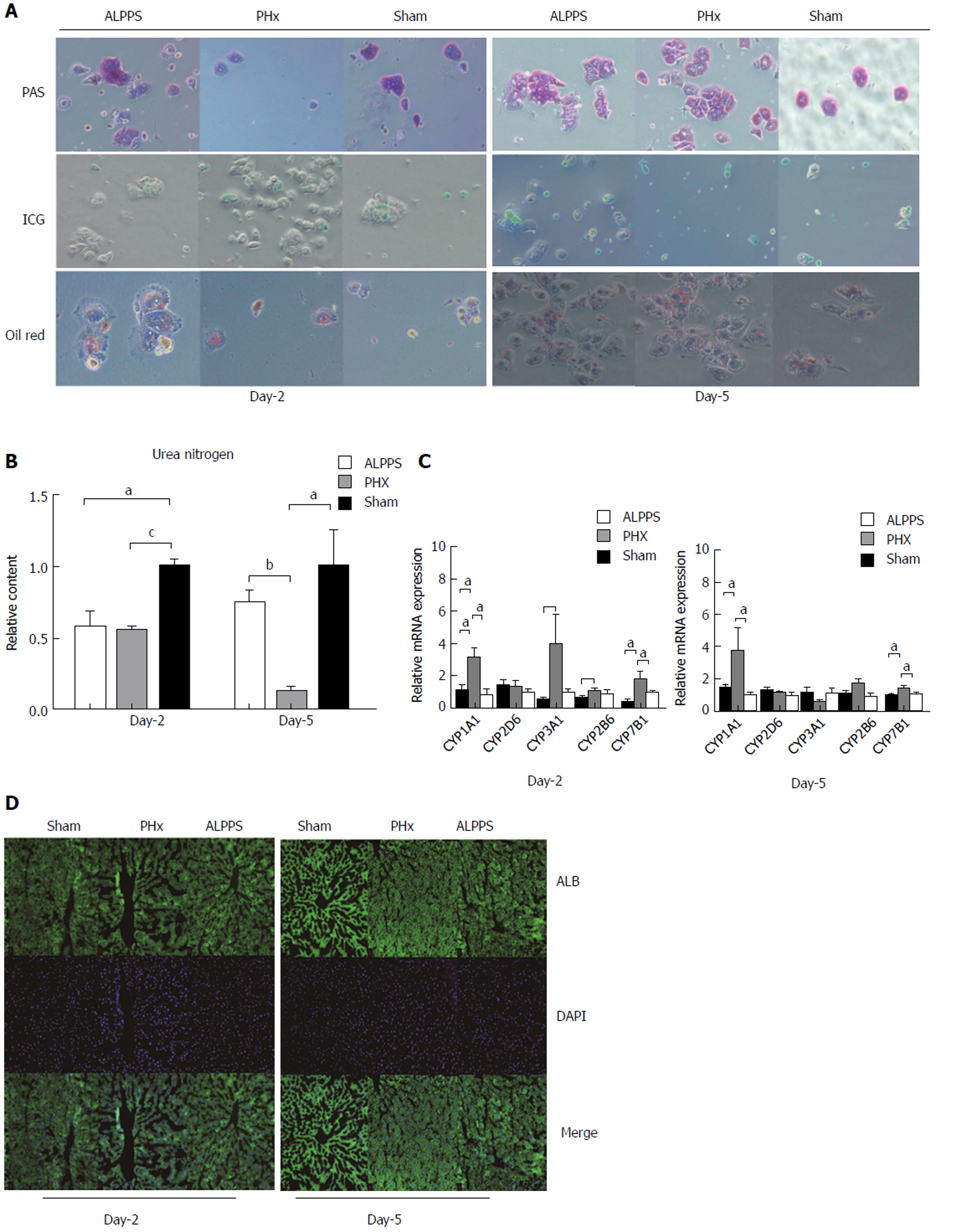
Given the proliferation in volume and the immaturity of the induced hepatocyte, the functional regeneration had to be naturally questioned. Despite the complexity of induced liver regeneration, an elaborate detection of hepatocyte function was performed as shown below. By primary hepatocyte isolation, the PAS stain, OilRed stain, ICG stain, and synthesis of urea nitrogen of each group in early and later stages were conducted (Figure 4A). As a result, a comparable capacity of glycogen synthesis and fat metabolism was presented between groups, even in the early stage of proliferation. Nevertheless, compared with sham group on the second day after operation, the PHx group had a decreased capability of ICG up-take. Furthermore, the ALPPS group showed a more inferior capacity of ingestion of ICG. Moreover, an insufficiency of urea nitrogen synthesis of hypertrophic groups existed in the whole course of liver regeneration (Figure 4B). With respect to the metabolism, the P450Y enzyme system was the most representative substance. In comparison of PHx group, the expression of CYP3A1 and CYP2B6 in ALPPS group was suppressed on the second day and the expression of CYP1A1 and CYP7B1 was down-regulated in both stages (Figure 4C). But in terms of expression of albumin, no inter-group discrepancy was clarified (Figure 4D).
In this section, we found that the ALPPS-derived liver regeneration postponed the functional maturity in aspects of ICG up-take, synthesis of urea nitrogen, and expression of P450Y enzyme in the early stage of proliferation.
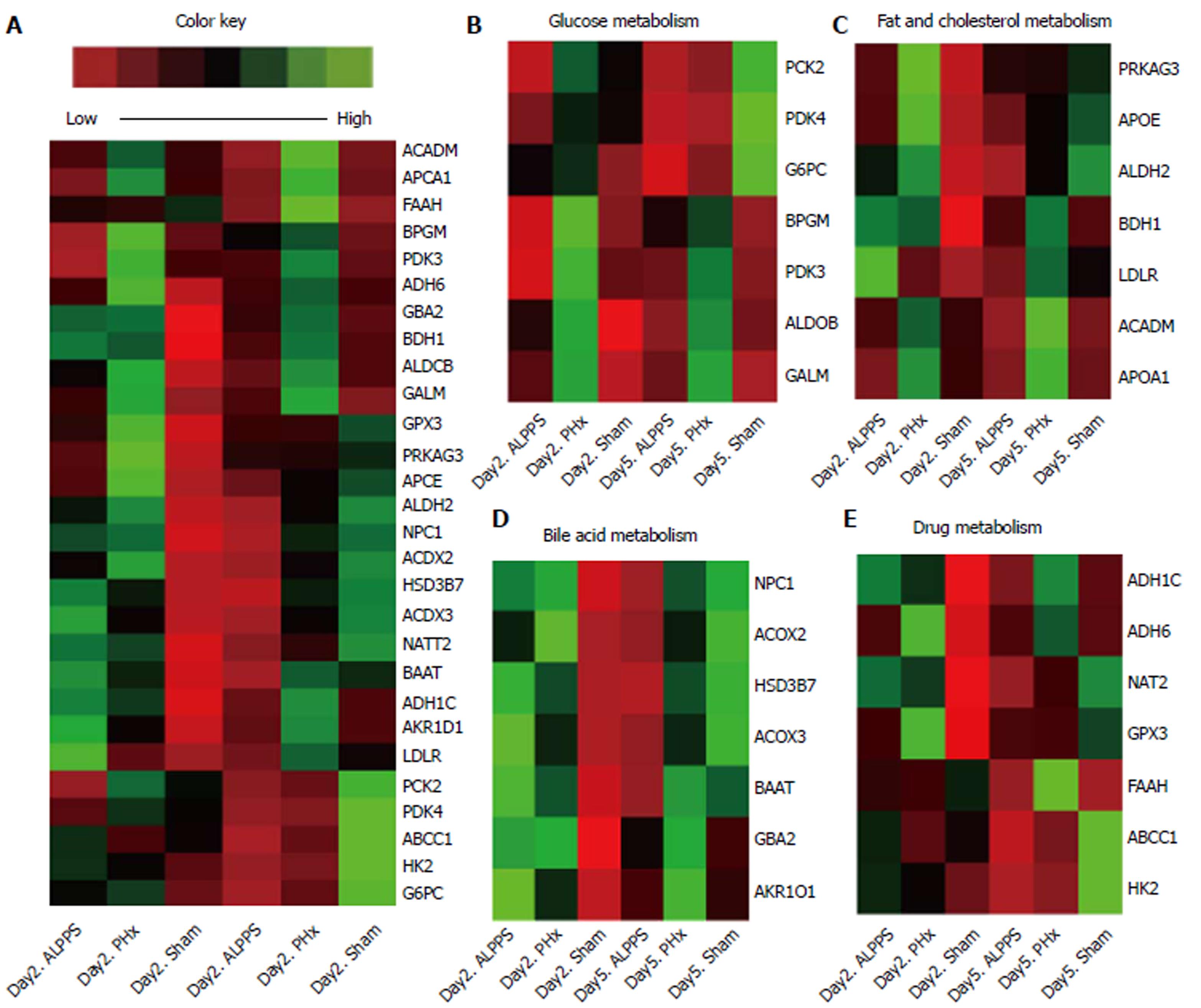
As seen from above, because the ALPPS-derived liver regeneration might be not completely mature in early stage, cluster analysis of different expression of functional genes was therefore performed. All expression of functional genes were summarized in Figure 5A and Supplement Table 2. In the initial stage of liver regeneration, almost all functional genes were up-regulated and were accompanied by an increase in the amount of hepatocyte, which either was induced by ALPPS or PHx procedure. Intriguingly, although the PAS stain showed comparable capacity of synthesis of glycogen in ALPPS and PHx groups, the cluster analysis indicated a relatively low expression of genes in regard to glycogen metabolism. Until the later stage of proliferation, almost all genes had relatively lower expression in the ALPPS group (Figure 5B-E).
Together, these results suggested the expression of functional genes in hepatocytes derived from ALPPS procedure were relatively lower, even in the later stage of proliferation.
Based on the first ALPPS Consensus Meeting held in Hamburg in February 2015, the timing of stage II was recommended until the FLR reached 40% in liver with cirrhosis or 25% to 30% in normal liver[25,26]. Nevertheless, the incidence of postoperative hepatic failure remains up to 31% even when sufficient FLR volumes were achieved[27]. A rational explanation is the fact that rapid increase in volume derived from ALPPS lags behind the functional proliferation. And thereby, the timing of stage II could be better addressed and the rate of mortality could be minimized more efficiently on the basis of functional evaluation. Till now, several technologies including ICG disappearance rate, fluorodeoxyglucose imaging, and 99mTc-mebrofenin hepatobiliary scintigraphy (HBS) had been established[28,29]. Among them, the widespread application of HBS is of great help in the assessment of functional maturity before and after hepatectomy, even in the ALPPS procedure[8]. However, the functional maturity in the process of liver regeneration is too complicated to be determined by merely the uptake and excretory function. For instance, the synthesis of urea nitrogen and albumin, vitality of P450Y enzyme, and fat metabolism are vital parameters in the assessment of liver function. Clinically, it is quite difficult to recruit adequate recipients to fulfill the study of functional detection in ALPPS procedure. Thus, we conducted a rat model mimicking ALPPS procedure to evaluate the functional proliferation, which could be of great help in clinical decision-making.
As suggested by previous results presenting that the volumetric proliferation of FLR could be induced in patients who underwent ALPPS procedure, it was remarkable that the right middle lobe in our rat model was rapidly hypertrophic through the same manipulation. Nonetheless, we found that the newborn hepatocytes for liver restoration remained poorly characterized. First of all, our study revealed that in ALPPS and PHx models, of the majority of cells with a large ratio of nucleus to cytoplasm appeared enriched around the portal vein. In addition, a significant up-regulation of Sox9 on the second day after ALPPS and subsequent decrease in the later stage indicated the potential role of HPC in triggering the activation of regeneration[30]. Based on previous reports, the HPC, a stem-like cell around the portal triads, presented a dramatic capacity to restore liver function and replenish liver mass in liver regeneration[31-33]. Thus, we inferred that the activity of HPC promoted the accelerated liver restoration. To clarify the immaturity of newborn hepatocyte, we detected several transcription factors associated with the destiny of hepatocytes, and found that the peak of HNF4, which plays a critical role in regulating the expression of differentiation-related genes and maintenance of liver function in mammals, was postponed[34-36]. Meanwhile, the inferior expression of AAT was manifested on the second day of ALPPS and PHx groups, which supported the immaturity of hepatocytes. To sum up, the induced hepatocytes were still in the process of differentiation, which suggested the induced-hepatocytes were defective functionally in early regenerative response.
To further evaluate the maturity of neonatal hepatocytes, function detection was performed. In this section, a mild inefficiency of ICG uptake capacity was presented in ALPPS group in early stage. And the insufficient synthesis of P450Y enzymes indicated a deficient capacity of ALPPS-derived hepatocyte in early stage. As seen from the above, the metabolic function is not recovered in early stage of ALPPS procedure. Hence, for patients with biliary obstruction or disorder of renal function, the accumulation of detrimental substances always aggravates the burden of detoxification, and thereby induces the hepatic failure theoretically. Correspondingly, the patients with hilar cholangiocarcinoma or gallbladder cancer, always combined with biliary obstruction, presented a higher mortality and morbidity clinically. Intriguingly, the cluster analysis of different genes expression revealed a paradoxical result. The lower abundance of functional genes relevant to glycogen metabolism presented an insufficiency of synthesis in the ALPPS group, even though the PAS stain presented a comparable capacity in terms of glycogen synthesis. Moreover, relatively low expression of genes in regards to fat, cholesterol, bile acid, and drugs in later stage reminded us that liver function of hepatocytes derived from ALPPS procedure lagged behind the increase in liver volume. Taken together, the assessment of ICG clearance and hepatic metabolites could improve the comprehensiveness of liver functional evaluation, avoiding the process by which the volumetric increase overestimates the functional gain in liver.
In the present study, we demonstrated that the liver regeneration derived from ALPPS was not completely mature in early stage, but the functional proliferation was mainly completed on the fifth day after ALPPS. Besides, the 0% of mortality after stage II indirectly clarified both volumetric and functional regeneration were achieved. Nevertheless, whether the results could be generalized to clinical patients are warranted definitely. To begin with, a majority of ALPPS procedure are performed in patients with diseased liver (e.g., fibrosis, non-alcoholic steatohepatitis, post-chemotherapy, etc). Given that the capacity of liver regeneration was attenuated in the diseased liver, the maturity has to be queried subsequently. In this setting, the timing of stage II should be prolonged as well. Additionally, the rate of metabolism is apparently faster in rats than in humans. The optimal time to perform stage II in human is still controversial, despite that extended hepatecotmy was performed smoothly with an interval time of five days in rat. Theoretically, the species-specific characteristics and inconsecutive observation point time could contribute to a confounding effect on the conclusion.
Given the mechanism of ALPPS-derived liver regeneration is scarce, our follow-up work will verify the hypothesis that the dramatic capacity of regeneration is derived from HPC by lineage tracing method (Sox9-Cre-GFP mouse)[32]. Despite these limitations, our study revealed the immaturity of ALPPS-derived proliferation in early regenerative response, which indicated the volumetric assessment overestimated the functional proliferation. This could be a convincing evidence that the stage II of ALPPS should be performed prudently in patients with marginally adequate FLR, as the ALPPS-derived proliferation in volume lags behind the functional regeneration.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been increasingly popular worldwide recently. However, the high mortality makes surgeons reconsider the difference between functional and volumetric proliferation in ALPPS-derived liver regeneration. In this study, we therefore establish a rat model to mimic the ALPPS, exploring whether the functional proliferation lags behind the hypertrophy in volume.
This was a preliminary study with regard to the maturity of ALPPS-derived liver regeneration. On the basis of developing a rat model, we found the volumetric assessment overestimated the functional proliferation in ALPPS procedure, which indicated the stage II of ALPPS should be performed prudently in patients with marginally adequate future liver remnant.
This study was to evaluate the maturity of ALPPS-derived liver regeneration. In our rat model, the postponed maturity in function might be an important reason for high mortality of ALPPS even when the adequate future liver remnant was achieved before stage II of ALPPS. Likewise, the functional proliferation should be performed to time the stage II of ALPPS clinically.
In this study, ALPPS, partial hepatectomy (PHx) and sham models were conducted. The ratio of right middle lobe to body weight as well as proliferative markers were used for assessing the liver regeneration. Morphological changes by HE stain and detection of specific markers of progenitor or mature hepatocytes were adopted to identify the characteristics of newborn hepatocytes. Eventually, the liver function in vivo and vitro was measured, followed by the cluster analysis of expression of functional genes to detect the maturity of liver regeneration from different models.
By establishment of ALPPS, PHx and sham models, we demonstrated that ALPPS could induce an accelerated proliferative response. However, the characteristics of newborn hepatocytes seemed to be not mature completely. Sox9 positive hepatocyte, as well as different expression of other specific markers, indicated the potential role of progenitor hepatic cell in ALPPS-derived regeneration. Parts of limited liver function and different expression of functional genes supported the above-mentioned immaturity in ALPPS-induced proliferation.
As the mortality remains unsatisfactory even in patients with adequate future liver remnant after stage I of ALPPS, this study presented the immaturity of ALPPS-derived proliferation in early regenerative response, which indicated that the volumetric assessment overestimated the functional proliferation. To the best of our knowledge, this is the first study to evaluate the maturity of ALPPS-derived liver regeneration in a rat model. Meanwhile, Sox9 positive hepatocyte indicated the potential role of hepatic progenitor cell in the ALPPS rather than conventional PHx model. Therefore, a more detailed research about the hepatic progenitor cell promotes the ALPPS-derived liver regeneration and its mechanism would be done in our next work.
The stage II of ALPPS should be performed prudently in patients with marginally adequate future liver remnant, as the ALPPS-derived proliferation in volume lags behind the functional regeneration. By the way, as the hepatic progenitor cell might be an important role in ALPPS-derived liver regeneration, our future work is to further demonstrate the fate of Sox9 positive hepatocyte with ALPPS procedure and its underlying mechanism by lineage tracing method.
Thanks to Professor Li-jian Hui, PhD, Laboratory of Molecular Cell Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy for Sciences, for guidance of primary hepatocyte isolation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Campos RR, Starlinger P S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21363] [Article Influence: 2136.3] [Reference Citation Analysis (3)] |
| 2. | Zhong BY, Ni CF, Chen L, Zhu HD, Teng GJ. Early Sorafenib-related Biomarkers for Combination Treatment with Transarterial Chemoembolization and Sorafenib in Patients with Hepatocellular Carcinoma. Radiology. 2017;284:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Tong Y, Li Z, Liang Y, Yu H, Liang X, Liu H, Cai X. Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: friend or foe? a propensity score analysis. Oncotarget. 2017;8:26671-26678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Langiewicz M, Schlegel A, Saponara E, Linecker M, Borger P, Graf R, Humar B, Clavien PA. Hedgehog pathway mediates early acceleration of liver regeneration induced by a novel two-staged hepatectomy in mice. J Hepatol. 2017;66:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, Topp SA, Vivarelli M, Aldrighetti LA, Robles Campos R. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017;19:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Lang H, de Santibanes E, Clavien PA. Outcome of ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017;19:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Chan ACY, Chok K, Dai JWC, Lo CM. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepatocellular carcinoma: Complete-ALPPS versus partial-ALPPS. Surgery. 2017;161:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Linecker M, Björnsson B, Stavrou GA, Oldhafer KJ, Lurje G, Neumann U, Adam R, Pruvot FR, Topp SA, Li J. Risk Adjustment in ALPPS Is Associated With a Dramatic Decrease in Early Mortality and Morbidity. Ann Surg. 2017;266:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Olthof PB, Huiskens J, Wicherts DA, Huespe PE, Ardiles V, Robles-Campos R, Adam R, Linecker M, Clavien PA, Koopman M. Survival after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for advanced colorectal liver metastases: A case-matched comparison with palliative systemic therapy. Surgery. 2017;161:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Sun Z, Tang W, Sakamoto Y, Hasegawa K, Kokudo N. A systematic review and meta-analysis of feasibility, safety and efficacy of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH). Biosci Trends. 2015;9:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bertens KA, Hawel J, Lung K, Buac S, Pineda-Solis K, Hernandez-Alejandro R. ALPPS: challenging the concept of unresectability--a systematic review. Int J Surg. 2015;13:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22:3109-3120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Han X, Yu H, Huang D, Xu Y, Saadatpour A, Li X, Wang L, Yu J, Pinello L, Lai S. A molecular roadmap for induced multi-lineage trans-differentiation of fibroblasts by chemical combinations. Cell Res. 2017;27:386-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Chaudhari P, Tian L, Deshmukh A, Jang YY. Expression kinetics of hepatic progenitor markers in cellular models of human liver development recapitulating hepatocyte and biliary cell fate commitment. Exp Biol Med (Maywood). 2016;241:1653-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Truant S, Baillet C, Deshorgue AC, El Amrani M, Huglo D, Pruvot FR. Contribution of hepatobiliary scintigraphy in assessing ALPPS most suited timing. Updates Surg. 2017;69:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Stockmann M, Bednarsch J, Malinowski M, Blüthner E, Pratschke J, Seehofer D, Jara M. Functional considerations in ALPPS - consequences for clinical management. HPB (Oxford). 2017;19:1016-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Shi H, Yang G, Zheng T, Wang J, Li L, Liang Y, Xie C, Yin D, Sun B, Sun J. A preliminary study of ALPPS procedure in a rat model. Sci Rep. 2015;5:17567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Wei W, Zhang T, Zafarnia S, Schenk A, Xie C, Kan C, Dirsch O, Settmacher U, Dahmen U. Establishment of a rat model: Associating liver partition with portal vein ligation for staged hepatectomy. Surgery. 2016;159:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Schlegel A, Lesurtel M, Melloul E, Limani P, Tschuor C, Graf R, Humar B, Clavien PA. ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg. 2014;260:839-846; discussion 846-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Linecker M, Kambakamba P, Reiner CS, Linh Nguyen-Kim TD, Stavrou GA, Jenner RM, Oldhafer KJ, Björnsson B, Schlegel A, Györi G. How much liver needs to be transected in ALPPS? A translational study investigating the concept of less invasiveness. Surgery. 2017;161:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Almau Trenard HM, Moulin LE, Padín JM, Stringa P, Gondolesi GE, Barros Schelotto P. Development of an experimental model of portal vein ligation associated with parenchymal transection (ALPPS) in rats. Cir Esp. 2014;92:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Zhou Y, Tao F, Chai S, Xu X, Yang Y, Yang Y, Xu H, Wang K. N-myc downstream regulated gene 1(NDRG1) promotes the stem-like properties of lung cancer cells through stabilized c-Myc. Cancer Lett. 2017;401:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 660] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 24. | Nishikawa T, Bell A, Brooks JM, Setoyama K, Melis M, Han B, Fukumitsu K, Handa K, Tian J, Kaestner KH. Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest. 2015;125:1533-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (2)] |
| 25. | Oldhafer KJ, Stavrou GA, van Gulik TM; Core Group. ALPPS--Where Do We Stand, Where Do We Go?: Eight Recommendations From the First International Expert Meeting. Ann Surg. 2016;263:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Cai X, Tong Y, Yu H, Liang X, Wang Y, Liang Y, Li Z, Peng S, Lau WY. The ALPPS in the Treatment of Hepatitis B-Related Hepatocellular Carcinoma With Cirrhosis: A Single-Center Study and Literature Review. Surg Innov. 2017;24:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-785; discussion 785-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 28. | Olthof PB, Coelen RJS, Bennink RJ, Heger M, Lam MF, Besselink MG, Busch OR, van Lienden KP, van Gulik TM. 99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford). 2017;19:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Serenari M, Collaud C, Alvarez FA, de Santibañes M, Giunta D, Pekolj J, Ardiles V, de Santibañes E. Interstage Assessment of Remnant Liver Function in ALPPS Using Hepatobiliary Scintigraphy: Prediction of Posthepatectomy Liver Failure and Introduction of the HIBA Index. Ann Surg. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Itoh T. Stem/progenitor cells in liver regeneration. Hepatology. 2016;64:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Li D, Li W, Hui L. Hybrid hepatocyte: A newly identified player for regeneration in hepatic injuries. Hepatology. 2016;64:2244-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, Nakagawa H, Valasek MA, Ye L. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 33. | Iacob R, Rüdrich U, Rothe M, Kirsch S, Maasoumy B, Narain N, Verfaillie CM, Sancho-Bru P, Iken M, Popescu I. Induction of a mature hepatocyte phenotype in adult liver derived progenitor cells by ectopic expression of transcription factors. Stem Cell Res. 2011;6:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 35. | Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 750] [Cited by in RCA: 1052] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 36. | Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 480] [Article Influence: 40.0] [Reference Citation Analysis (0)] |