Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1093
Peer-review started: December 12, 2017
First decision: December 27, 2017
Revised: December 31, 2017
Accepted: January 24, 2018
Article in press: January 24, 2018
Published online: March 14, 2018
Processing time: 91 Days and 19.2 Hours
To design colon-targeted codrugs of mycophenolic acid (MPA) and aminosugars as a safer option to mycophenolate mofetil (MMF) in the management of inflammatory bowel disease.
Codrugs were synthesized by coupling MPA with aminosugars (D-glucosamine and D-galactosamine) using EDCI coupling. The structures were confirmed by infrared radiation, nuclear magnetic resonance, mass spectroscopy and elemental analysis. The release profile of codrugs was extensively studied in aqueous buffers, upper gastrointestinal homogenates, faecal matter and caecal homogenates (in vitro) and rat blood (in vitro). Anti-colitic activity was assessed in 2,4,6-trinitrobezenesulfonic acid-induced colitis in Wistar rats by the estimation of various demarcating parameters. Statistical evaluation was performed by applying one-way and two-way ANOVA when compared with the disease control.
The prodrugs resisted activation in HCl buffer (pH 1.2) and stomach homogenates of rats with negligible hydrolysis in phosphate buffer (pH 7.4) and intestinal homogenates. Incubation with colon homogenates (in vitro) produced 76% to 89% release of MPA emphasizing colon-specific activation of codrugs and the release of MPA and aminosugars at the site of action. In the in vitro studies, the prodrug of MPA with D-glucosamine (MGLS) was selected which resulted in 68% release of MPA in blood. in vitro studies on MGLS revealed its colon-specific activation after a lag time of 8 h which could be ascribed to the hydrolytic action of N-acyl amidases found in the colon. The synthesized codrugs markedly diminished disease activity score and revived the disrupted architecture of the colon that was comparable to MMF but superior to MPA.
The significant attenuating effect of prodrugs and individual aminosugars on colonic inflammation proved that the rationale of the codrug approach is valid.
Core tip: Mycophenolic acid (MPA), an immunosuppressant and its morpholinoethyl ester prodrug: mycophenolate mofetil are under investigation for the treatment of inflammatory bowel disease (IBD). Diarrhea and local gut toxicity are their major setbacks. The present work focused on the synthesis of colon-targeted prodrugs wherein MPA was bio-reversibly linked with aminosugars to mask the carboxyl group of MPA responsible for gastrointestinal side effects. The synthesized prodrugs exhibited a significant mitigating effect on 2,4,6-trinitrobezenesulfonic acid-induced colitis in Wistar rats compared to MPA. The absence of pancreatitis, hepatitis and the gastro-sparing nature of the prodrugs emphasize their potential which could be investigated further for the management of IBD.
- Citation: Chopade SS, Dhaneshwar SS. Determination of the mitigating effect of colon-specific bioreversible codrugs of mycophenolic acid and aminosugars in an experimental colitis model in Wistar rats. World J Gastroenterol 2018; 24(10): 1093-1106
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1093.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1093
Mycophenolic acid (MPA) was synthesized as a fermentation product of Penicillium stoloniferum cultures by Gosio in 1896. Since its discovery, MPA has been the subject of intensive in vivo and in vitro screening for antifungal[1], antibacterial[2,3] and immunosuppressive activities[4-8]. Currently it is under investigation for the treatment of inflammatory bowel disease (IBD) and rheumatoid arthritis (RA). MPA is a potent, non-competitive, reversible inhibitor of eukaryotic inosine monophosphate dehydrogenase which is necessary for the growth of T cells and B cells. Due to its local gastric irritant side effect, it is being marketed in two forms i.e. mycophenolate mofetil (MMF) and mycophenolate sodium (enteric coated) to improve its gastrointestinal tolerance[7] and enhance bioavailability. MMF is an immunosuppressant of choice due to its multifaceted spectrum of activities and the apparent advantage of enhanced oral bioavailability. However, MMF-induced diarrhoea and local gut toxicity lead to poor patient compliance. It is under investigation for the treatment of IBD patients refractory or intolerant to steroids.
Since the isolation of MPA, it has been shown to possess a broad spectrum of activities due to which continuous attempts were made to improve its specificity by replacement of the lactone ring, modification of aromatic substituents, ester or amide derivatization, but these failed to show any benefits in most cases. Various derivatives of MPA, their synthesis and uses in the treatment of autoimmune diseases, psoriasis, inflammatory diseases including IBD, RA, tumors, virus and allograft rejection are described in many United States Patents[9-12]. Hydroxamic acid derivatives of MPA as histone deacetylases at the cellular level have been reported by Batovska et al[9]. 4 and 6-substituted derivatives of MPA[10], 5-hexanoic acid side chain derivatives of MPA[11] and a parenteral formulation of MPA, a salt or prodrug[12] thereof have been filed in the last two decades. Recently, Iwaszkiewicz-Grzes et al[13] in search of new immunosuppressants, synthesized amino acid derivatives of MPA as methyl esters using EDCI as the coupling reagent. With the exception of MMF, the morpholinoethyl ester prodrug of MPA, there are no reports of any prodrugs in the literature that are designed with the objective of enhancing its efficacy, reducing its effective dose or rendering a gastro-sparing effect.
The routine drug therapy of IBD involves the use of 5-aminosalicylic acid (5-ASA), sulfasalazine, corticosteroids (hydrocortisone, prednisolone, betamethasone, beclometasone and budesonide) and immunosuppressive agents (6-mercaptopurine, azathioprine, methotrexate and cyclosporine), whereas 4-ASA, broad-spectrum antibiotics, MMF, metronidazole, nicotine and thalidomide are other choices available for the treatment of disease or its secondary effects. Short chain fatty acids such as butyric acid are presently under investigation as treatment options for the safer management of IBD. Alternative treatments for IBD involve the use of colestyramine, sodium cromoglycate, bismuth and arsenical salts, methotrexate, lidocaine (lignocaine), sucralfate, new steroid entities, cytoprotective agents, fish oils and human growth hormone. During the last decade, a huge number of biological agents against tumor necrosis factor-α, as well as many biochemical substances, have been developed for the effective treatment of patients with IBD. Among the new biologic agents, natalizumab is currently in regular use in the United States. Several nutritional deficiencies have been indicated in IBD and there are reports of improvement in the symptoms by taking daily supplements of nutraceuticals like vitamin A and carotenoids, vitamin E, vitamin C, vitamin K, folic acid, calcium, iron, selenium, zinc, magnesium and copper. Patients with active ulcerative colitis (UC) have a reduction in the amounts of obligate anaerobes, facultative organisms and micro-aerobes, when compared to UC patients in remission. Supplementation with probiotics is considered an effective option in UC. Leukocytapheresis has shown satisfactory results in steroid-refractory patients with UC. Unfractionated heparin and low molecular weight heparins (LMWH) have been used in patients with active UC with encouraging results. Microbes and microbial products including eggs of helminths seem to reduce disease activity in patients with UC or Crohn’s disease[14].
5-ASA and its colon-targeted prodrug sulphasalazine, are associated with various serious side effects including ulcerogenicity, hypersensitivity, skin rash, blood disorders, pancreatitis and hepatitis. Corticosteroids and immunosuppressants are also known to produce many serious side effects so there is an urgent need for steroid-sparing, non-nephrotoxic, safe anti-inflammatory agents to treat inflammatory diseases such as IBD, RA, psoriasis and organ transplantations[14-18].
The present work was focused on exploring colon-targeting prodrugs of MPA for their possible application in the management of IBD. For the study, aminosugars such as D-galactosamine and D-glucosamine were selected as promoieties. An extensive literature review revealed that aminosugars play a vital role in resisting chemical attack and improving the tenacity of colon mucus. Moreover, glucosamine is involved in the biosynthesis of glucosaminoglycans and intestinal mucus. Colonic mucus is distinguished from mucus in the proximal intestine not only by its greater sialylation but also by its marked degree of sulphation[19]. The mucin molecule bristles with oligosaccharide side chains, the initial sugar of which is always N-acetyl galactosamine O-linked onto serine or threonine in the protein core. Onto this initial galactosamine may be attached up to 10 or more sugar moieties which can include N-acetyl glucosamine, galactose, N-acetyl galactosamine, fucose or the acid sugar sialic acid (usually as N-acetyl neuraminic acid). Alterations in sialylation and sulphation of mucins and O-acetylation of the mucin sialic acids could all have important effects on the resistance of mucus to bacterial enzymatic attack leading to colorectal cancer and IBD. Clamp and colleagues demonstrated that in UC and Crohn’s disease, the oligosaccharide side chain of mucin is greatly shortened[20]. Therefore, we thought of linking glucosamine and galactosamine with MPA which when released in the colon would help to retain the architecture of mucin and form a protective layer on the colon mucosa. In addition to this, the polyhydroxy nature of these aminosugars would increase the hydrophilicity of MPA to such an extent that absorption of the intact prodrug in the upper gastrointestinal tract (GIT) would be minimized assuring efficient delivery to the site of action.
In the present study, we synthesized colon-specific mutual prodrugs involving the formation of a covalent amide linkage between MPA and aminosugars (glucosamine and galactosamine) in such a manner that upon oral administration the linkage remains intact in the upper GIT, but releases MPA in the colon through enzymatic activation[14,21]. Site-specific drug delivery through site-specific prodrug activation may be accomplished by the utilization of specific enzymes secreted by the colonic microflora such as N-acyl amidases. The present work was aimed at the rational design, pharmacokinetic and pharmacodynamic comparison of colon-targeting, mutual prodrugs of MPA with the marketed prodrug MMF.
Mycophenolate sodium was generously gifted by Emcure Pharmaceuticals Pvt. Ltd., Pune, India. D-(+)-glucosamine hydrochloride and D-(+)-galactosamine hydrochloride were purchased from Sigma-Aldrich, St. Louis, United States and Himedia Laboratories Pvt. Ltd., Nashik, India, respectively. All other chemicals used in the synthesis were purchased from Merck Speciality Pvt. Ltd., Mumbai, India. The reactions were monitored on TLC, which was performed on pre-coated silica gel plates-60 F264 (Merck). Nuclear magnetic resonance (NMR) spectra were recorded on a NMR Varian Mercury 400 MHz at SAIF, Panjab University, Chandigarh, India. The IR spectra were recorded on a Jasco, V-530 FTIR in potassium bromide (anhydrous IR grade). A Jasco V530, UV-Visible double-beam spectrophotometer was used for determination of aqueous solubility and partition coefficient. The partition coefficient was determined in n-octanol/distilled water, whereas aqueous solubility was determined in distilled water at room temperature (25 ± 1 °C). In vitro and in vivo studies were carried out using HPLC. Before analysis, the mobile phase was filtered through a 0.45 μm membrane filter and degassed using a sonicator. Sample solutions were also filtered through the same system. The HPLC system consisted of a pump (Jasco PU-1580, Serial No. B179460677), with an autosampler injector (Jasco AS-1555) and a UV/VIS detector (Jasco UV 1575, Serial No. A083360681). Data were incorporated using Jasco Borwin version 1.5. The Waters Xterra RP 18 (4.6 mm I.D. × 150 mmL; Part No. 186000442) column along with a Hypersil guard column were used in the reversed phase partition chromatographic condition. The system was used in an air-conditioned HPLC laboratory atmosphere (20 ± 1 °C). The column was monitored for UV absorbance at a detection wavelength of 225 nm for estimation of both prodrugs. All kinetic studies were carried out in triplicate. The K values from the plots were calculated separately and the average K and SD values were determined. Histopathological studies of the colon, liver and pancreas were carried out at PRADO Pathology Laboratory, Pune. The pathologist was unaware of the experimental protocols. The histopathological sections were stained with haematoxylin and eosin. Colour microphotographs of the sections were captured on a Nikon optical microscope, Eclipse E-200, with resolution 10 × 40 ×, and a trinocular camera at Poona College of Pharmacy, Pune.
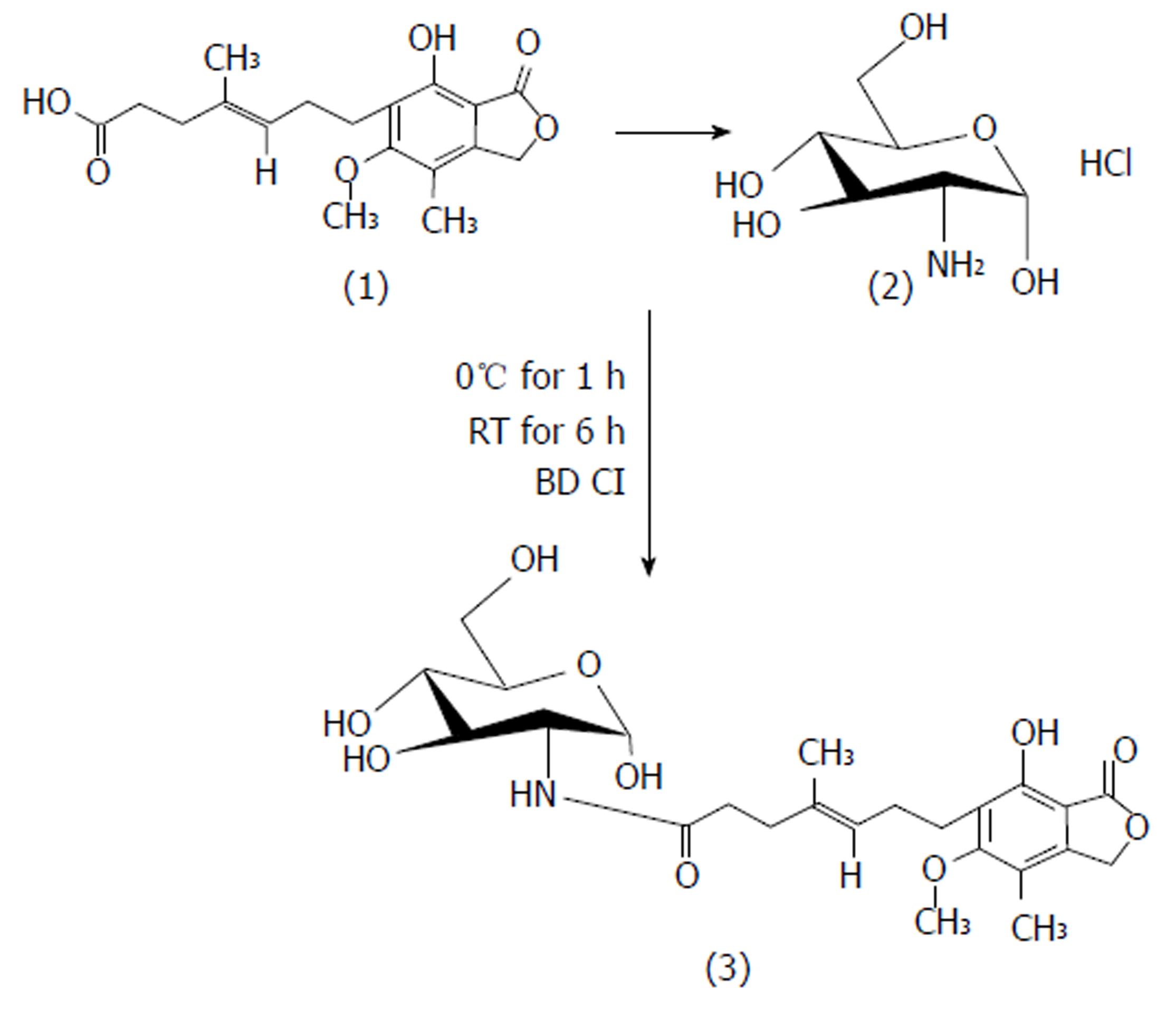
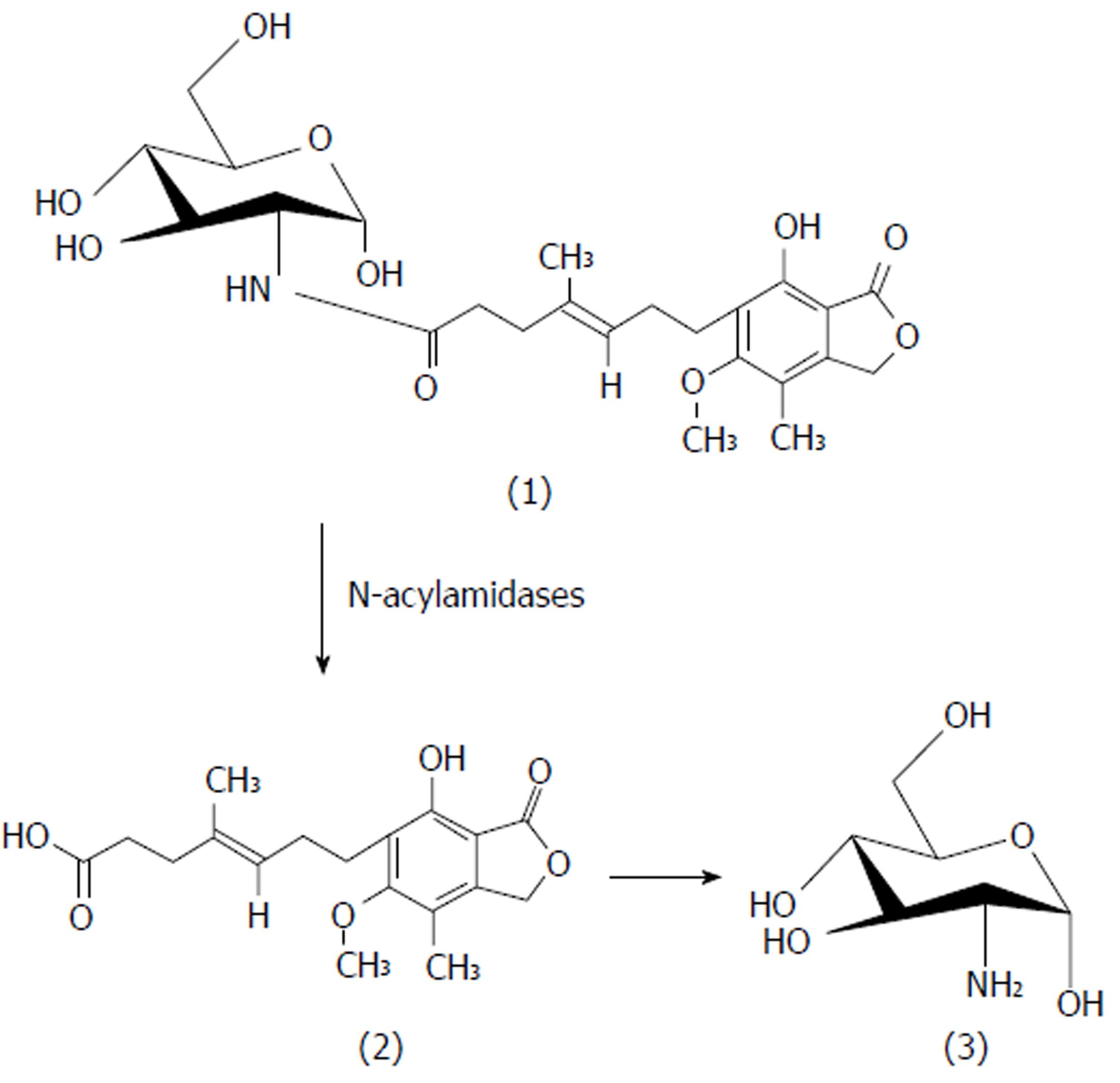
MPA (1 mol) in DMF was reacted with EDCI (2 mol) at 0 °C. The reaction was continued for 1 h until the MPA-EDCI activated complex was formed. Then in a separate beaker, the aminosugar (1.2 mol/L) in methanol was mixed with 2 drops of triethylamine until basic to litmus and the resulting solution was added to the MPA-EDCI activated complex and stirred at room temperature for 6 h. After completion of the reaction, the solution was poured into 15 mL of cold water and extracted with ethyl acetate. The organic layer was separated and dried with sodium sulphate, filtered and concentrated. The crude product was purified by preparative chromatography using chloroform:methanol:glacial acetic acid (90 V:9 V:1 V). The structures of the synthesized prodrugs (MPA with D-glucosamine: MGLS and MPA with D-galactosamine: MGAS) were established by spectroscopic methods. The scheme of synthesis[13] is presented in Figure 1.
In vitro release profiling of the synthesized prodrugs was carried out in aqueous buffers (0.05 mol/L HCl buffer pH 1.2 and 0.05 mol/L phosphate buffer pH 7.4), faecal matter and tissue homogenates of the upper GIT and colon of male Wistar rats by a validated HPLC method. The Rt values for MPA, MGAS and MGLS were 5.9, 9.7 and 11.6, respectively. Concentrations of the parent drug and prodrugs were calculated by equations generated from their respective calibration curves. All the kinetic studies were carried out in triplicate and their standard deviation was calculated.
Prodrug (50 mg) was introduced into 100 mL of HCl buffer in a flask which was kept in a constant temperature bath at 37 ± 1 °C under continuous stirring and 2 mL aliquots were withdrawn at different time intervals (0-180 min), each time reloading with an equal volume of fresh buffer. The aliquots were filtered through a membrane filter (0.45 μm) and directly estimated by HPLC at 225 nm using phosphate buffer (pH 4.5):acetonitrile (40 V:60 V) as mobile phase. The same procedure as described above was followed to study the release kinetics of prodrugs in 0.05 mol/L phosphate buffer pH 7.4.
Wistar rats (250 g) were anesthetized by ether, sacrificed and then midline incisions were made. Sections of stomach, small intestine and colon were collected separately, homogenized using a Remi overhead homogenizer and diluted to half concentration with isotonic HCl buffer (pH 1.2) for stomach homogenates and with isotonic phosphate buffer (pH 7.4) for small intestinal homogenates and colon homogenates. To each Eppendorf tube, 0.8 mL of prodrug solution (1 mg/mL) and 0.2 mL of homogenate solution were added and incubated at 37 ± 1 °C. Subsequently, at 15 min intervals for the first 1 h and then hourly for the next 3 h (stomach homogenates), 7 h (small intestinal homogenates) and 10 h (colon homogenates), the Eppendorf tubes were withdrawn and mixed in a vortex mixer, centrifuged at 10000 rpm for 10 min and the supernatants were filtered through a membrane filter (0.45 μm). The filtrate (20 μL) was injected into the HPLC system and the amount of MPA released estimated by the UV detector using the same mobile phase as above at 225 nm.
To a 0.1 mL suspension of fresh rat faecal matter and caecal homogenate (homogenised and diluted with phosphate buffer pH 7.4), prodrug solution (0.9 mL) was added and the tubes were incubated at 37 ± 1 °C. The samples were removed from the incubator at predetermined time intervals over a period of 13 h and the same procedure was followed for analysis as mentioned in the above section.
Of the two prodrugs, MGLS was selected for in vivo study. All the experimental procedures and protocols used for the in vivo release studies were reviewed and approved by the Institutional Animal Ethical Committee (IAEC) of Poona College of Pharmacy, Pune (CPCSEA/PCH/15/2014-15) and were in accordance with the guidelines of the Committee for the purpose of Control and Supervision of Experiment on Animals (CPCSEA), Government of India.
Male Wistar rats fasted for 24 h (average weight 200-230 g; 12-15 wk; n = 6/group) were provided with only water ad libitum. The retro-orbital bleeding technique was used to withdraw blood from the rats (1 mL) and this was considered the zero hour reading. Then an oral dose of MGLS prodrug (28.53 mg/kg) in 1.0 mL of physiological saline was administered and blood samples were collected in EDTA tubes at 15 min intervals for 1 h and then on an hourly basis until the 11th hour and finally at the 24th hour. The EDTA tubes were centrifuged at 5000 rpm at 0-5 °C for 10 min. The supernatant solution of centrifuged blood was added to the Eppendorf tube and 0.9 mL of methanol was added for immediate plasma precipitation. All the solutions were then vortexed for 2 min, centrifuged at 10000 rpm for 5 min at 0-5 °C, filtered through a membrane filter and analysed by HPLC.
Faeces and urine were collected from metabolic cages, pooled together as 24 h samples, diluted 100- and 10-fold, respectively, with isotonic phosphate buffer solution pH (7.4) and then the same procedure was followed as described above.
Induction of colitis: To induce inflammation in rat colon, all the groups except the healthy control group were catheterized using a rubber cannula (8 cm intra-rectal) and 0.25 mL of 2,4,6-trinitrobezenesulfonic acid (TNBS) (100 mg/kg of body weight in 50% v/v ethanol solution) was administered after light narcotizing with ether. The animals were then maintained in a vertical position for 30 s and returned to their cages. The rats were housed without treatment for 3 d to maintain the development of a full inflammatory bowel disease model. The animals received standard and test compounds orally, once daily for five continuous days. The doses of prodrugs were calculated on an equimolar basis to MPA and are shown in Table 1. The healthy control and colitis control groups were given only saline instead of free drug or prodrug[22].
| Groups | Dose1 (mg/kg) |
| HC | 0.9% saline (1 mL) |
| MPA | 18.5 |
| MMF | 25.0 |
| MGLS | 28.5 |
| MGAS | 28.5 |
| D- Glucosamine | 12.4 |
| D- Galactosamine | 12.4 |
| MPA + GL | 18.5 + 12.4 |
| MPA + GA | 18.5 + 12.4 |
During the course of 11 d, three parameters were observed; weight loss, stool consistency and rectal bleeding Table 2. Anti-colitic activity was estimated by assessment of the disease activity score rate[23] (Table 3). As previously applied by Krawisz et al[24], the disease activity score was determined by calculating the average of the above three parameters for each group on each day in the range of 0 (healthy) to 4 (maximal activity of colitis). On the 11th day the animals were sacrificed by isoflurane anaesthesia and their colon/body weight ratio was calculated using dissected sections of colon. Anti-colitic activity of the prodrugs was compared with MPA and MMF as standards. Rat stomach, colon, liver and pancreas were dissected. Gastric ulcers were scanned and the ulcer index was calculated by scoring the ulcers as per the method reported by Cioli et al[25] (1979). Specimens of colon, liver and pancreas were fixed in formalin for histopathological analysis.
| Prodrug | Incubation media | |||||||
| Aqueous buffers pH 1.2 and pH 7.4 | Stomach and intestinal homogenates | Colon homogenate | Faecal matter | |||||
| K ± SD (min-1)1 | t1/2 (min) | % Prodrug hydrolysed | % MPA released | % Prodrug hydrolysed | % MPA released | |||
| MGLS | Stable | 1.30% | 0.0025 ± 0.000978 | 275 | 92.6 | 86.7 | 63 | 54 |
| MGAS | Stable | 14-19% | 0.000108 ± 0.00001 | 305 | 79.15 | 76.4 | 47 | 52 |
| Groups | Days | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| HC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DC | 0 | 0.67 ± 1.15 | 0.87 ± 1.50 | 2.53 ± 0.50 | 2.63 ± 0.65 | 2.63 ± 0.65 | 2.97 ± 0.85 | 3.12 ± 1.80 | 3.22 ± 1.70 | 3.23 ± 2.00 | 3.30 ± 2.0 |
| MMF | 0 | 0.33 ± 0.58 | 0.67 ± 1.15 | 1.07 ± 1.29 | 1.53 ± 0.50 | 2.53 ± 0.0 | 2.10 ± 0.85 | 1.07 ± 0.51 | 0.73 ± 0.81 | 0.23 ± 0.68 | 0c |
| MPA | 0 | 0.43 ± 0.75 | 0.67 ± 1.15 | 2.50 ± 0.17 | 2.63 ± 0.35 | 2.63 ± 0.35 | 2.33 ± 0.58 | 1.97 ± 0.65 | 1.63 ± 0.91 | 0.97 ± 0.85 | 0.77 ± 0.68c |
| MGLS | 0 | 0.33 ± 0.58 | 0.33 ± 0.58 | 1.77 ± 0.40 | 1.97 ± 0.65 | 2.53 ± 1.08 | 1.73 ± 1.03 | 1.06 ± 0.50 | 0.63 ± 0.65 | 0.33 ± 0.17 | 0c |
| MGAS | 0 | 0.33 ± 0.58 | 0.43 ± 0.75 | 1.53 ± 0.81 | 2.10 ± 0.17 | 2.33 ± 0.58 | 1.73 ± 1.03 | 0.93 ± 0.58 | 0.73 ± 0.81 | 0.50 ± 0.35 | 0.30 ± 0.1c |
| GL | 0 | 0.33 ± 0.58 | 0.67 ± 1.15 | 2.1 ± 0.85 | 2.3 ± 0.89 | 2.53 ± 0.50 | 2.1 ± 0.85 | 1.73 ± 0.75 | 1.40 ± 0.17 | 1.20 ± 0.40 | 1.02 ± 0.2b |
| GA | 0 | 0.43 ± 0.75 | 0.67 ± 1.15 | 2.17 ± 0.75 | 2.53 ± 0.50 | 2.53 ± 0.52 | 2.33 ± 0.58 | 2.10 ± 0.85 | 1.50 ± 1.01 | 1.39 ± 1.00 | 1.30 ± 0.4b |
| GL + MPA | 0 | 0.33 ± 0.58 | 0.33 ± 0.58 | 1.83 ± 0.68 | 2.17 ± 0.75 | 2.17 ± 0.75 | 1.87 ± 0.51 | 1.30 ± 0.70 | 0.93 ± 0.58 | 0.53 ± 0.50 | 0.40 ± 0.35c |
| GA + MPA | 0 | 0.33 ± 0.58 | 0.667 ± 1.15 | 1.97 ± 0.65 | 1.97 ± 0.654 | 2.3 ± 0.89 | 1.77 ± 0.51 | 1.17 ± 0.51 | 0.73 ± 0.23 | 0.53 ± 0.50 | 0.40 ± 0.35c |
All data were expressed as mean ± SEM; (n refers to the number of animals in each group). For the disease activity score rate, statistical differences between groups were calculated by two-way ANOVA followed by Bonferroni’s test, whereas statistical analysis of ulcerogenic activity was carried out using one-way ANOVA followed by the Dunnett’s post- hoc test. Differences were considered significant at P < 0.001-0.05.
The aqueous solubility was determined experimentally and was found to be 0.42 mg/mL and 0.21 mg/mL for MGLS and MGAS, respectively. Log P in n-octanol/ distilled water was found to be 2.1 and 2.3, respectively.
Structures of the synthesized prodrugs were confirmed by IR, NMR, mass spectroscopy and elemental analysis.
MGLS: (E)-N-((2S, 3R, 4R, 5S)-tetrahydro-2,4,5-trihydroxy-6-(hydroxymethyl)-2H-pyran-3-yl)-7-1,3-dihydro-7-hydroxy-5-methoxy-4-methyl-1-oxoisobenzofuran-6-yl)-4-methylhept-4-enamide, M.P: 78 °C (uncorrected), Rf 0.42; chloroform: methanol: glacial acetic acid (90:9:1; v/v/v), Aq. sol.: 0.42 mg/mL, Log Poct: 2.1, FTIR (anhydrous KBr; cm-1): 3530 (OH stretch), 3284 (NH stretch sec. amide), 3048 (C=C stretch aromatic ring), 1765 (C=O stretch ester), 1698 (C=O stretch sec. amide), 1536 (C=C bend aromatic ring), 1133 (C-O stretch ester), 1H NMR (CDCl3 ; 400MHz) MPA backbone : δ 1.71 [s,3H] C-CH3 , 1.80-1.89 [t, 2H] C-CH2, 2.33-2.38 [q, 2H]-CH2, 2.50 [s, 3H] -CH3 Ar-H, 2.59-2.63 [m, 4H] O=C-CH2 and Ar-CH2, 3.71 [t, 3H] -OCH3, 3.77-3.78 [d, H] = CH, 5.20 [s, 2H] lactone ring, 5.26 [s, H] -OH glucosamine backbone: 2.1 [s, 2H] -OH tetrahydropyran, 2.17-2.22 [t, 2H] -OH group tetrahydropyran, 3.06-3.36 [m, 5H]-CH, 3.79-3.82 [d, H]-CH2-OH, 9.2 [s, H] -NH sec. amide, 13C NMR (400 MHz, CDCl3): δ 11.00, 11.40, 14.42, 16.06, 22.34, 25.74, 34.09, 60.41, 68.65, 78.31, 78.64, 78.84, 78.97, 106.63, 115.71, 122.16, 122.82, 123.14, 133.29, 145.03, 162.60, 170.53, Mass: m/z M+1: 496. 99, (molecular weight: 495.52), Elemental analysis: calculated for C24H33NO10; C, 58.17; H, 6.71; N, 2.83. Found: C, 58.24; H, 6.72; N, 2.82.
MGAS: (E)-N-((2S, 3R, 4R, 5R)-tetrahydro-2,4,5-trihydroxy-6-(hydroxymethyl)-2H-pyran-3-yl)-7-(1,3-dihydro-7-hydroxy-5-methoxy-4-methyl-1-oxoisobenzofuran-6-yl)-4-methylhept-4-enamide. M.P: 80 °C (uncorrected), Rf 0.41; chloroform: methanol: glacial acetic acid (90:9:1; v/v/v), Aq. sol.: 0.21 mg/mL, Log Poct: 2.3, FTIR (anhydrous KBr ; cm-1): 3652 (OH stretch) , 3284 (NH stretch sec. amide group), 2997 (C=C stretch aromatic), 1765 (C=O stretch ester), 1698 (C=O stretch sec. amide), 1536 (C=C bend aromatic), 1132 (C-O stretch ester), 1H NMR (CDCl3; 400MHz) MPA backbone : δ 1.88 [s, 3H] =C-CH3, 2.09 [s, 3H] Ar-CH3 , 2.17-2.20 [m, 4H]-CH2-CH2-, 2.29-2.36 [q, 2H]=C-CH2, 2.59-2.70 [t, 2H]Ar-CH2, 3.1 6[s, 3H] -OCH3 , 5.13 [t, 1H] =C-H, 5.14 [s, 2H] lactone ring, 5.29 [s, 1H] OH group, galactosamine backbone: 1.79 [s, 1H] -OH tetrahydropyran, 2.50-2.52 [s, 3H] -OH tetrahydropyran , 3.20-3.38 [m, 7H] C-H tetrahydropyran ring, 9.1 [s, 1H] -NH sec. amide, 13C NMR (400 MHz, CDCl3, δ ppm): 11.01, 14.46, 15.82, 22.35, 32.15, 34.09, 34.80, 51.02, 60.49, 60.93, 68.59, 78.53, 78.86, 79.19, 106.81, 115.83, 122.21, 123.05, 133.06, 145.46, 152.73, 162.54, 170.24, 172.75, Mass: m/z M+2 497. 09, (molecular weight: 495.52), Elemental analysis: calculated for C24H33NO10; C, 58.17; H, 6.71; N, 2.83. Found: C, 58.21; H, 6.72; N, 2.82.
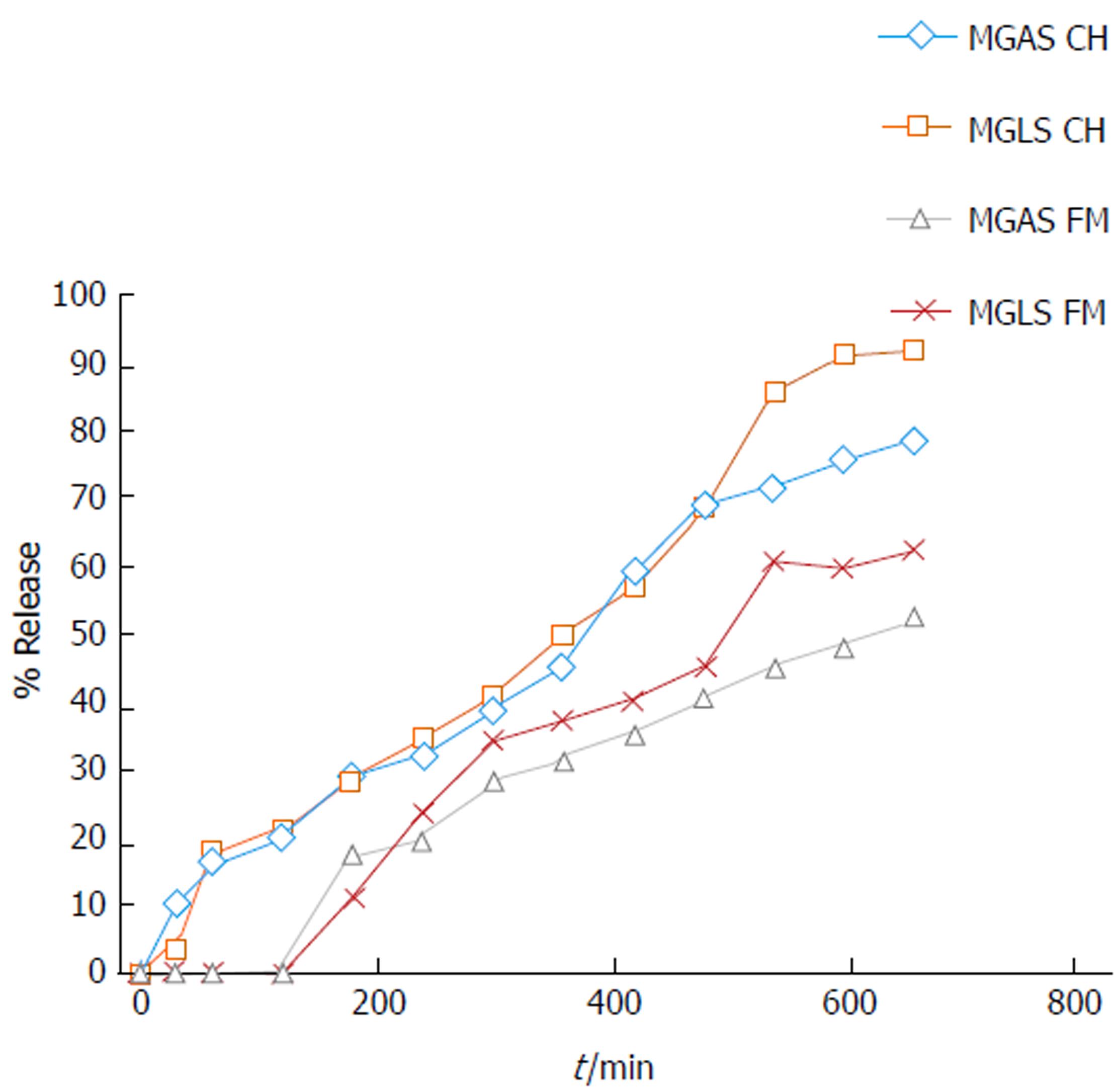
In vitro release kinetics are an important tool in understanding the behaviour of prodrugs with respect to their stability and ability to release drugs under in vitro conditions that simulate those in the body with respect to pH and enzymes. For the in vitro study, prodrugs were incubated at 37 °C in aqueous buffers at pH 1.2 and 7.4 representing the pH of stomach and small intestine, respectively. The prodrugs were also incubated with homogenates of stomach and small intestine at 37 °C and the release of MPA was studied. Approximately 1%-3% and 14%-19% release of MPA was observed in homogenates of stomach and small intestine, respectively, suggesting negligible hydrolysis by peptidases in the upper GIT. MGLS and MGAS showed 63% and 52% release of MPA in rat faecal matter, respectively, and 87% and 76% release in colon homogenate, respectively (Figure 2). The half-lives of MGLS and MGAS were found to be 275 min and 305 min in rat colon homogenates and 523 min and 641 min in rat faecal matter following first order kinetics (Table 2).
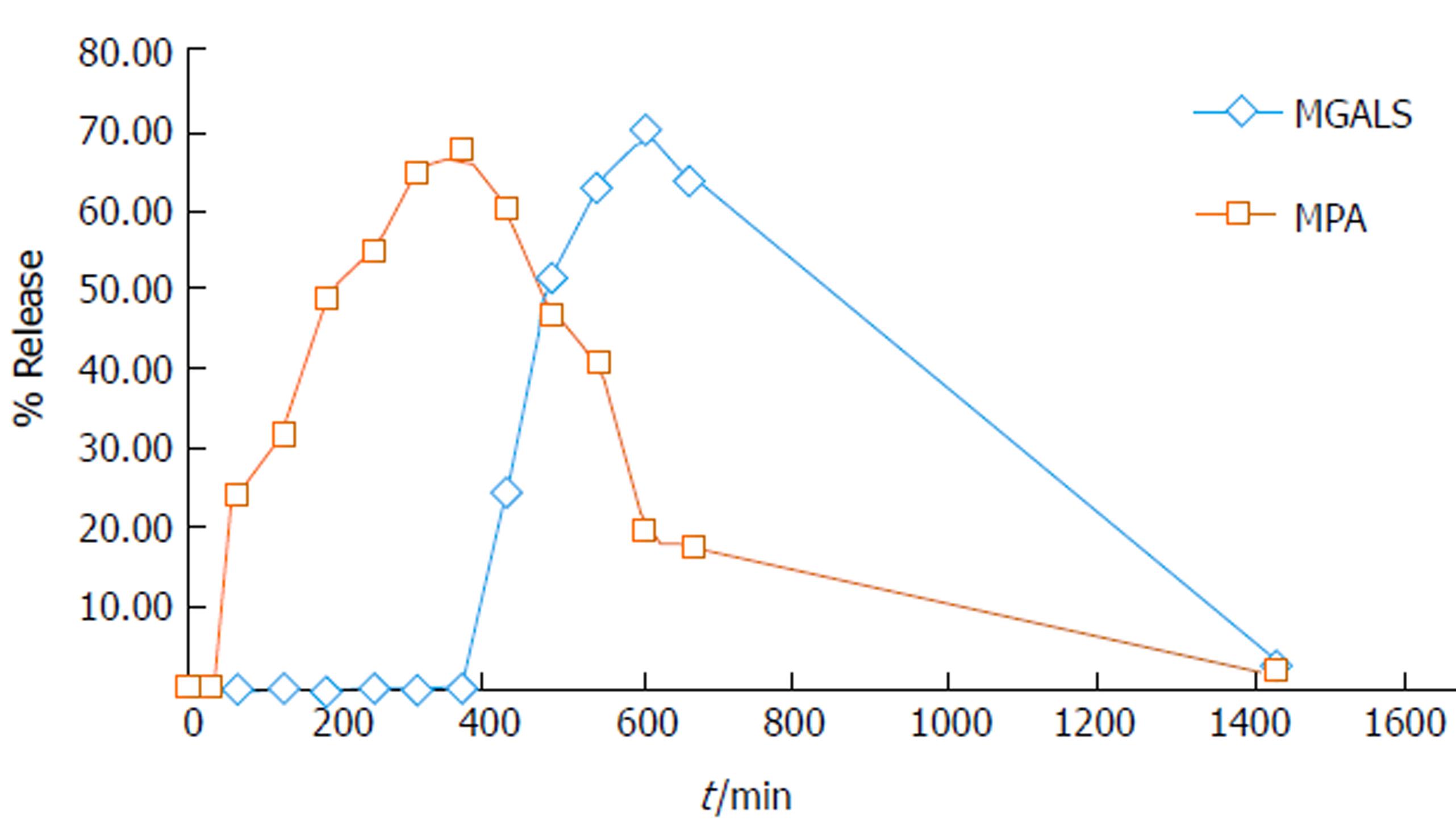
MGLS was selected as a representative of the two synthesized prodrugs to study in vivo behaviour which was compared with standard MPA. The study was carried out by oral administration of 28.53 mg/kg MGLS and 18.5 mg/kg plain MPA in male Wistar rats housed in metabolic cages. The blood samples were withdrawn by retro-orbital puncture at pre-set time intervals. Urine/faeces samples were collected at various time intervals and pooled together over a period of 24 h. The release pattern of the prodrug was determined by injecting these biological samples into the HPLC system using the newly developed and validated method. After oral administration, MPA appeared in the blood after 1 h, reached a maximum at 7 h (68%) and then gradually declined with disappearance at 24 h. After administration of MGLS, neither MPA nor intact prodrug was observed until 6 h. At 7 h, MGLS was observed in the blood. MGLS showed 70% concentration at 10 h. MPA was observed in the blood at 10 h and reached 68% at 13 h. The concentration of MPA and MGLS started to decline in the blood and was negligible at 24 h (Figure 3).
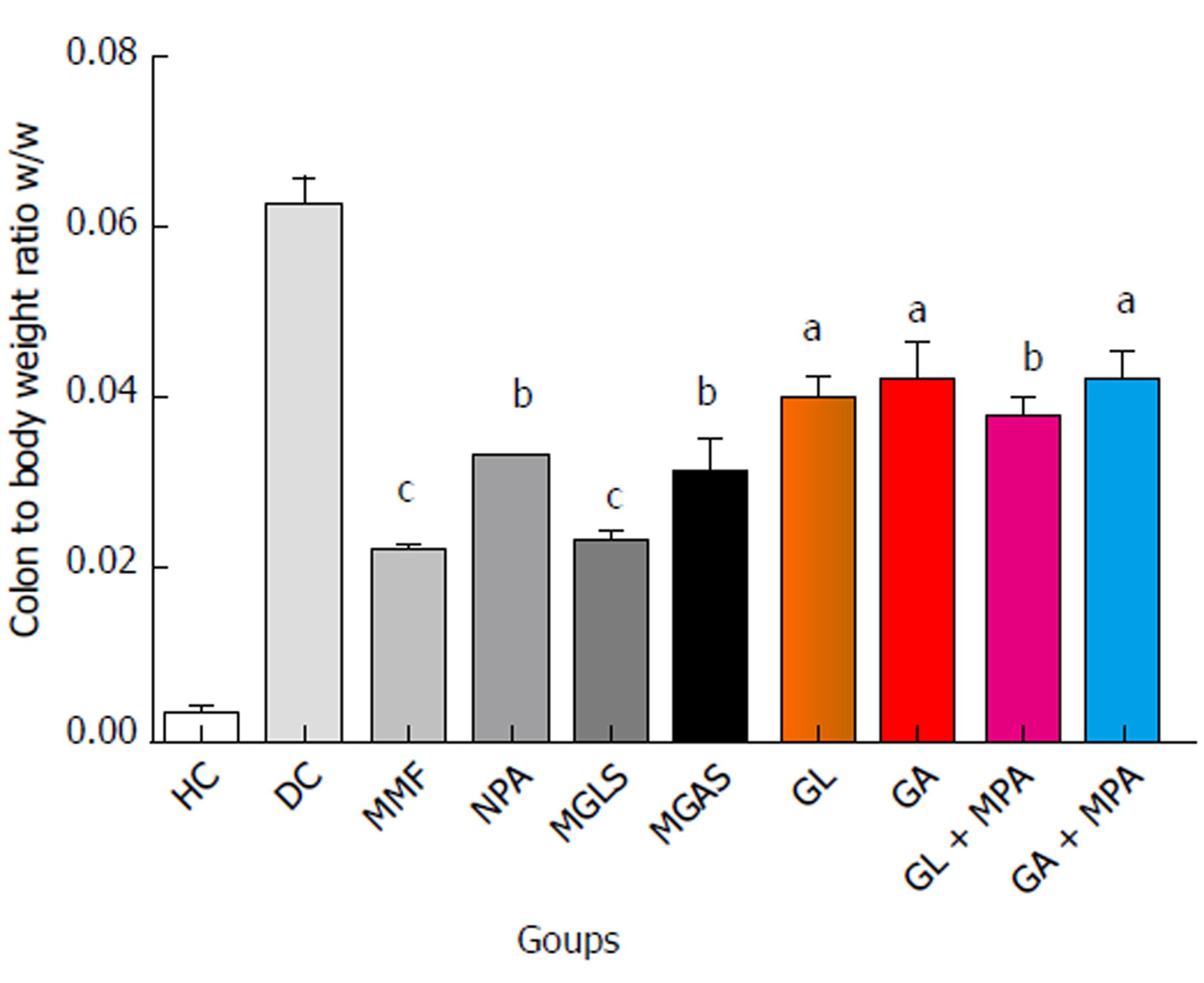
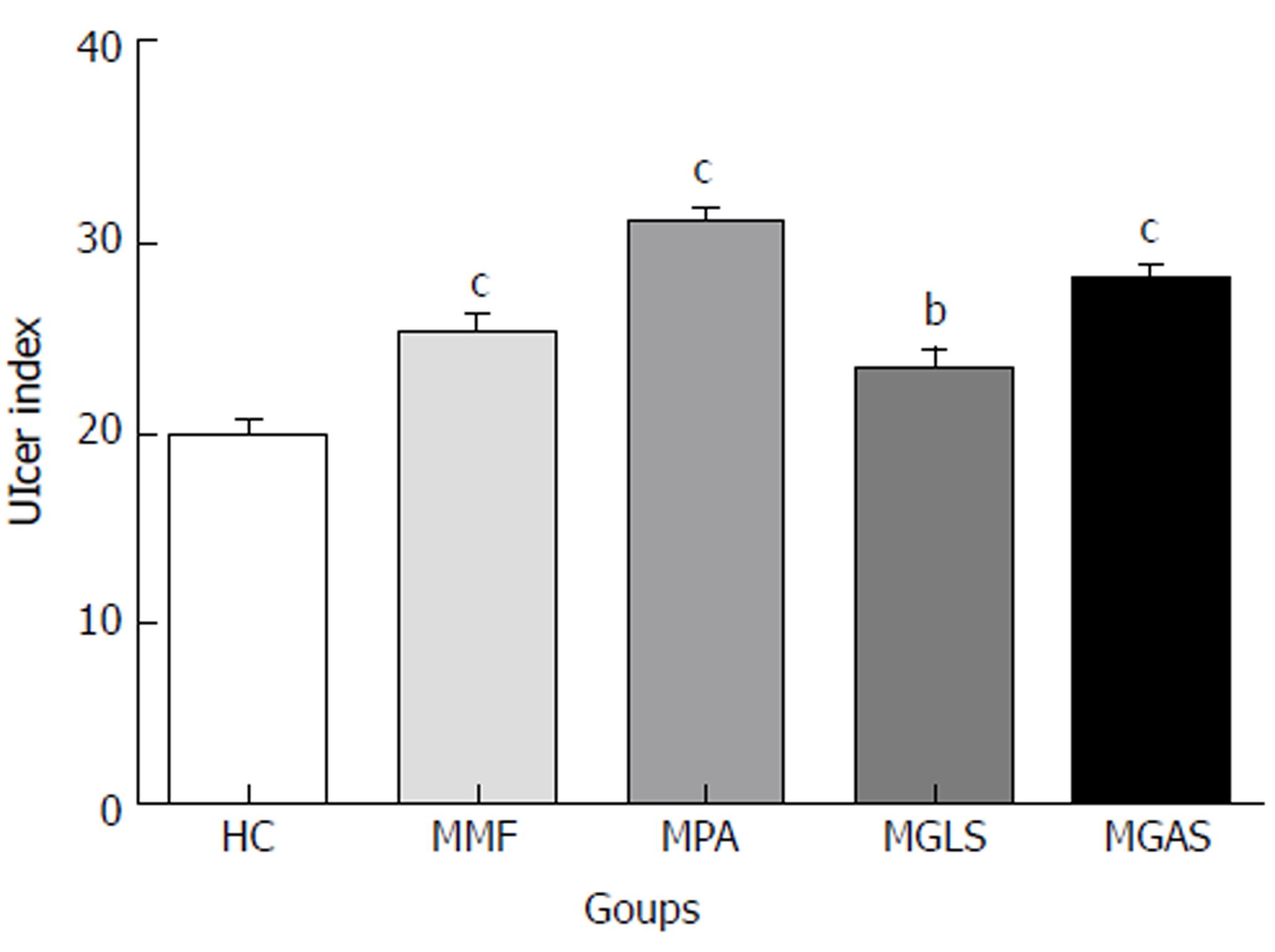
The TNBS model efficiently imitates both acute and chronic colitis resembling human UC[26]. All animals in each group were examined for stool consistency, rectal bleeding and weight loss for 11 d to determine the disease activity score(Figure 4). On the 11th day, all animals were sacrificed and the colon/body weight ratio was calculated to quantify inflammation (Figure 5). The mitigating effect of synthesized prodrugs as well as standards was evaluated for disease activity score rate and colon/body weight ratio in the TNBS-induced experimental colitis model in Wistar rats[24,27]. The results for disease activity score, colon/body weight ratio and ulcerogenic activity are shown in Table 3, Figures 5 and 6, respectively, while photomicrographs of rat colon, pancreas, and liver are shown in Figures 7-9, respectively.
Two mutual latentiated amide derivatives (MGLS and MGAS) of MPA were synthesized with aminosugars D-glucosamine and D-galactosamine, respectively. As anticipated, the outcomes of physico-chemical characterisation revealed that the aqueous solubility of MPA (practically insoluble in water) was significantly improved with a corresponding decrease in the partition coefficient (2.8) in prodrugs. This feature would minimize trans-cellular absorption of prodrugs, thus facilitating their passage directly to the colon. Increased aqueous solubility was attributed to the polyhydroxy nature of the aminosugars.
The IR spectra showed characteristic 2 amide stretches at 3284 and 3283 and C=O stretches of amide at 1698 and 1700 cm-1. 1H NMR of both prodrugs showed characteristic chemical shifts for protons of amide between δ 5 to 8. 13C NMR showed all the relevant chemical shifts as per number of anticipated carbon atoms present in the prodrugs. Disappearance of C=O stretch and OH stretch of carboxylic acid confirmed the formation of an amide bond between MPA and the aminosugars.
The outcomes of 1H NMR and 13C NMR spectral studies supported the formation of MGLS and MGAS prodrugs. The results of elemental analysis and mass spectroscopy revealed that the calculated molecular weights of the prodrugs were in accordance with their predicted molecular weights.
The aim of the present work was to achieve colon-targeted drug delivery of MPA through its site-specific activation in the colon by resident microflora. The in vitro studies showed that the prodrugs were stable in aqueous buffers at pH 1.2 and 7.4 over a period of 3 h and 7 h, respectively, confirming that the prodrugs were not released in the upper GIT. Further studies revealed that both prodrugs exhibited faster activation in colon homogenates as compared to faecal content which was attributed to greater microbial populations in the colon than in faeces. The release of MPA from prodrugs in rat colon homogenates and faecal matter confirmed their colon-specific activation.
The in vivo study indicated quick absorption of MPA in the upper GIT. In contrast, when MGLS was administered, neither MPA nor intact prodrug was observed until 6 h indicating that prodrug was not absorbed from the stomach and remained intact there. MGLS was observed in the blood at 7 h indicating minimal absorption through the small intestine. The concentration of MGLS consistently increased in the blood reaching a maximum of 70% at 10 h indicating its absorption from the large intestine (colonic mucosa) into the systemic circulation. MPA was observed in the blood at 10 h indicating hydrolysis of MGLS to MPA in the large intestine which was due to its high lipophilicity and it may have transversed the colonic mucosa into the systemic circulation. MPA concentration reached 68% at 13 h. The concentration of MPA and MGLS started to decline in the blood and was negligible at 24 h (Figure 3). The in vivo release profile clearly indicated that activation of the prodrug was pH-dependent (pH 7.4) and by N-acyl amidase present in the colon (Figure 4). The 24 h pooled faeces and urine samples also showed peaks of prodrug and MPA, which confirmed that the prodrug passed through the entire length of the GIT and hence appeared in the faeces; thus, the objective of targeting MPA to the colon was achieved. Moreover, the systemic availability of MPA at high concentration at 13 h revealed that MGLS can be used effectively for the treatment of inflammatory diseases associated with the circadian rhythm such as rheumatoid arthritis.
An approximately 11 d TNBS-induced colitis model was used for the pharmacological evaluation. The anti-colitic activity of prodrugs was compared with MMF and MPA. Up to the 5th day the disease activity score increased rapidly and consistently for all TNBS-treated groups (Table 3). From day 6 to day 10, standard MMF and MPA, prodrugs, carriers and physical mixtures were administered orally to the animals. Full-blown colonic inflammation was demonstrated by the high disease activity score (3.30 ± 2.0) in the colitis control group. Prodrug-treated groups showed a marked decrease in disease activity score rate (99%-100%) that was comparable with the MMF-treated groups. The results of the animal study showed that the disease activity-lowering effect of both prodrugs was significantly superior to MPA (77%), individual carriers (61%-69%) and physical mixtures (88%). Overall, the maximum decrease in colon/body weight ratio was observed in the prodrug-treated groups indicating their significant protective effect on colon inflammation, which was significantly better than MMF and MPA. The lowering effect of carriers and physical mixtures was also better than that of both standards.
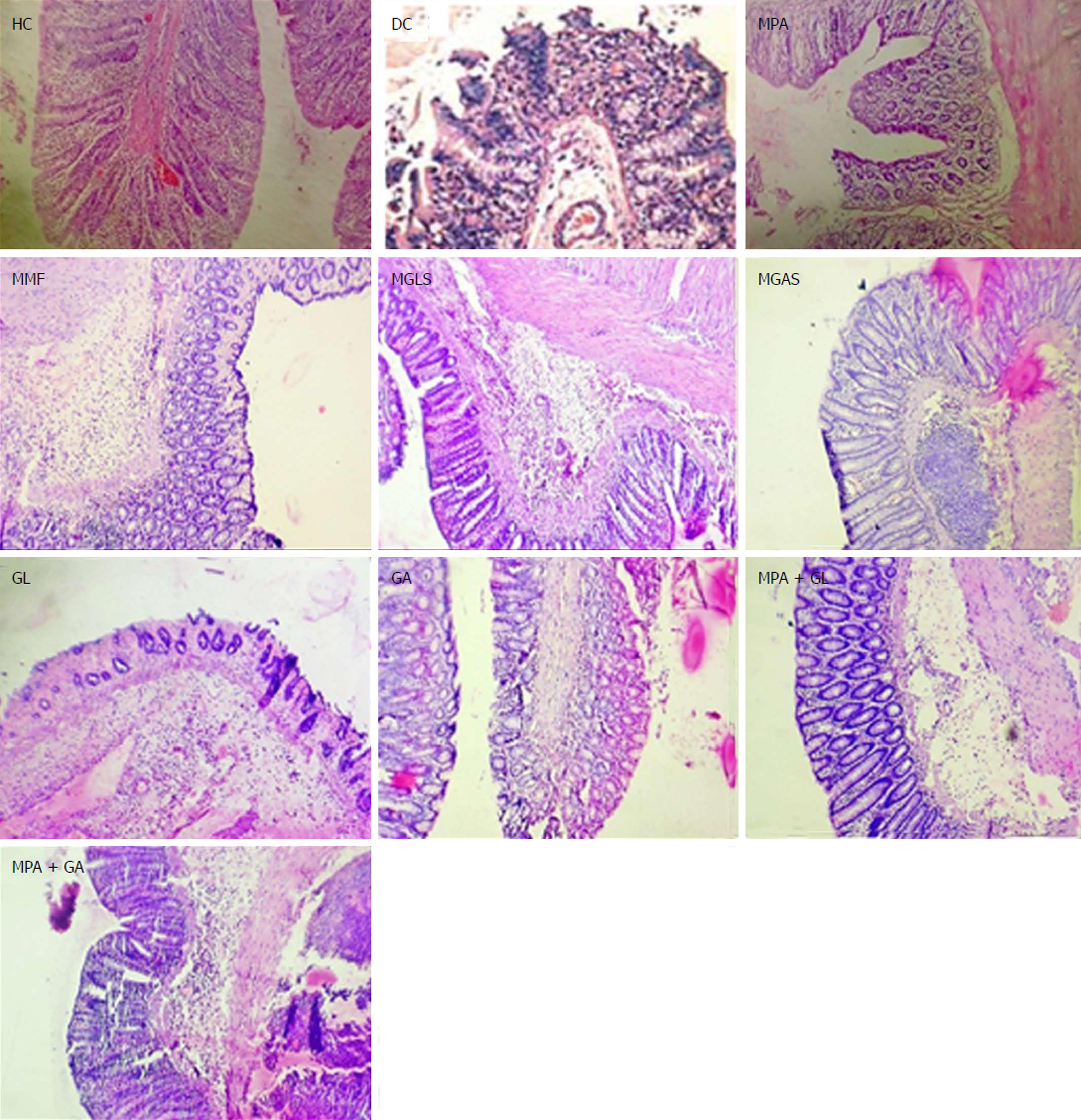
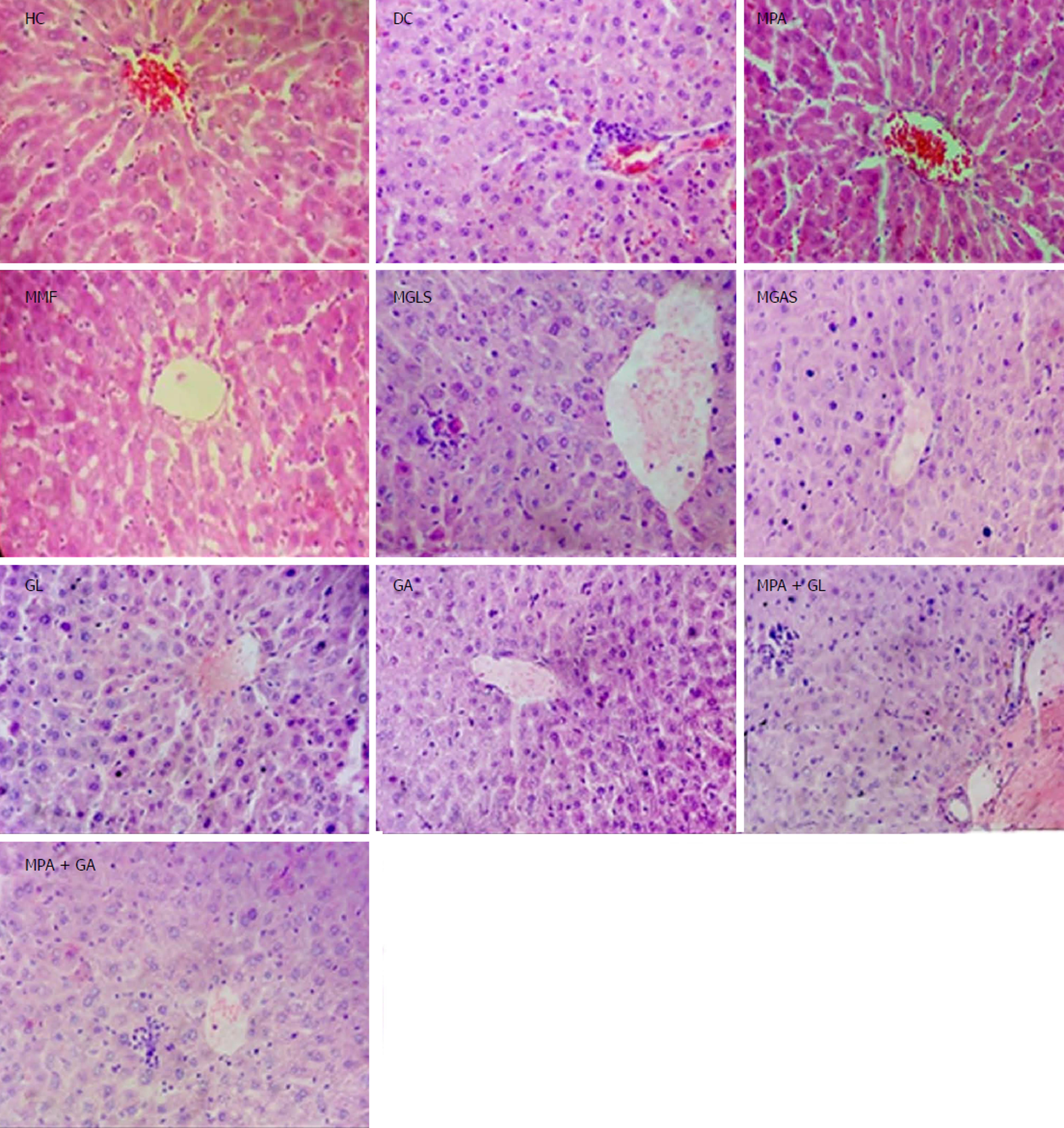
Microscopic characterisation of a colon section was performed by considering the following four important parameters, mucosal distortion, inflammation, fibrosis and cryptitis (Figure 7). The colitis control showed severe erosion with absence of the mucosal layer, goblet cell depletion, distorted crypt architecture, lymphocytic infiltration, and thickening of the muscularis mucosa. The lamina propria was also infiltrated with leukocytes. Due to the destruction of crypts, normal mucosal architecture was totally lost. Treatment with the synthesized prodrugs resulted in a marked decrease in the extent and severity of colonic damage (Figure 7) with normal morphology and mild lymphocytic infiltration, which may have been due to activation of T lymphocytes triggered by immune-stimulation of D-glucosamine as well as D-galactosamine as reported by Sadeghi et al[28], while the colon of animals treated with a physical mixture of MPA and aminosugars, appeared congested with ulcerated mucosa. MGLS and MGAS proved to be better than the physical mixtures as they were able to release MPA and aminosugars locally in the colon in effective concentrations, thus having a protective effect, while MPA and aminosugars administered orally were unable to reach the colon in required concentrations to mitigate colonic inflammation.
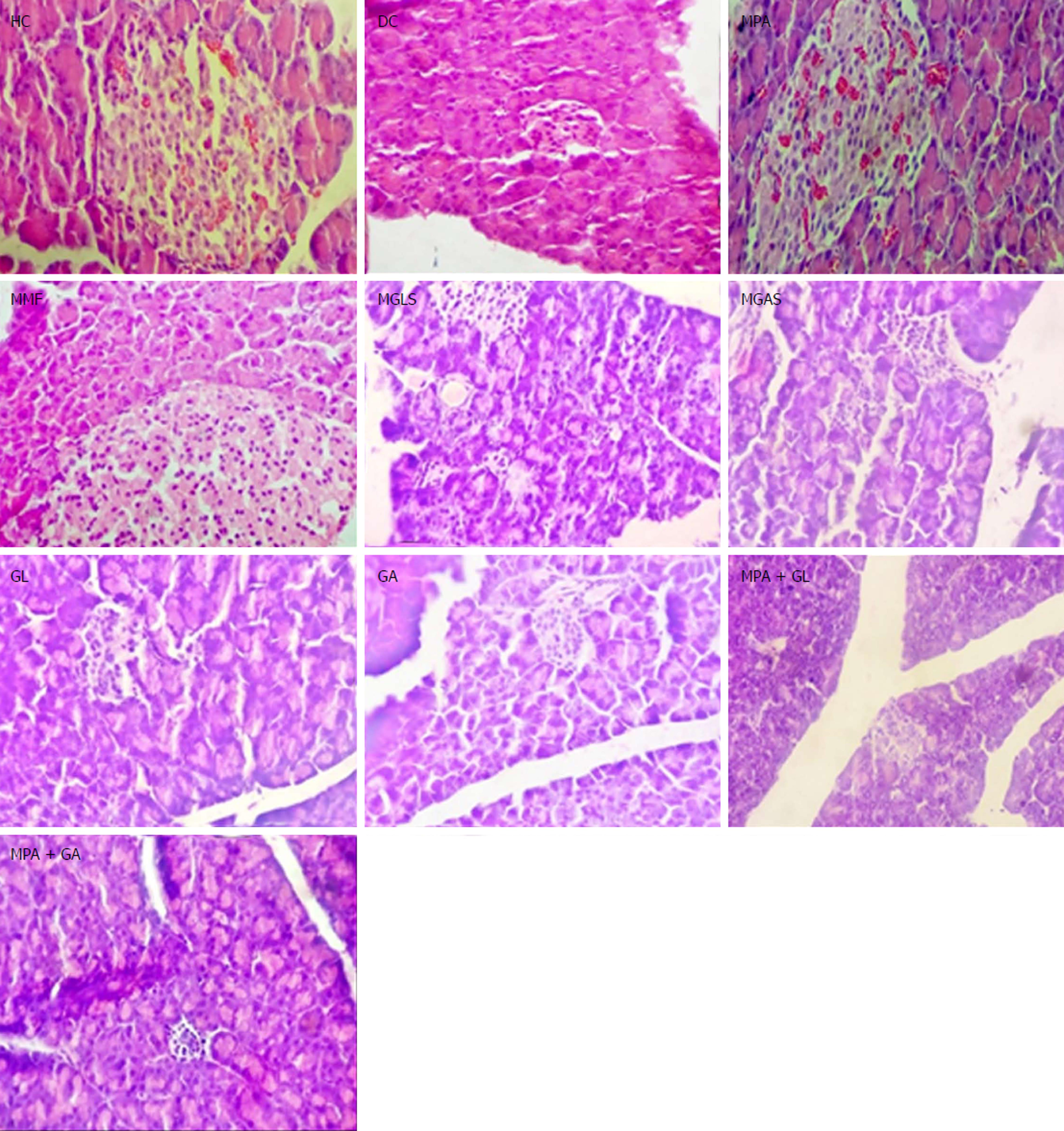
All groups except DC exhibited normal pancreas morphology (Figure 8). Mild infiltration of inflammatory cells in the liver was observed in the groups treated orally with prodrugs and physical mixtures (Figure 9).
The synthesized prodrugs resulted in substantial lowering of ulcer indices as compared to MMF and MPA (Figure 6). These results were consistent with their stability in upper GIT homogenates.
In conclusion, in the present study carrier-linked codrug platforms were successfully designed for efficient colon-specific transport of MPA in order to investigate their potential in mitigating local inflammation in the colon induced by TNBS. Tethering aminosugars to MPA as promoieties resulted in a significant outcome in terms of a marked protective effect compared to MPA without the perpetual diarrhoea observed with MMF therapy. The gastro-sparing nature of the prodrugs and the absence of adverse effects on the pancreas and liver make them promising candidates which should be exploited further as mainstream therapy in the management of IBD.
Defects in the innate immune system are key factors in the pathogenesis of inflammatory bowel disease (IBD). The most commonly used immunomodulators are azathioprine (AZA) or 6-mercaptopurine; however, approximately 10% of patients exhibit intolerance to these drugs, resulting in either withdrawal or prescription of an alternative immunomodulator. Discovered by Gosio in 1893, mycophenolic acid (MPA) was the first antibiotic to be synthesized in a pure and crystalline form. It was used as an immunosuppressant to prevent rejection in organ transplantation, but poor oral bioavailability of MPA was an issue of serious concern. To overcome this setback, mycophenolate mofetil (MMF), the only marketed prodrug of MPA was introduced in 1995 (Trade name: Cellcept) which was used in the treatment of refractory Crohn’s disease. Unfortunately MMF also induced GI side effects such as diarrhoea and local gut toxicity leading to poor patient compliance. The role of MMF as an immunomodulator in managing IBD is yet to be fully defined. It is reported in the literature that it may represent a promising treatment for inducing and maintaining remission in IBD patients intolerant of thiopurines. It may be of more value and relevance in ulcerative colitis, as few alternative proven therapies are available.
Hence, it is necessary to design novel colon-targeted prodrugs of MPA with improved bioavailability, lower gastrointestinal (GI) side effects and enhanced efficacy. Many derivatives of MPA have been patented but none has cleared the clinical trials. Therefore, in the present study, colon-specific mutual prodrugs were synthesized by the formation of a covalent amide linkage between MPA and aminosugars. To confirm attainment of an effective concentration of MPA in the colon, in vivo pharmacokinetic studies were performed. A 2,4,6-trinitrobezenesulfonic acid (TNBS)-induced colitis model in Wistar rats was used for biological screening of prodrugs and to confirm their mitigating effect on colonic inflammation. The present study underlines the potential of colon-targeting co-therapy of IBD using a novel strategy of delivering MPA with aminosugars simultaneously at the site of action without causing the GIT distress associated with MMF therapy.
Immunosuppressants such as AZA are an inherent constituent of therapy for 50% of patients with Crohn’s disease (CD) who develop steroid-dependent or refractory disease. MMF is of proven efficacy and safety in transplantation and in some autoimmune disorders. It has been reported that both AZA and MMF are effective in inducing remission, but AZA seems to be more effective in maintaining remission while onset of the therapeutic effect is delayed less under MMF treatment. Both drugs have steroid-sparing potential, which is delayed under AZA. It seems that AZA is still the immunosuppressant of choice in chronic active CD, but MMF is a reasonable alternative in patients who do not tolerate AZA. One of the major side effects of MMF is gastric distress and diarrhoea that may worsen the symptoms of IBD. The morpholino moiety in MMF is implicated in these side effects. This particular observation inspired us to design codrugs of MPA; replacing the morpholino moiety of MMF with aminosugars which could help to maintain the integrity of the colonic mucosal wall. It was envisaged that these prodrugs might find applicability in those cases of CD which are intolerant to AZA.
The main objectives of this work were to minimize gastric discomfort caused by MMF, and improve the bioavailability and efficacy of MPA in the management of IBD. As gastric side effects are known to be caused by the morpholino moiety of MMF, replacing this moiety with nontoxic aminosugars may have a mitigating effect on inflammation, a stabilizing effect on the colonic mucosal layer and the requisite hydrophilic nature that would impart the desired aqueous solubility to MPA ensuring its efficient delivery to the colon. As anticipate, effective targeting of MPA to the colon using glucosamine and galactosamine as carriers was achieved due to enhanced aqueous solubility of the prodrugs and a resistant amide linkage between MPA and the aminosugars. MMF-related diarrhoea was not evident in the prodrug-treated groups, which was one of the important objectives of the present study. Site-specific delivery of MPA resulted in improved bioavailability of MPA. Future research should be directed at investigating the potential of these prodrugs on refractory CD and for inducing and maintaining remission in IBD patients intolerant of thiopurines.
A clean, single step synthesis of target codrugs was achieved through optimization of the EDCI coupling reaction to avoid complex purification procedures. The synthesized compounds were extensively characterized by spectral analysis. New HPLC methods were developed and validated for simultaneous estimation of MPA and aminosugars in the presence of intact codrugs in order to study the release profiles of codrugs in buffers of various pH, homogenates of gastro-intestinal tract, faecal matter, rat blood, urine and faeces. The TNBS-induced experimental colitis model was optimized to investigate the mitigating effect of codrugs of MPA and aminosugars in comparison to standard drugs (MPA and MMF) and physical mixtures (MPA plus aminosugars) to prove the codrug hypothesis. All data were analysed using relevant statistical tests.
Colon-targeted release of MPA, absence of gastric distress, diarrhoea, maintenance of the integrity of colonic mucosa by released aminosugars and a significant marked amelioration of TNBS-induced colitis in Wistar rats as compared to MPA were the promising outcomes of this study. These codrugs should be explored further as an alternative to MMF for inducing and maintaining remission in IBD patients, those intolerant to thiopurines as well as those with refractory CD.
A comparative analysis of efficacy of these codrugs against the established anticolitics such as aminosalicylates and corticosteroids is required. In addition, screening of combinations of these prodrugs with established therapies would help to prove their potential in the management of IBD. Acute toxicity and estimation of various pro-inflammatory mediators such as interleukins, tumour necrosis factor-α and myeloperoxidase enzyme are several studies which are underway.
In the present work, the morpholino moiety of MMF was replaced by novel aminosugars which were tethered with MPA as a literature review revealed that glucosamine and galactosamine play a vital role in resisting chemical attack and improving the tenacity of colon mucus. The novel strategy of codrug therapy proved to be beneficial in terms of lowering the disease activity, colon to body weight ratio and markedly improving the degenerated colon morphology induced by TNBS. MMF-related gut toxicity and diarrhoea were not evident with these codrugs, which was a very promising outcome. This study underlined the utility of aminosugars in maintaining the integrity of colonic mucosa and supported the significant role of an abnormal immune response in the pathophysiology of IBD. These codrugs have the potential to be screened further to determine their efficacy in refractory CD and in the induction and maintenance of IBD remission in patients who are intolerant to thiopurines or MMF.
A mutual prodrug approach was proven to be superior to physical mixtures of two active drugs i.e., MPA and aminosugars in this study as the efficacy of the codrugs was found to be significantly better than physical mixtures. There is a scope to explore the possibility of conjugation of other nontoxic, biocompatible carrier moieties and established drugs with MPA for a synergistic advantage over administering them in the form of a physical combination.
The authors are grateful to Department of Science and Technology, New Delhi (DST- WOS-A) for financial assistance to carry out this project and Emcure Pharmaceuticals Pvt. Ltd. Pune, Maharashtra for providing a gift sample of mycophenolate sodium. The authors would like to thank Dr. K.R. Mahadik, Principal, Poona College of Pharmacy, Pune for providing the necessary facilities to carry out the work.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gassler N, Mijandrusic-Sincic B, Sergi CM S- Editor: Wang JL L- Editor: Webster JR E- Editor: Ma YJ
| 1. | Hohenleutner U, Mohr VD, Michel S, Landthaler M. Mycophenolate mofetil and cyclosporin treatment for recalcitrant pyoderma gangrenosum. Lancet. 1997;350:1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Wilkins WH. Investigation into the production of bacteriostatic substances by fungi; preliminary examination of the fifth 100 species, all basidiomycetes, mostly of the wood-destroying type. Br J Exp Pathol. 1946;27:140-142. [PubMed] |
| 3. | Abraham EP. The Antibiotics. Comprehensive Biochemistry. Oxford: Sir William Dunn School of Pathology, University of Oxford (Great Britain) 1963; 181-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Wilasrusmee C, Da Silva M, Singh B, Siddiqui J, Bruch D, Kittur S, Wilasrusmee S, Kittur DS. Morphological and biochemical effects of immunosuppressive drugs in a capillary tube assay for endothelial dysfunction Suppl 9. Clin Transplant. 2003;17:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Mitsui A, Suzuki S. Immunosuppressive effect of mycophenolic acid. J Antibiot (Tokyo). 1969;22:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Eugui EM, Almquist SJ, Muller CD, Allison AC. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol. 1991;33:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Eugui EM, Mirkovich A, Allison AC. Lymphocyte-selective antiproliferative and immunosuppressive effects of mycophenolic acid in mice. Scand J Immunol. 1991;33:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 271] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Cioli V, Putzolu S, Rossi V, Corradino C. A toxicological and pharmacological study of ibuprofen guaiacol ester (AF 2259) in the rat. Toxicol Appl Pharmacol. 1980;54:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Batovska DI, Kim DH, Mitsuhashi S, Cho YS, Kwon HJ, Ubukata M. Hydroxamic acid derivatives of mycophenolic acid inhibit histone deacetylase at the cellular level. Biosci Biotechnol Biochem. 2008;72:2623-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Patterson JW, inventor . Syntex (USA) Inc., assignee. 4 and 6-substituted derivatives of MPA. United States patent No.5,554,612.1996. . |
| 11. | Morgans J, inventor . Syntex (USA) Inc., assignee. 5-hexanoic acid side chain derivatives of MPA. United States patent No. 5,633,279.1997. . |
| 12. | Ahlheim M, inventor . Parenteral formulation of mycophenolic acid, a salt or prodrug thereof. United States Patent No. 20060189683 A1.2006. . |
| 13. | Iwaszkiewicz-Grzes D, Cholewinski G, Kot-Wasik A, Trzonkowski P, Dzierzbicka K. Synthesis and biological activity of mycophenolic acid-amino acid derivatives. Eur J Med Chem. 2013;69:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Dhaneshwar SS, Vadnerkar G. Rational design and development of colon-specific prodrugs. Curr Top Med Chem. 2011;11:2318-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Jung YJ, Lee JS, Kim YM. Synthesis and in vitro/in vivo evaluation of 5-aminosalicyl-glycine as a colon-specific prodrug of 5-aminosalicylic acid. J Pharm Sci. 2000;89:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Jung YJ, Kim HH, Kong HS, Kim YM. Synthesis and properties of 5-aminosalicyl-taurine as a colon-specific prodrug of 5-aminosalicylic acid. Arch Pharm Res. 2003;26:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kim H, Huh J, Jeon H, Choi D, Han J, Kim Y, Jung Y. N,N'-Bis(5-aminosalicyl)-L-cystine is a potential colon-specific 5-aminosalicylic acid prodrug with dual therapeutic effects in experimental colitis. J Pharm Sci. 2009;98:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Nagpal D, Singh R, Gairola , N , Bodhankar SL, Dhaneshwar SS. Mutual azo prodrug of 5-aminosalicylic acid for colon targeted drug delivery: synthesis, kinetic studies and pharmacological evaluation. Ind J Pharm Sci. 2006;68:171-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Rhodes JM. Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut. 1989;30:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Clamp JR, Fraser G, Read AE. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond). 1981;61:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Dhaneshwar S, Gautam H. Exploring novel colon-targeting antihistaminic prodrug for colitis. J Physiol Pharmacol. 2012;63:327-337. [PubMed] |
| 22. | Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K, Eigler A, Lehr HA, Endres S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J Pharmacol Exp Ther. 2000;292:22-30. [PubMed] |
| 24. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 25. | Cioli V, Putzolu S, Rossi V, Scorza Barcellona P, Corradino C. The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol Appl Pharmacol. 1979;50:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Barbier M, Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut. 1998;43:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Sadeghi B, Hagglund H, Remberger M, Al-Hashmi S, Hassan Z, Abedi-Valugerdi M, Hassan M. Glucosamine Activates T Lymphocytes in Healthy Individuals and may Induce GVHD/GVL in Stem Cell Transplanted Recipients. Open Transplant J. 2011;5:1-7. [DOI] [Full Text] |