Published online Jan 7, 2018. doi: 10.3748/wjg.v24.i1.112
Peer-review started: October 13, 2017
First decision: November 8, 2017
Revised: December 1, 2017
Accepted: December 12, 2017
Article in press: December 12, 2017
Published online: January 7, 2018
Processing time: 86 Days and 22.8 Hours
To quantify the components in biofilms and analyze the predisposing factors involved in occlusion of biliary stents.
In a prospective study conducted from April 2011 to March 2014 at a tertiary care hospital, all consecutive patients who required endoscopic biliary stent exchange/removal were included. Etiology of the biliary disease was diagnosed by imaging, cytology and on follow-up. Clinical details of patients with biliary stent retrieval were noted. All extracted stents were collected in sterile containers and immediately processed for quantification of biofilm proteins and polysaccharides. Molecular identification of commonly known and unknown bacteria was performed by polymerase chain reaction and density gradient gel electrophoresis methods.
Eighty one patients (41 males) with age range of 20-86 years were studied. The underlying causes for stent insertion were bile duct stones (n = 46; 56.8%) benign stricture (n = 29; 35.8%) and malignancy (n = 6; 7.4%) with cholangitis in 50 (61.7%) patients. The retrieved stent sizes were 7 Fr (n = 62; 76.5%) and 10 Fr (n = 19; 23.5%) with 65 days median insertion duration. Polybacterial consortia were detected in 90.1% of the stents. The most common bacteria identified by polymerase chain reaction alone and/or sequencing were Pseudomonas (n = 38), Citrobacter (n = 23), Klebsiella (n = 22), Staphylococcus (n = 20), Serratia (n = 16), Escherichia coli (n = 14), Streptococcus (n = 13), Enterococcus (n = 13), Aeromonas (n = 12), Proteus (n = 10) and Enterobacter (n = 9). Protein concentration according to gender (0.547 ± 0.242 mg/mL vs 0.458 ± 0.259 mg/mL; P = 0.115) as well as age > 60 years and < 60 years (0.468 ± 0.295 mg/mL vs 0.386 ± 0.238 mg/mL; P = 0.205) was non-significant. However, polysaccharide concentration was significant both according to gender (0.052 ± 0.021 mg/mL vs 0.049 ± 0.016 mg/mL; P < 0.0001) and age (0.051 ± 0.026 mg/mL vs 0.038 ± 0.016 mg/mL; P < 0.011). Protein concentration in the biofilm was significantly higher (0.555 ± 0.225 mg/mL vs 0.419 ± 0.276 mg/mL; P = 0.018) in patients with cholangitis, lower (0.356 ± 0.252 mg/mL vs 0.541 ± 0.238 mg/mL; P = 0.005) in the 10 Fr group than the 7 Fr group, and significantly higher (0.609 ± 0.240 mg/mL vs 0.476 ± 0.251 mg/mL; P = 0.060) in stents of ≥ 6 mo of indwelling time. However presence/absence of cholangitis, size of stent, indication of stent insertion and indwelling time did not affect the quantity of polysaccharide concentration.
Plastic stents retrieved from patients with biliary tract disease showed polymicrobial organisms with higher protein content among patients with cholangitis and those with smaller diameter stents. Longer indwelling duration had more biofilm formation.
Core tip: This prospective study evaluated the components in biofilms of retrieved biliary stents and analyzed predisposing factors involved in the process. A majority of stents showed growth of polymicrobial consortia. Polymerase chain reaction and sequencing helped to detect several microorganisms in most of the stents. Presence of cholangitis, smaller diameter of stents and longer indwelling time of stents were associated with higher chance of biofilm formation. To prevent stent occlusion, longer diameter stents with an indwelling time of 3 to 6 mo should be used.
- Citation: Vaishnavi C, Samanta J, Kochhar R. Characterization of biofilms in biliary stents and potential factors involved in occlusion. World J Gastroenterol 2018; 24(1): 112-123
- URL: https://www.wjgnet.com/1007-9327/full/v24/i1/112.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i1.112
Biliary strictures are responsible for severe complications which can be serious or life threatening to the patients[1]. Transpapillary endoscopic stent placement helps in the relief of obstructed biliary system by a non-surgical approach in patients with benign or malignant biliary disease[2,3]. The natural microbial barrier posed by the sphincter of Oddi is breached when a stent is placed across it and creates a low resistance pathway for colonization by the intestinal microbes[4]. Plastic biliary stents often get occluded by biofilms formed due to adhering microorganisms embedded in an exopolysaccharide (EPS) matrix[5]. A biofilm is defined as a collection of microbial communities enclosed by a matrix of EPS, separated by a network of open water channels and attached to man-made or natural surfaces. Bacterial biofilms are formed when unicellular bacteria come together to form a community that is attached to a solid surface and get encased in an exopolymeric substance largely comprising of proteins and different extracellular polymers[6]. The proposed mechanism of biofilm formation initiates with the process of priming of the stent surface with various proteins followed by microbial adherence and subsequently formation of an EPS matrix to embed the microbial colonies and other “foreign bodies” to give rise to the final mature biofilm[2,3]. Biofilms formed inside biliary stents consist of a mixed spectrum of bacterial communities[2]. Most of these bacteria, generally coming from the enterocolon, are uncultivable by standard culture methods.
Clinical stent occlusion leads to jaundice and bacterial cholangitis with polymicrobial infections in up to 90% of patients[7,8]. Improper use of antimicrobial agents against these microbes leads to antimicrobial resistance and consequently to ineffective treatment of stent-associated cholangitis[9]. Moreover occluded stents need repeat procedures and subsequently lead to increased medical costs as well as poor quality of life. Microorganisms isolated from blocked biliary stents include both aerobic and anaerobic species apart from fungi[2] and reveal their intestinal origin[10-12]. The material properties of the biofilm are heavily dependent on the composition of the EPS which consists of proteins, polysaccharides, nucleic acids and lipids in varying proportions depending on the milieu in which the biofilm grows[13]. Materials derived from bacteria and the host form a conditioning film which lays the foundation for the biofilm development and initiates the process of bacteria-driven sludge formation[3].
In spite of multiple studies on isolation of various organisms in the formation of biofilms, factors involved in the formation of these biofilms are not well studied. Proper characterization of biofilm formation in plastic stents is yet to be adequately explained before steps for its prevention can be made successful. In this study we elucidated (1) the various bacteria in biofilm formation in biliary plastic stents by molecular identification inclusive of polymerase chain reaction (PCR) and sequencing; (2) principal constituents of biofilms viz. proteins and polysaccharides; and (3) the possible predisposing factors in relation to biofilm formation in the stents.
This was a prospective study conducted over a three year period at a tertiary care hospital in Northern India (Postgraduate Institute of Medical Education and Research, Chandigarh, India) from April 2011 to March 2014. During this period, all consecutive patients who required an elective or emergency biliary stent exchange/removal were included in the study. The study was reviewed and approved by the Institutional Ethics Committee which operates according to the Declaration of Helsinki. Written informed consent was taken from all the patients prior to study enrollment. Clinical details of each patient were noted with reference to age, sex, etiology, presence of cholangitis and duration for which the stents had been in situ. Cholangitis was diagnosed as per the Tokyo guidelines[14]. Etiology of the biliary disease was diagnosed by imaging, cytology and on follow-up. All the stents had been placed endoscopically earlier in our institution.
Stent exchange or removal was carried out by first confirming the position of the stent under fluoroscopy. Thereafter, the stents were retrieved endoscopically after grasping with sterile foreign body forceps or a snare and withdrawal of the instrument wholly. The retrieved stents were immediately transferred to a sterile container and transported to the Microbiology Division of the department for processing in order to provide good pre-analytic conditions.
For molecular identification of bacterial species, the central part of the biliary stents were cut and divided horizontally under sterile conditions. The encrusted sludge within the stent was then cultured aerobically in Brain Heart Infusion broth. For identification of anaerobic bacteria, the crust from stents were cultured in Brucella broth under anaerobic conditions. The microbial DNA was extracted from the culture growth by phenol-chloroform method. Briefly 1.5 mL media containing the growth was centrifuged at 12000 g for 10 mins. The supernatant was discarded and the pellet obtained was resuspended in Tris-EDTA (TE). Sodium dodecyl sulfate (0.5%) and proteinase K (200 μg/mL) was added and incubated for 30 min. Next 100 μL sodium chloride was added followed by an equal amount of chloroform-iso-amyl alcohol. The solution was mixed thoroughly and centrifuged. The supernatant was transferred to fresh tube and equal amount of phenol: chloroform: iso-amyl alcohol was added. After mixing again the solution was centrifuged and the supernatant obtained was transferred to another tube to which 0.6 volume of iso-propanol was added. Centrifugation was repeated and the pellet was washed with 70% ethanol. The pellet obtained was air dried, dissolved in TE and run in 0.8% agarose gel for checking for DNA.
Identification of commonly known bacteria involved in biofilm formation: PCR was standardized using the universal 16S rRNA gene specific primers for determining the DNA sequence for commonly known bacteria such as Pseudomonas, Escherichia coli, Citrobacter, Streptococcus, Aeromonas, Enterococcus, Staphylococcus, Proteus, Bacillus, Klebsiella, Enterobacter, Serratia, Vibrio, Yersinia, Bacteroides and Clostridium using standard strains obtained from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India, as positive controls. Following standardization, PCR was done for identification of the above known bacteria that could be responsible for biofilm formation in the stents. The primers used for determination of bacteria are given in Table 1. The amplicons were visualized on 1.5% agarose gels stained with ethidium bromide and compared to a database of known sequences.
| Sr. No | Organism | Primer sequences |
| 1 | Pseudomonas | F: 5'-GACGGGTGAGTAATGCCTA- 3' |
| R: 5'-CACTGGTGTTCCTTCCTATA -3' | ||
| 2 | Staphylococcus | F: 5'- AAC TCT GTT ATT AGG GAA GAA CA- 3' |
| R: 5'- CCA CCT TCC TCC GGT TTG TCA CC-3' | ||
| 3 | E. coli | F: 5'-GAAGCTTGCTTCTTTGCT- 3' |
| R: 5'-GAGCCCGGGGATTTCACAT- 3' | ||
| 4 | Enterococcus | F: 5'- GTTTATGCCGCATGGCATAAGAG -3' |
| R: 5'-CCGTCAGGGGACGTTCAG -3' | ||
| 5 | Citrobacter | F: 5′-TCAGATTTGAACGCTGGCGGCA -3′ |
| R: 5′-CGTATTACCGCGGCTGCTGCCAC -3′ | ||
| 6 | Proteus | F: 5'-AGA GTT TGA TCC TGG CTC AG-3' |
| R: 5'-AAG GAG GTG ATC CAG CC-3' | ||
| 7 | Klebsiella | F: 5'-AGA GTT TGA TCC TGG CTC AG-3' |
| R: 5'-AAG GAG GTG ATC CAG CC-3' | ||
| 8 | Clostridium | F: 5'-TGG CTC AGA TTG AAC GCT GGC GGC -3' |
| R: 5'-TAC CTT GTT ACG ACT TCA CCA CA-3' | ||
| 9 | Bacillus | F:5'- AGA GTT TGA TCC TGG CTC AG -3' |
| R: 5'- AAG GAG GTG ATC CAG CCG CA-3' | ||
| 10 | Vibrio | F: 5'-AGA GTT TGA TCA TGG CTC AG -3' |
| R:5'-GAA ATT CTA CCC CCC TCT ACA G-3' | ||
| 11 | Aeromonas | F: 5'-GCT GGT CTG AGA GGA TGA TC-3' |
| R: 5'-CTT TAC GCC CAG TAA TTC CG-3' | ||
| 12 | Bacteroides | F: 5'- ATT CTA GAG TTT GAT CAT GGC TCA-3' |
| R: 5'-ATG GTA CCG TGT GAC GGG CGG TGT GTA-3' | ||
| 13 | Enterobacter | F: 5'-AGTTTGATCCTGGCTCAG -3' |
| R: 5'-TAC CTT GTT ACG ACT TCG TCC CA-3' | ||
| 14 | Streptococcus | F: 5'-TAA CCA GAA AGG GAC GGC TA-3' |
| R: 5'-CAC TCT CCG CTT CTG CAC TC-3' | ||
| 15 | Serratia | F : 5'-GCGGTTTGTTAAGTCAGATG -3' |
| R: 5'-CGAATTAAACCACATGCTCC-3' | ||
| 16 | Yersinia | F : 5′-AAT ACC GCA TAA CGT CTT CG-3′ |
| R: 5′-CTT CTT CTG CGA GTA ACG TC-3′ |
Identification of unknown bacteria involved in biofilm formation: Molecular identification of unknown bacteria involved in biofilm formation was done using the Density Gradient Gel Electrophoresis (DGGE). The DNA isolated from the biofilms were used for creating multiple copies of the 16S rRNA genes of similar but not identical bacteria for identifying unknown bacteria. The variable regions V3 to V5 were amplified using the following universal primers: 341-F (5′-CCT ACG GGA GGC AGC AG-3′) with a 40 bp GC sequence clamped to its 5′ end (5′-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC G-3′) and 907-R (5′-CCG TCA ATT CMT TTG AGT TT-3′). This set of primers was designed to be specific for most bacteria[15]. The reaction mixture (50 μL) contained 50 ng microbial DNA, 200 μmol/L of each deoxynucleoside triphosphate, 0.5 pmol/L of each of the primers, 2.5 mmol/L MgCl2, 3 mg/mL BSA and 3 U DNA Taq polymerase. The touchdown PCR was performed in eppendorf thermocycler using a program described by Sánchez et al[16]. Following an initial denaturation at 95 °C for 3 min, a touchdown program began with 15 cycles consisting of one minute denaturation at 95 °C, one minute annealing beginning at 65 °C and ending at 50 °C (decreasing 1 °C per cycle), and a one minute extension at 72 °C. A final extension of 5 min at 72 °C was done. PCR products were quantified on 1.5% (w/v) agarose gel. The desired PCR product was 594 bp (including the GC clamp).
The sample was loaded in the DGGE gel solution consisting of 6% (w/v) acrylamide/bisacrylamide (37.5:1) in 0.5 × TAE buffer containing 40% to 60% of the denaturant. The gels were prepared, loaded and run according to the instructions of the manufacturers of DGGE system (D-Code, BioRad, United States) for analysis of PCR products.
For sequencing, the selected DGGE bands were excised from the gels using sterile scalpel and placed in a sterile eppendorf containing 20 μL of sterile water. The amplified PCR products were sequenced commercially (Chromous Biotech, Bengaluru, India) using bands which were different from commonly known bands. Data obtained after sequencing in fasta format were compared with the National Center of Biotechnology Information (NCBI) GenBank data base using standard nucleotide blast search tools (BLAST N 2.2.29+).
The major molecules like proteins and carbohydrates which act as a molecular glue for biofilm formation were measured as follows.
Protein estimation: Protein estimation in the biofilm mass was done by modified Lowry’s method as described by Raunkjær et al[17]. Briefly, one centimeter of the stent was put into a 1.5 mL centrifuge tube and 500 μL sodium hydroxide (0.5 mol/L) was added to it. The tube with the stent was heated at 80 °C for 30 min in a water bath. Centrifugation at 4238 g at 4 °C for 15 min was done and supernatant was transferred to another micro-centrifuge tube. 50 μL supernatant was put into a test tube and 1 mL reagent comprising of CuSO4.5H2O and sodium tartrate was added. It was incubated for 5 min at room temperature and absorbance was read at 620 nm using a colorimeter (Electronics India). Bovine serum albumin served as the standard for the assay.
Polysaccharide estimation: Polysaccharide estimation in the biofilm mass was done using the anthrone method as described by Ahimou et al[13]. Briefly, one centimeter of biliary stent was put into 1.5 mL centrifuge tube and 500 μL sodium hydroxide (1 N) was added to it and heated at 80 °C for 30 min in a water-bath. Centrifugation was done at 4238 g and 500 μL supernatant was transferred to another micro-centrifuge to which 500 μL distilled water and 4 mL of 0.2% anthrone reagent in concentrated sulfuric acid was added and mixed well. It was incubated for 10 min in boiling water-bath and allowed to cool at room temperature. Glucose (1 mg/10 mL) was used as a standard and absorbance was read at 620 nm using a colorimeter (Electronics India).
Statistical analysis for this study was performed using SPSS version 20.0 (IBM Corp., United States). The distribution of quantitative and qualitative data was presented as median (range) or absolute and relative frequencies. χ2 test and Fisher’s exact test were used to investigate the relationship between each parameter. Significance was defined as a P value < 0.05.
A total of 81 patients (41 males) with age-range of 20-86 years were included in the study. The underlying causes for stent insertion were bile duct stones (n = 46, 56.8%) benign stricture (n = 29, 35.8%), and malignant stricture (n = 6, 7.4%). All the stents were double pig-tailed and made of polyethylene (Wilson-Cook Medical, Ireland) and had been placed endoscopically at our Institute. The diameter of the stents retrieved was 7Fr (n = 62, 76.5%) or 10 Fr (n = 19, 23.5%). The median duration of stent insertion was 65 days (range 5-1095 d). Cholangitis was present in 50 (61.7%) patients, at the time of stent insertion.
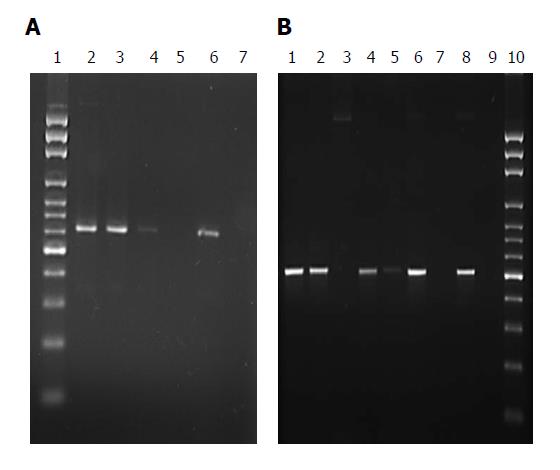
Microbiological analysis: Of the 81 stents retrieved, organisms were detected in 73 by PCR alone, whereas all 81 stents had organisms detected by touchdown PCR and sequencing which included uncultured bacteria in 12 stents (Table 2). Polybacterial consortia were detected in majority of the stents (n = 73, 90.1%) whereas single species were found in the remaining 8 (9.9%) stents. The most common Gram-negative bacteria detected by both PCR alone and by sequencing were Pseudomonas (n = 38), Citrobacter (n = 23), Klebsiella (n = 22), Serratia (n = 16), Escherichia coli (n = 14), Aeromonas (n = 12), Proteus (n = 10), Enterobacter (n = 9). The most prevalent Gram-positive bacteria were Staphylococcus sp. (n = 20) Streptococcus (n = 13) and Enterococcus (n = 13). Figure 1A and B show amplification of 541 bp of Pseudomonas sp and 500 bp of Citrobacter sp. as representative bacteria detected by PCR. Table 3 depicts the number of biliary stents in which Gram negative and Gram positive bacteria were detected.
| Sr. No. | Organisms identified by touchdown PCR and sequencing | Organisms identified by PCR alone |
| BF 1 | Stenotrophomonas maltophilia | Klebsiella |
| BF 2 | Pseudomonas stutzeri | Pseudomonas, Proteus, Aeromonas |
| BF 3 | Bacillus tequilensis | Staphylococcus, Bacillus |
| BF 4 | Uncultured bacterium clone DolRC 17069 | Streptococcus, Aeromonas, Serratia |
| BF 5 | Bacillus cereus | Staphylococcus, Bacillus |
| BF 6 | Micrococcus yunnanensis | Streptococcus, Proteus, Serratia |
| BF 7 | Staphylococcus epidermidis | Staphylococcus, Bacillus |
| BF 8 | Citrobacter sp. | Citrobacter, Escherichia coli |
| BF 9 | Stenotrophomonas maltophilia | Proteus |
| BF 10 | Anaerosalibacter sp. | Pseudomonas, Citrobacter |
| BF 11 | Enterobacteriales bacterium | Staphylococcus, Streptococcus, Klebsiella |
| BF 12 | Uncultured bacterium clone PS B346 | Staphylococcus, Aeromonas |
| BF 13 | Stenotrophomonas maltophilia | —— |
| BF 14 | Stenotrophomonas maltophilia | Streptococcus |
| BF 15 | Stenotrophomonas maltophilia | Serratia |
| BF 16 | Uncultured organism clone ELU0026 | Citrobacter |
| BF 17 | Uncultured organism clone ELU0020 | —— |
| BF 18 | Stenotrophomonas maltophilia | Citrobacter, Streptococcus |
| BF 19 | Uncultured bacterium clone ELU0020 | Klebsiella, Aeromonas, Enterococcus |
| BF 20 | Bacillus mojavensis | Pseudomonas, Bacillus, Enterobacter |
| BF 21 | Paenibacillus sp. A1006 | Proteus, Klebsiella, Serratia |
| BF 22 | Bacillus cereus | Streptococcus, Serratia |
| BF 23 | Stenotrophomonas maltophilia | —— |
| BF 24 | Bacillus sp. | Proteus, Yersinia, Aeromonas |
| BF 25 | Pseudomonas stutzeri | Pseudomonas, Escherichia coli |
| BF 26 | Enterobacteriales bacterium | Pseudomonas, Proteus, Klebsiella |
| BF 27 | Uncultured bacterium clone DolRc DL35rect19C08 | Proteus, Klebsiella |
| BF 28 | Enterococcus faecalis | Citrobacter, Enterococcus |
| BF 29 | Micrococcus luteus | —— |
| BF 30 | Staphylococcus epidermidis | Staphylococcus, Serratia |
| BF 31 | Staphylococcus epidermidis | Citrobacter, Escherichia coli, Staphylococcus |
| BF 32 | Enterococcus durans | Escherichia coli, Klebsiella, Enterococcus |
| BF 33 | Enterococcus durans | Staphylococcus, Streptococcus, Serratia |
| BF 34 | Stenotrophomonas maltophilia | Pseudomonas |
| BF 35 | Uncultured bacterium clone B64 | Citrobacter, Proteus, Klebsiella |
| BF 36 | Pseudomonas otitidis | Pseudomonas, Aeromonas |
| BF 37 | Enterobacteriales bacterium | Streptococcus, Klebsiella, Enterobacter |
| BF 38 | Pseudomonas alcaligenes | Citrobacter, Escherichia coli, Yersinia |
| BF 39 | Enterococcus faecalis | Citrobacter, Streptococcus, Klebsiella |
| BF 40 | Enterococcus faecalis | Streptococcus, Klebsiella , Aeromonas |
| BF 41 | Enterococcus sp. | Citrobacter, Enterobacter |
| BF 42 | Bacillus subtilis | Pseudomonas, Streptococcus, Aeromonas |
| BF 43 | Pseudomonas aeruginosa | Pseudomonas, Escherichia coli, Yersinia |
| BF 44 | Uncultured bacterium | Pseudomonas, Citrobacter, Yersinia |
| BF 45 | Uncultured bacterium | Pseudomonas, Citrobacter, Escherichia coli |
| BF 46 | Pseudomonas sp. | Escherichia coli, Enterobacter |
| BF47 | Bacillus cereus | Proteus, Klebsiella |
| BF 48 | Stenotrophomonas maltophilia | Pseudomonas, Citrobacter, Streptococcus |
| BF 49 | Stenotrophomonas maltophilia | Pseudomonas, Staphylococcus, Proteus |
| BF 50 | Bacillus cereus | Pseudomonas , Staphylococcus |
| BF 51 | Pseudomonas aeruginosa | Pseudomonas, Citrobacter, Escherichia coli |
| BF 52 | Pseudoxanthomonas icgebensis | Citrobacter, Serratia |
| BF 53 | Enterobacteriales bacterium | Klebsiella, Enterobacter, Vibrio |
| BF 54 | Citrobacter freundii | Citrobacter, Staphylococcus |
| BF 55 | Uncultured bacterium clone PS | Staphylococcus, Serratia |
| BF 56 | Enterobacteriales bacterium | Pseudomonas, Citrobacter, Streptococcus |
| BF 57 | Morganella morganii | —— |
| BF 58 | Pseudomonas aeruginosa | Pseudomonas, Escherichia coli, Staphylococcus |
| BF 59 | Pseudomonas sp. | Pseudomonas, Staphylococcus, Serratia |
| BF 60 | Pseudomonas putida | Pseudomonas, Klebsiella , Aeromonas |
| BF 61 | Morganella morganii | —— |
| BF 62 | Enterobacteriales bacterium | Pseudomonas, Aeromonas |
| BF 63 | Enterococcus faecalis | Enterococcus |
| BF 64 | Pseudomonas sp. | Pseudomonas, Staphylococcus, Serratia |
| BF 65 | Bacillus sp. | Citrobacter, Enterobacter |
| BF 66 | Uncultured organism clone | —— |
| BF 67 | Pseudomonas sp. | Pseudomonas |
| BF 68 | Bacillus licheniformis | Pseudomonas, Enterobacter |
| BF 69 | Enterococcus sp. | Klebsiella , Serratia |
| BF 70 | Enterococcus faecalis | Citrobacter, Enterobacter, Serratia |
| BF 71 | Pseudomonas stutzeri | Pseudomonas, Staphylococcus, Aeromonas |
| BF 72 | Escherichia coli | Escherichia coli |
| BF 73 | Klebsiella sp. | Pseudomonas, Klebsiella |
| BF 74 | Uncultured Klebsiella sp. | Escherichia coli |
| BF 75 | Enterobacteriales bacterium | Escherichia coli, Enterobacter, Yersinia |
| BF 76 | Stenotrophomonas maltophilia | —— |
| BF 77 | Enterobacteriales bacterium | Klebsiella |
| BF 78 | Citrobacter sp. enrichment clone | Citrobacter |
| BF 79 | Serratia marcescens | Staphylococcus, Serratia |
| BF 80 | Klebsiella sp. BAB-3527 | Klebsiella, Aeromonas |
| BF 81 | Exiguobacterium aurantiacum | Klebsiella |
| Number of stents positive for the isolates | |
| Gram-positive microorganism | |
| Bacillus sp. | 11 |
| Enterococcus sp. | 9 |
| Micrococcus sp. | 2 |
| Streptococcus sp. | 11 |
| Staphylococcus sp. | 17 |
| Gram-negative microorganism | |
| Citrobacter sp. | 20 |
| Escherichia coli | 12 |
| Enterobacter sp. | 9 |
| Klebsiella sp. | 19 |
| Morganella morganii | 2 |
| Proteus sp. | 10 |
| Pseudomonas sp. | 27 |
| Serratia sp. | 15 |
| Stenotrophomonas maltophila | 11 |
| Vibrio sp. | 1 |
| Yersinia sp. | 5 |
| Aeromonas sp. | 12 |
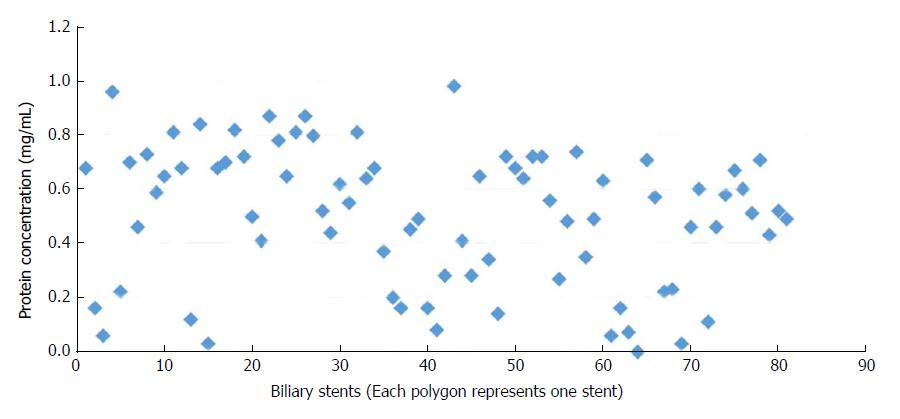
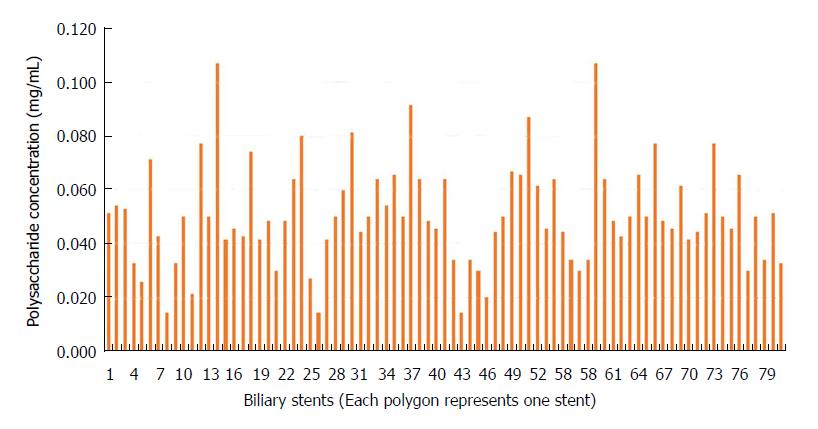
Quantification of protein and polysaccharide in the biofilms: Protein content in the biofilm formation ranged from 0 to 0.98 mg/mL with a mean of 0.50 ± 0.25 mg/mL (Figure 2) while the polysaccharide content ranged from 0.014 to 0.107 mg/mL with a mean of 0.051 ± 0.018 mg/mL (Figure 3).
The relation of biofilm constituents with various predisposing factors was analyzed and is summarized in Table 4.
| Parameters | Protein (mg/ml) | P value | Polysaccharide concentration (mg/mL) | P value |
| Gender | ||||
| Male (n = 41) | 0.547 ± 0.242 | 0.115 | 0.052 ± 0.021 | < 0.0001 |
| Female (n = 40) | 0.458 ± 0.259 | 0.049 ± 0.016 | ||
| Age | ||||
| Below 60 (n = 60) | 0.386 ± 0.238 | 0.205 | 0.038 ± 0.016 | 0.011 |
| Above 60 (n = 21) | 0.468 ± 0.295 | 0.051 ± 0.026 | ||
| Etiology of stenting | ||||
| Cholangitis (n = 50) | 0.555 ± 0.225 | 0.018 | 0.0512 ± 0.021 | 0.790 |
| No cholangitis (n = 31) | 0.419 ± 0.276 | 0.050 ± 0.014 | ||
| Indication of stent insertion | ||||
| CBD stone (n = 46) | 0.518 ± 0.256 | 0.530 | 0.051 ± 0.022 | 0.785 |
| Benign stricture (n = 29) | 0.453 ± 0.256 | 0.050 ± 0.012 | ||
| Indwelling stent size | ||||
| 7 Fr (n = 62) | 0.541 ± 0.238 | 0.005 | 0.049 ± 0.015 | 0.674 |
| 10 Fr (n = 19) | 0.356 ± 0.252 | 0.052 ± 0.020 | ||
| Duration of indwelling stents | ||||
| < 3 mo (n = 39) | 0.481 ± 0.242 | 0.472 | 0.0489 ± 0.015 | 0.385 |
| ≥ 3 mo (n = 42) | 0.523 ± 0.264 | 0.0525 ± 0.022 | ||
| < 6 mo (n = 65) | 0.476 ± 0.251 | 0.060 | 0.0501 ± 0.017 | 0.560 |
| ≥ 6 mo (n = 16) | 0.609 ± 0.240 | 0.0533 ± 0.026 | ||
| No of microorganisms detected monomicrobial (n = 13) | 0.502 ± 0.263 | 0.996 | 0.051 ± 0.018 | 0.968 |
| Polymicrobial (n = 68) | 0.501 ± 0.050 | 0.049 ± 0.015 |
Gender and age groups: Male gender had higher protein concentration (P = 0.115) and polysaccharide concentration (P < 0.0001) than female gender. Patients > 60 years of age had higher protein concentration (P = 0.205) and polysaccharide concentration (P < 0.011) than those < 60 years of age.
Cholangitis: The quantity of biofilm components was compared in the stents retrieved from patients with (n = 50) and without cholangitis (n = 31). Protein concentration was found to be significantly higher (P = 0.018) in stents with cholangitis (0.555 mg/mL) as compared to those without cholangitis (0.419 mg/mL). Polysaccharide content was however not different in patients with or without cholangitis.
Indication of stent insertion: Biofilm constituents were also compared with etiology of biliary disease (benign stricture vs stone). However there was no statistical significance observed between CBD stone or benign stricture as regards to both protein and polysaccharide quantity.
Size of indwelling stents: Biofilm constituents were analyzed between two stent size groups of 7 Fr vs 10 Fr. Protein concentration in the 10 Fr group was significantly lower than in the 7 Fr group (0.356 ± 0.252 mg/mL vs 0.541 ± 0.238 mg/mL, P = 0.005). However there was no significant difference in the quantity of polysaccharide concentration (P = 0.674) in the stents of the two different sizes.
Duration of indwelling stents: When the stents with an indwelling time of ≥ 3 mo were compared with those < 3 mo, it was found that there was no significant difference in the protein concentration (P = 0.472) or polysaccharide concentration (P = 0.385) between the two groups. When the stents with an indwelling time of ≥ 6 mo were compared with those < 6 mo, it was found that protein concentration was significantly higher in the stents of ≥ 6 mo of indwelling time (0.609 ± 0.240 mg/mL vs 0.476 ± 0.251 mg/mL, P = 0.060), but there was no difference in polysaccharide concentration (P = 0.560).
Number of microorganisms detected: When the number of microorganisms isolated i.e. single vs multiple by PCR alone was analyzed no significant difference was seen with respect to the protein (P = 0.996) and the polysaccharide parameters (P = 0.968).
Most of the annotated DNA sequences obtained by sequencing from biofilms of each biliary stent have been deposited with the GenBank at NCBI, United States (Accession Nos. KP198519-43; KP205043-80; KP212173-77).
Biofilm formation is an important step in the occlusion of biliary stents and depends on a number of factors, inclusive of bacterial colonization[2,3]. Swidsinski et al[18] had demonstrated that neither the gall bladder wall nor the bile duct wall had any biofilm, denoting that opportunistic attachment of the microbes occurs later with subsequent biofilm formation on the biliary stents. In the natural setting, bacteria composed of a single species are seldom found in biofilms and most of the biofilms are multispecies consortia with a synergistic effect on the biofilm formation[19]. Aerobic Enterococcus, E. coli and Klebsiella as also anaerobic Clostridia are the most common microorganisms isolated from biliary sludge[2,20]. In our study, polybacterial consortia were seen in 90.1% of the biliary stents with most common microorganisms being Pseudomonas, Citrobacter, Klebsiella, and Staphylococcus. Similar frequency of polymicrobial consortia was found in patients with or without cholangitis. Schneider et al[20] also reported that occluded stents have higher proportion of Staphylococcus sp. as compared to the non-occluded ones. Lübbert et al[9] reported that enterococci plays a significant part in the microbial colonization of biliary stents. In our study enterococci were found in 16% of the biliary stents. Though anaerobes are reported by some authors to have important role in the formation of biofilms[20], we found no anaerobe in the occluded biliary stents in the present study.
The proposed mechanism of biofilm formation is initiated with the process of priming of the stent surface with various proteins followed by microbial adherence and subsequent formation of an EPS matrix to embed the microbial colonies and other particles to give rise to the final mature biofilm. Yu et al[21] reported attachment of fibronectin to the inner surface of the stents within 24 h of exposure to bile. Another contributing factor is the bile immunoglobulin-bacteria complex which further promotes the binding of the bacteria to the inner surface of the stents[22]. Thus, the basic ingredients of a biofilm include the adherence proteins, the bacteria and the EPS. In patients with cholangitis, wherein these factors are expected to be high, there are higher chances of biofilm formation and stent occlusion. In the current study, the protein concentration of the biofilms was found to be significantly higher in stents placed in patients with cholangitis than those without cholangitis. Polysaccharide concentration was also higher among the cholangitis group, although it was not statistically significant. This highlights the phenomenon of higher propensity of stent occlusion due to biofilm formation in an infected biliary system as compared to the non-infected ones. The higher risk of stent occlusion in cholangitis can also be explained by the increased bile viscosity of the infected bile, causing decrease in the bile flow velocity leading to bile stasis and increased spontaneous and bacteria-driven bile salt precipitation[2].
The diameter of the biliary stents has always been a key issue governing the dynamics of bile flow and stent occlusion. An increase in the inner stent diameter of 0.2 mm leads to a 300% increase in the bile flow[23]. The maximum diameter of plastic stent that can be placed endoscopically is 11.5 Fr[24]. This limitation of the maximum diameter of stents placed endoscopically is the reason why stents up to 10 Fr size are used. Smaller diameter stents have a higher tendency to get occluded due to biofilm formation. Larger diameter entails greater bile flow velocity and subsequently less predisposition to bile salt precipitation, protein accumulation and biofilm formation. Thus, large diameter stents have always fared better than smaller diameter ones in terms of durability[25] and one of the major advantages of metallic stents is in fact its large diameter[26]. In the current study, 10 Fr stents were found to have significantly lower protein concentration in their biofilm formation as compared to the 7 Fr ones. This highlights a probable lower propensity for protein deposition - one of the key events for initiation of biofilm formation due to high bile flow velocity in the 10 Fr groups.
The process of biofilm formation is a time-dependent one and risk of standard polyethylene stent occlusion increases progressively after 3 mo[2]. We did not find any difference in the protein and polysaccharide concentration in relation to stents removed < 3 mo and ≥ 3 mo. However when the stents placed for < 6 mo were compared with those of ≥ 6 mo, the protein concentration was found to be higher in stents kept for ≥ 6 mo. The nature of protein (human/bacterial origin, immunoglobulins, fibrinogen etc.) could also not be further analyzed as this was not the aim of the study. Quantitative assessment of the number of bacteria present in the stents could not be made as molecular identification was carried out after culture of bacteria from the stent segments in fluid culture media. The process of biofilm formation in general is very complex (obstruction of biliary stents is more complex and involves not only bacteria and their products but bilirubin complexes, cholesterol complexes and ingrowth of tissue). Schneider et al[20] in a multivariate analysis have shown that sludge formation had significant relationship with stent indwelling time.
Understanding pathophysiology of biofilm formation in plastic biliary stents is important in preventing their occlusion and complications thereof. From our data stents indwelling time of ≤ 3 mo or > 3 mo did not correlate with biofilm formation. However stents placed for > 6 mo had higher biofilm formation. Hence stents should not be left indwelling beyond 6 mo. Also larger diameter stents (10 Fr) should be preferred. A number of other options have been studied to prevent biofilm formation. Several studies have shown the effects of antibiotic coatings on medical devices effective against biofilm formation; however, such data has not been as successfully replicated for biliary stents[27]. Recently some workers have found that biliary plastic stents coated with silver nano particles or ions have antibacterial activity against several organisms and extends the period of use of biliary stents[28,29].
Our study had a few limitations. Data on comorbidities were not available, so we could not study the predisposition, if any, of biofilm formation in patients with diabetes. We had only a few patients with malignancy who were excluded from analysis. A larger number of patients with malignant obstruction could have given us a comparison between benign and malignant etiology. Culture results of patients with cholangitis at the time of stent insertion were not available to correlate with organisms grown in the biofilms.
Ours is one of the first studies of its kind to measure the biofilm components, namely protein and polysaccharide in biliary stents. Presence of cholangitis at the time of stent insertion and smaller diameter of stents were found to have higher protein concentration, whereas male gender and age above 60 years had higher polysaccharide concentration, predisposing to higher propensity of biofilm formation. Longer (≥ 6 mo) indwelling time of stents was associated with higher biofilm formation and protein concentration elucidating the time-dependent process of biofilm formation. Our data suggest that plastic stents should be replaced between 3-6 mo.
Since its introduction in 1979, biliary plastic stents have been a landmark achievement in the field of endoscopic retrograde cholangiopancreatography for the relief of obstructed biliary system by a non-surgical approach. The limiting factors for these plastic stents are their diameter and the tendency to get occluded. The maximum diameter of plastic stent that can be placed is 11.5 Fr requiring a duodenoscope accessory channel diameter of 4.2 mm. This limitation of the maximum diameter leads to the tendency for them to get occluded due to the formation of biofilm causing recurrent obstruction and need for repeat procedures subsequently leading to increased medical costs and poor quality of life. The cardinal step in the process of stent occlusion is bacterial colonization. Various studies including scanning electron microscopic observations have shown that the clogging material found in biliary stents consists of bacterial biofilm, biliary sludge and duodenal refluxate of dietary fibers. Biofilm is formed by microbes embedded in an exopolysaccharide matrix which also engulfs “foreign bodies” of various sizes. Its ultrastructure reveals voids and channels required for nutrient diffusion and molecular signaling.
Despite multiple studies elucidating the various organisms and the formation of biofilms, various factors involved in the formation of these biofilms are not well studied. The proposed mechanism of biofilm formation initiates with the process of priming of the stent surface with various proteins followed by microbial adherence and subsequently formation of an exopolysaccharide matrix to embed the microbial colonies and other “foreign bodies” to give rise to the final mature biofilm. However, proper characterization of biofilm formation in plastic stents has to be adequately elucidated before steps for its prevention can be made successful. Components of the biofilm such as protein and polysaccharides developing in biliary stents have never been quantified in previous studies.
The main objectives of this study were to elucidate the various bacteria implicated in biofilm formation in biliary plastic stents, to quantify the principal constituents (namely proteins and polysaccharide) of biofilm mass and the possible predisposing factors in relation to biofilm formation in the stents. This prospective study evaluated the extracellular polymeric substance such as protein and polysaccharide in the biofilms as well as microbes occluding the biliary stents in patients who had retrieval of biliary stents (7 Fr and 10 Fr) and analyzed predisposing factors involved in the process of occlusion of the stents. Our results showed that the presence of cholangitis at the time of stent insertion and smaller diameter of stents were found to have higher protein concentration, whereas male gender and age above 60 years had higher polysaccharide concentration, predisposing to higher propensity of biofilm formation. Longer (≥ 6 mo) indwelling time of stents was associated with higher biofilm formation and protein concentration, elucidating the time-dependent process of biofilm formation. Our data suggest that plastic stents should be replaced between 3-6 mo. Further studies can be done to explore the origin of the bacteria grown in biofilms. Strategies to prevent biofilm formation can also be planned and investigated.
This was a prospective study conducted at a tertiary care hospital in Northern India (Postgraduate Institute of Medical Education and Research, Chandigarh, India) from April 2011 to March 2014. All consecutive patients who required an elective or emergency biliary stent exchange/removal were enrolled and clinical details of each patient were noted. The stents were retrieved through video duodenoscope and transferred into sterile containers for processing. For molecular identification of bacterial species, the encrusted material enclosed within the stent was cultured aerobically and anaerobically and the microbial DNA was extracted. PCR was standardized using the universal 16S rRNA gene-specific primers for determining the DNA sequence for commonly known bacteria. Molecular identification of unknown bacteria involved in biofilm formation was done using the Density Gradient Gel Electrophoresis. The amplified PCR products were sequenced commercially using bands which were different from commonly known bands. Data obtained after sequencing were compared with the National Center of Biotechnology Information GenBank data base, using standard nucleotide blast search tools. The major molecules in the biofilms such as protein and polysaccharide were estimated in the biofilm mass by modified Lowry’s method and anthrone method respectively. The outcome measures were quantification of biofilm protein, polysaccharides and the organisms and their relation with gender, age, etiology of biliary diseases, stent indwelling time, stent size and the presence of cholangitis. Statistical analysis for this study was performed using SPSS version 20.0 using χ2 test and Fisher’s exact test to investigate the relationship between various parameters.
Higher protein concentration in the biofilm was noted in patients with cholangitis as compared to those without cholangitis. Cholangitis and protein concentration increased the likelihood of biofilm formation in these patients explaining higher stent occlusion rates in infected bile. Male gender and age above 60 years had higher polysaccharide concentration, predisposing to higher propensity of biofilm formation. Smaller diameter stents depicted higher protein concentration predisposing to early biofilm formation thereby indicating the use of larger diameter stents. 10 Fr stents had lower concentration of protein deposition in the biofilm compared to 7 Fr stents and hence explains the longer patency rates. PCR and sequencing helped to detect several commonly known and unknown microorganisms in most of the stents. Time dependent process of biofilm formation was demonstrated by greater quantity of biofilm mass deposition on increasing length of stent indwelling time. Longer indwelling time of stents has a greater likelihood of accumulating higher biofilm formation and patients should be followed-up between 3-6 mo to avoid complications.
Presence of cholangitis at the time of stent insertion and smaller diameter of stents were found to have higher protein concentration, whereas male gender and age above 60 years had higher polysaccharide concentration, predisposing to higher propensity of biofilm formation. Longer indwelling time of stents was associated with higher biofilm formation and protein concentration elucidating the time-dependent process of biofilm formation. Our study suggests that plastic stents should be replaced between 3-6 mo. Plastic stents retrieved from patients with biliary tract disease showed polymicrobial organisms with higher protein content among patients with cholangitis and those with smaller diameter stents. Longer indwelling duration had more biofilm formation. Presence of cholangitis at the time of stent insertion and smaller diameter of stents were found to have higher protein concentration, whereas male gender and age above 60 years had higher polysaccharide concentration, predisposing to higher tendency of biofilm formation. Longer indwelling stent duration was associated with higher biofilm formation and protein concentration, revealing the time-dependent progression of biofilm formation. Longer indwelling time of stents, smaller diameter stents, male gender and age above 60 years are associated with more biofilm formation. Data on comorbidities such as diabetes in patients should be checked for predisposition, if any, of biofilm formation. Culture results of patients with cholangitis at the time of stent insertion will help to correlate with organisms grown in the biofilms. Protein concentration in the biofilm was significantly higher in patients with cholangitis, lower in the 10 Fr group than the 7 Fr group, and significantly higher in stents of ≥ 6 mo of indwelling time. Polysaccharide concentration in biofilms of stents of male gender as well as in patients with age > 60 years was significant. The most common bacteria identified by PCR alone and/or sequencing were Pseudomonas (n = 38), Citrobacter (n = 23), Klebsiella (n = 22), Staphylococcus (n = 20), Serratia (n = 16), Escherichia coli (n = 14), Streptococcus (n = 13), Enterococcus (n = 13), Aeromonas (n = 12), Proteus (n = 10) and Enterobacter (n = 9). Longer indwelling time of stents and smaller diameter stents are associated with more biofilm formation. Larger diameter (10 Fr) stents should be preferred for the relief of obstructed biliary system by a non-surgical approach in patients with benign or malignant biliary disease. Stents should not be kept in-situ for more than 3-6 mo.
Our study suggests that 10 Fr stents should be preferred over the 7 Fr stents and stents should be replaced between 3-6 mo. Attempts to prevent biofilm formation should be investigated. Ultrastructural characterization of biofilms in occluded stents should also be done.
Mr. Prashant Kapoor is acknowledged for his technical help and Ms Megha Sharma for her secretarial help.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Liu QD, Mendez-Sanchez N, Nakano H S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, Neuhaus H, Roy A, González-Huix Lladó F, Barkun AN. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385-395; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin Med Res. 2007;5:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Kwon CI, Lehman GA. Mechanisms of Biliary Plastic Stent Occlusion and Efforts at Prevention. Clin Endosc. 2016;49:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Sung JY, Leung JW, Shaffer EA, Lam K, Olson ME, Costerton JW. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J Gastroenterol Hepatol. 1992;7:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Yeoh KG, Zimmerman MJ, Cunningham JT, Cotton PB. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49:466-471. [PubMed] |
| 6. | Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-745. [PubMed] |
| 7. | Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest Endosc. 2002;56:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Prat F, Cosson C, Domingo N, Chapat O, Fompeydie D, Nassar N, Fritsch J, Choury AD, Pelletier G, Buffet C. Study of the mechanisms of biliary stent occlusion: an analysis of occluded and nonoccluded stents, with emphasis on the role of antinucleating biliary anionic Peptide factor. Endoscopy. 2004;36:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Lübbert C, Wendt K, Feisthammel J, Moter A, Lippmann N, Busch T, Mössner J, Hoffmeister A, Rodloff AC. Epidemiology and Resistance Patterns of Bacterial and Fungal Colonization of Biliary Plastic Stents: A Prospective Cohort Study. PLoS One. 2016;11:e0155479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Dowidar N, Kolmos HJ, Lyon H, Matzen P. Clogging of biliary endoprostheses. A morphologic and bacteriologic study. Scand J Gastroenterol. 1991;26:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Di Rosa R, Basoli R, Donelli A, Penni G, Salvatori A, Fiocca FM, Baldassarri FL. A microbiological and morphological study of blocked biliary stents. Microb Ecol Health Dis. 1999;11:84-88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Leung JW, Liu Y, Chan RC, Tang Y, Mina Y, Cheng AF, Silva J Jr. Early attachment of anaerobic bacteria may play an important role in biliary stent blockage. Gastrointest Endosc. 2000;52:725-729. [PubMed] |
| 13. | Ahimou F, Semmens MJ, Haugstad G, Novak PJ. Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness. Appl Environ Microbiol. 2007;73:2905-2910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Muyzer G, Brinkhoff T, NuÈ bel U, Santegoeds C, SchaÈfer H, Wawer C. Denaturing gradient gel electrophoresis (DGGE) in microbial ecology. Molecular Microbial Ecology Manual, Kluwer Academic Publishers, the Netherlands. 1997;1-27. |
| 16. | Sánchez O, Gasol JM, Massana R, Mas J, Pedrós-Alió C. Comparison of different denaturing gradient gel electrophoresis primer sets for the study of marine bacterioplankton communities. Appl Environ Microbiol. 2007;73:5962-5967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Raunkjær K, Jacobsen TH, Nielsen PH. Measurement of pools of protein, carbohydrate and lipid in domestic wastewater. Water Res. 1994;28:251-262. |
| 18. | Swidsinski A, Schlien P, Pernthaler A, Gottschalk U, Bärlehner E, Decker G, Swidsinski S, Strassburg J, Loening-Baucke V, Hoffmann U. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 2005;54:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Leung JW, Liu YL, Desta T, Libby E, Inciardi JF, Lam K. Is there a synergistic effect between mixed bacterial infection in biofilm formation on biliary stents? Gastrointest Endosc. 1998;48:250-257. [PubMed] |
| 20. | Schneider J, Hapfelmeier A, Fremd J, Schenk P, Obermeier A, Burgkart R, Forkl S, Feihl S, Wantia N, Neu B. Biliary endoprosthesis: a prospective analysis of bacterial colonization and risk factors for sludge formation. PLoS One. 2014;9:e110112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Yu JL, Andersson R, Ljungh A. Protein adsorption and bacterial adhesion to biliary stent materials. J Surg Res. 1996;62:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Chan FK, Suen M, Li JY, Sung JJ. Bile immunoglobulins and blockage of biliary endoprosthesis: an immunohistochemical study. Biomed Pharmacother. 1998;52:403-407. [PubMed] |
| 23. | Rey JF, Maupetit P, Greff M. Experimental study of biliary endoprosthesis efficiency. Endoscopy. 1985;17:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | ASGE Technology Assessment Committee, Pfau PR, Pleskow DK, Banerjee S, Barth BA, Bhat YM, Desilets DJ, Gottlieb KT, Maple JT, Siddiqui UD, Tokar JL, Wang A, Song LM, Rodriguez SA. Pancreatic and biliary stents. Gastrointest Endosc. 2013;77:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;34:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 136] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest Endosc. 2006;63:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Shatzel J, Kim J, Sampath K, Syed S, Saad J, Hussain ZH, Mody K, Pipas JM, Gordon S, Gardner T. Drug eluting biliary stents to decrease stent failure rates: A review of the literature. World J Gastrointest Endosc. 2016;8:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Yang F, Ren Z, Chai Q, Cui G, Jiang L, Chen H, Feng Z, Chen X, Ji J, Zhou L. A novel biliary stent coated with silver nanoparticles prolongs the unobstructed period and survival via anti-bacterial activity. Sci Rep. 2016;6:21714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Yamabe A, Irisawa A, Wada I, Shibukawa G, Fujisawa M, Sato A, Igarashi R, Maki T, Hoshi K. Application of a silver coating on plastic biliary stents to prevent biofilm formation: an experimental study using electron microscopy. Endosc Int Open. 2016;4:E1090-E1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |