Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1268
Peer-review started: May 30, 2016
First decision: July 29, 2016
Revised: August 26, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: February 21, 2017
Processing time: 269 Days and 21.7 Hours
To evaluate the effects of hydrotalcite combined with esomeprazole on gastric ulcer healing quality.
Forty-eight patients diagnosed with gastric ulcer between June 2014 and February 2016 were randomly allocated to the combination therapy group or monotherapy group. The former received hydrotalcite combined with esomeprazole, and the latter received esomeprazole alone, for 8 wk. Twenty-four healthy volunteers were recruited and acted as the healthy control group. Endoscopic ulcer healing was observed using white light endoscopy and narrow band imaging magnifying endoscopy. The composition of collagen fibers, amount of collagen deposition, expression of factor VIII and TGF-β1, and hydroxyproline content were analyzed by Masson staining, immunohistochemistry, immunofluorescent imaging and ELISA.
Following treatment, changes in the gastric microvascular network were statistically different between the combination therapy group and the monotherapy group (P < 0.05). There were significant differences (P < 0.05) in collagen deposition, expression level of Factor VIII and TGF-β1, and hydroxyproline content in the two treatment groups compared with the healthy control group. These parameters in the combination therapy group were significantly higher than in the monotherapy group (P < 0.05). The ratio of collagen I to collagen III was statistically different among the three groups, and was significantly higher in the combination therapy group than in the monotherapy group (P < 0.05).
Hydrotalcite combined with esomeprazole is superior to esomeprazole alone in improving gastric ulcer healing quality in terms of improving microvascular morphology, degree of structure maturity and function of regenerated mucosa.
Core tip: Gastric ulcer is mainly caused by an imbalance in defense mechanisms and injurious factors at the gastric mucosa, and has a high recurrence rate. Previous studies comparing the efficacy of hydrotalcite combined with esomeprazole vs esomeprazole therapy alone for the treatment of gastric ulcer have shown unclear results. In the present study, we conducted a clinical observation study involving 48 patients diagnosed with gastric ulcer treated with either hydrotalcite combined with esomeprazole or esomeprazole alone. The results showed that hydrotalcite combined with esomeprazole was superior to esomeprazole alone in improving gastric ulcer healing quality.
- Citation: Yang RQ, Mao H, Huang LY, Su PZ, Lu M. Effects of hydrotalcite combined with esomeprazole on gastric ulcer healing quality: A clinical observation study. World J Gastroenterol 2017; 23(7): 1268-1277
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1268
Gastric ulcer is mainly caused by an imbalance in defense mechanisms and injurious factors at the gastric mucosa. The use of Helicobacter pylori (H. pylori) eradication therapy and the wide application of proton pump inhibitors (PPI) have improved the clinical healing rate of gastric ulcer, but its recurrence rate remains high. Tarnawski et al[1] proposed the concept of quality of ulcer healing (QOUH) in 1991 for the first time, which considers the fact that tissue regeneration is often incomplete within ulcer scars. QOUH suggests that the evaluation of gastric ulcer healing should focus on whether the structure and function of mucosal and submucosal tissue have been completely regenerated in addition to endoscopic examination and evaluation of ulcer size. It has been shown that ulcer recurrence is closely related to QOUH. Esomeprazole is an H+-K+-ATP enzyme inhibitor, which can effectively inhibit acid, and thereby promotes ulcer repair. However, excessive acid suppression may cause indigestion, gastrointestinal bacterial infection and induce atrophic gastritis or even gastric cancer. Ulcers frequently recur following anti-acid drug treatment alone[2,3]. Therefore, combination therapies for gastric ulcer are favorable. To this end, hydrotalcite[4], a gastric mucosal protective agent, in which the mechanism of action includes the formation of a protective membrane at the ulcer site, provides an HCO3- reservoir and repairs the mucous bicarbonate barrier. Moreover, it upregulates various protective and repair factors in the gastric tissue such as promoting the formation of prostaglandins, improving mucosal blood flow and metabolism at the ulcer site, thereby enhancing mucosal functional repair. However, previous studies comparing the efficacy of hydrotalcite combined with esomeprazole vs esomeprazole therapy alone for the treatment of gastric ulcer did not lead to conclusive results, as these studies merely evaluated the endoscopic morphology of gastric mucosa and not the histologic and ultrastructural changes in the mucosal and submucosal layers. In addition, most studies were conducted using rat models[3,5-7]. Irregular punctate and linear gastric pits are usually abundant in normal gastric mucosa, surrounded by a rich and irregular microvascular network[8]. The morphology of gastric pits and the microvascular network may change corresponding to pathological changes in the gastric mucosa[9,10]. However, these changes cannot be observed under white light endoscopy, but are clearly visible under narrow band imaging (NBI) magnifying endoscopy[11]. Thus, in the present study, we used NBI magnifying endoscopy to observe morphological changes in the gastric pits and microvascular network of regenerated mucosa. The efficacy of hydrotalcite combined with esomeprazole in improving QOUH of adult gastric ulcers was assessed by the maturity of regenerated mucosal tissue. Eradication therapy was also administered to patients with a positive H. pylori test.
Forty-eight patients diagnosed with gastric ulcer[12] who received treatment in our hospital between June 2014 and December 2015 were randomly divided into the combination therapy group and the monotherapy group. Twenty-four healthy volunteers were recruited into the healthy control group. Exclusion criteria were as follows: (1) patients with bleeding, obstruction, perforation and cancer or other related complications of gastric ulcer; (2) complications such as heart, brain, kidney, lung and other major diseases; (3) recent treatment for gastric ulcer; and (4) contraindications to hydrotalcite or esomeprazole. All subjects were tested for H. pylori using the rapid urease test (CLO test; Delta West Pty Ltd., Western Australia). The study was approved by the ethics committee of our hospital (2014-XHNK-003) and registered in the Chinese Clinical Trial Registry (ChiCTR-IPR-16008317). Informed consent was obtained from all patients or their family members.
The combination group received oral hydrotalcite (1.0 g three times a day) combined with oral esomeprazole (20 mg twice a day), and the monotherapy group received oral esomeprazole alone (20 mg twice a day). Treatment duration was eight weeks. H. pylori-positive patients also received amoxicillin, clarithromycin and colloidal pectin bismuth for 10-14 d. The healthy control group received no treatment.
Endoscopic ulcer healing was observed first under white light endoscopy, and then gastric pits and microvascular morphology were observed using NBI magnifying endoscopy by the same experienced endoscopist. Following endoscopy, gastric mucosal tissues from patients were biopsied before and after treatment at the same location. Tissues were similarly biopsied from healthy volunteers. Part of the specimen was embedded in paraffin, sectioned and used for Masson, immunofluorescent and immunohistochemical staining. The remainder was preserved at -80 °C for subsequent determination of hydroxyproline content by ELISA.
Measurement data following normal distribution were expressed as mean ± SD. These data were compared by analysis of variance (ANOVA) followed by multiple comparisons using the LSD or Dunnett’s T3 method based on their determined homogeneity of variance. For data which were not normally distributed, the Kruskal-Wallis test was used followed by multiple comparisons by pairwise methods. Enumeration data were compared using the χ2 test. P values less than 0.05 were considered statistically significant. All data were analyzed using SPSS20.0 (SPSS Inc. United States).
No significant differences in gender and age were observed among the three groups (P > 0.05). H. pylori positive rates were similar between the combination therapy and monotherapy groups before and after treatment (P > 0.05) (Table 1).
| Group | No. | Gender | Age (yr) | H. pylori positive rate (BT) | H. pylori positive rate (AT) | Total effective rate (AT) | Gastric pits (AT) | Microvascular network changes (AT) |
| M/F | (mean ± SD)1 | n (%)2 | n (%)2 | n (%)2 | A + B (%)2 | Regular (%)2 | ||
| Healthy control | 24 | 14/10 | 55.5 ± 12.3 | 23 (95.8%) | 11 (45.8%) | |||
| Combination therapy | 24 | 13/114 | 54.5 ± 11.14 | 16 (66.7%)4 | 2 (8.3%)4 | 23 (95.8)4 | 22 (91.7%)4 | 20 (83.3%)3 |
| Monotherapy | 24 | 9/154 | 53.6 ± 11.24 | 14 (58.3%) | 3 (12.5%) | 22 (91.7) | 21 (87.5%) | 13 (54.2%) |
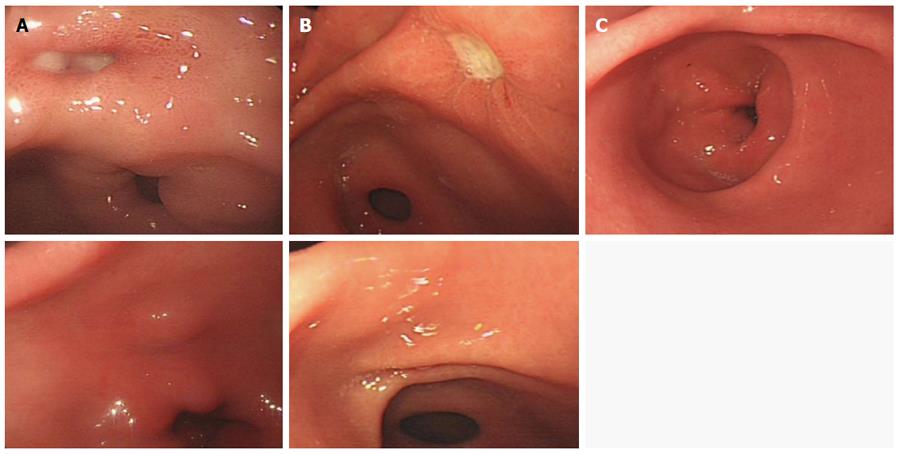
The standards of Japanese gastroscopy diagnosis and staging criteria were used[13]. A high total effective rate represents high endoscopic ulcer healing quality. The results are shown in Table 1 and Figure 1. The total effective rate was not significantly different between the combination therapy group (95.8%) and the monotherapy group (91.7%) after treatment (P > 0.05).
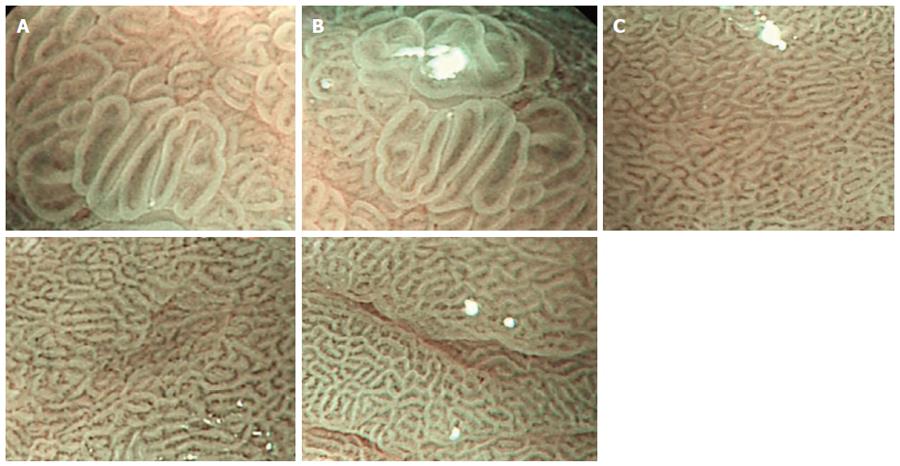
The results are shown in Table 1 and Figure 2. Based on the Sakak standards[14], most gastric pits in the normal gastric mucosa group were classified as type A and B, surrounded by a rich and irregular microvascular network, classified according to the Yao criteria[15]. In the combination therapy and monotherapy groups before treatment, gastric pits in the gastric ulcer mucosa were mostly type C and D, surrounded by a small microvascular network. The types of gastric pits and microvascular networks were not significantly different between the combination therapy and monotherapy groups before treatment (P > 0.05). After treatment, the percentage of type A and B gastric pits in the gastric mucosa was not significantly different between the combination therapy and monotherapy groups (PA+B > 0.05), but the percentage of regular microvascular network was significantly different between the two groups (P < 0.05), and was significantly higher in the combination therapy group than in the monotherapy group and normal control group.
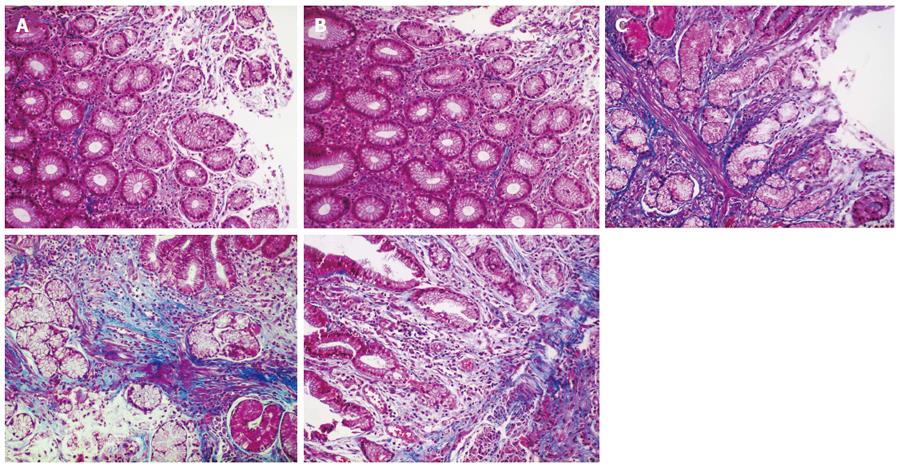
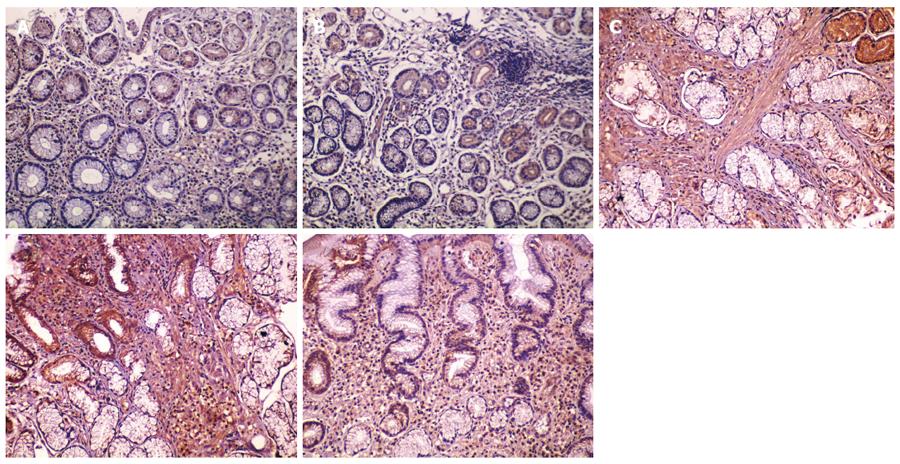
Collagen deposition area: Under normal circumstances, collagen fibers are usually distributed in the gastric mucosal and submucosal layers. The area of collagen fiber deposition was evaluated by Masson’s trichrome staining, which stains fibrotic tissues blue. Five randomly-chosen fields (× 200 times) of each specimen were imaged. All images were analyzed using Plus6.0 Image-Pro software. Collagen deposition area was measured using integral area and the average value was calculated. The area of collagen fiber deposition in the healthy control group was significantly larger than that in the combination therapy and monotherapy groups before treatment (P < 0.05). Areas of collagen fiber deposition were not significantly different between the combination therapy and monotherapy groups before treatment (P > 0.05, P = 0.951), but were significantly different after treatment (P < 0.05). Following treatment, the area of collagen fiber deposition in the combination therapy group (260909.51 ± 164713.88) was significantly higher than that in the monotherapy group (135700.75 ± 94175.38) (P < 0.05), but was not statistically significant compared with the healthy control group (244679.38 ± 210075.67) (P > 0.05) (Table 2, Figure 3).
| Group | No. | Collagen fiberarea | Collagen I to collagen III ratio | Hydroxyproline content (ng/mL) | FactorVIII-positivecells | TGF-β1expression | |||||
| mean ± SD1 | P value | mean rank2 | P value | mean ± SD1 | P value | mean ± SD1 | P value | mean ± SD1 | P value | ||
| Healthy control | 24 | 244679.38 ± | 0.0203 | 38.854 | 0.0313 | 1693.72 ± | 0.0133 | 23.25 ± 8.243 | 0.0003 | 93984.12 ± | 0.0163 |
| 210075.674 | 419.254 | 42066.644 | |||||||||
| Combination therapy (AT) | 24 | 260909.51 ± | 39.42 | 1909.53 ± | 33.54 ± 8.72 | 101163.85 ± | |||||
| 164713.88 | 828.78 | 38130.24 | |||||||||
| Monotherapy (AT) | 24 | 135700.75 ± | 25.963 | 1235.32 ± | 28.58 ± 6.943 | 72321.44 ± | |||||
| 94175.383 | 551.073 | 20030.293 | |||||||||
Ratio of collagen I to collagen III: The ratio of collagen I to collagen III in the healthy control group was significantly higher than that in the combination therapy and monotherapy groups before treatment (P < 0.05). The ratio of collagen I to collagen III was not significantly different between the combination therapy and monotherapy groups (P > 0.05, P = 0.553). In contrast, the ratio of collagen I to collagen III in the combination therapy (mean rank = 39.42) and monotherapy groups (mean rank = 25.96) after treatment were significantly different compared with the healthy control group (mean rank = 38.85) (P < 0.05, P = 0.031). The ratio in the combination therapy group was significantly higher than that in the monotherapy group (P < 0.05), but there was no statistically significant difference between the combination therapy group and the healthy control group (P > 0.05, P = 0.746), (Table 2, Figure 4).
Hydroxyproline content in gastric mucosa: Hydroxyproline content in the healthy control group was significantly higher than that in the combination therapy group and monotherapy group before treatment (P < 0.05). The difference in hydroxyproline content was not statistically significant between the combination therapy and monotherapy groups before treatment (P > 0.05, P = 0.996), but was significantly different after treatment (P < 0.05). Following treatment, hydroxyproline content in the combination therapy group (1909.53 ± 828.78 ng/mL) was significantly higher than that in the monotherapy group (1235.32 ± 551.07 ng/mL) (P < 0.05), but was not significantly different compared to the healthy control group (1693.72 ± 419.25 ng/mL), (P > 0.05, P = 0.592) (Table 2).
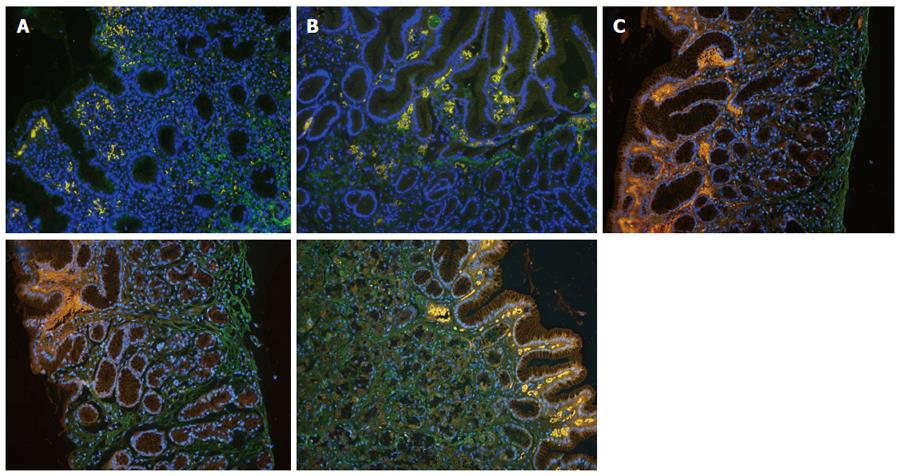
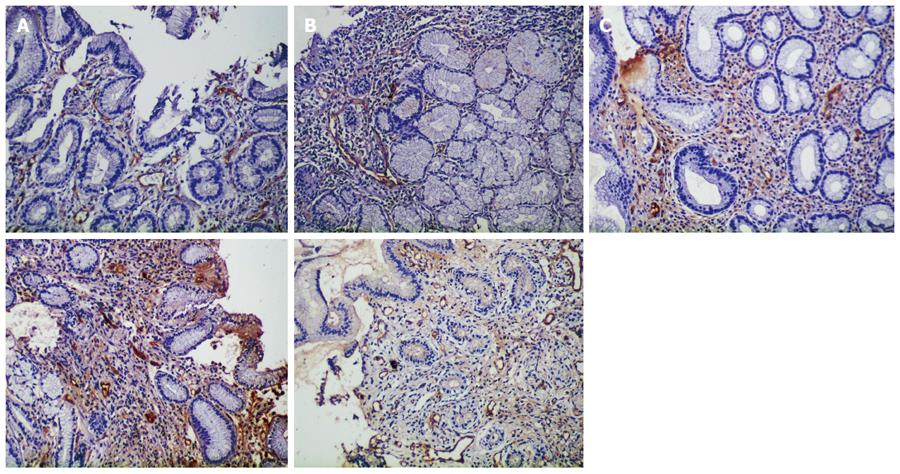
Expression of Factor VIII: The number of Factor VIII-positive cells in the healthy control group was significantly higher than that in the combination therapy and monotherapy groups before and after treatment (P < 0.05). The number of Factor VIII-positive cells was not significantly different between the combination therapy and monotherapy groups before treatment (P > 0.05, P = 0.947), while that in the combination therapy group (33.54 ± 8.72) was significantly higher than that in the monotherapy group (28.58 ± 6.94) and the healthy control group (23.25 ± 8.24) after treatment (P < 0.05), (Table 2, Figure 5).
Expression of TGF-β1: The integrated optical density (IOD) of TGF-β1 staining in the healthy control group was significantly higher than that in the combination therapy and monotherapy groups before treatment (P < 0.05). The IOD of TGF-β1 staining was not significantly different between the combination therapy and monotherapy groups (P > 0.05, P = 0.992), but was significantly different after treatment (P < 0.05). TGF-β1 expression in the combination therapy group (IOD = 101163.85 ± 38130.24) and the monotherapy group (IOD = 72321.44 ± 20030.29) after treatment was significantly different compared with the healthy control group (IOD = 93984.12 ± 42066.64) (P < 0.05). TGF-β1 in the combination therapy group was significantly higher than that in the monotherapy group (P < 0.05), but there was no statistically significant difference when compared with the healthy control group (P < 0.05), (Table 2, Figure 6).
The gastric ulcer healing process includes the clearance of necrotic debris, growth of granulation tissues, angiogenesis, collagen fiber deposition, scar tissue formation, and epithelial reconstruction with reestablishment of the mucosal microvascular network[16,17]. H. pylori eradication therapy and the wide application of PPIs have improved the clinical cure rate of gastric ulcer, but its high recurrence rate remains a clinical challenge. Gastric ulcer mainly recurs in situ or at areas adjacent to the healed ulcer. Tarnawski et al[1] showed that the recurrence of gastric ulcer was closely related to QOUH. These authors believe that compromised histological and ultrastructural healing of gastric mucosa impairs the function of mucosal defense, accounting for the pathological basis of ulcer recurrence[5]. In the present study, we observed morphological changes in gastric pits and the microvascular network of regenerated mucosa using NBI magnifying endoscopy, and evaluated the effects of hydrotalcite combined with esomeprazole on the healing quality of gastric ulcer following H. pylori eradication therapy. The results showed that hydrotalcite combined with esomeprazole improved the maturity of regenerated mucosal tissue both structurally and functionally.
This study also showed that the H. pylori-positive rate and endoscopic ulcer healing were similar between the combination therapy group and the monotherapy group after treatment. In addition, morphology of the gastric pits under the NBI magnifying endoscope was similar to the normal gastric mucosa in the combination therapy and monotherapy groups. This indicates that both treatments promoted superficial mucosal healing and improved gastric pit morphology.
Previous research[18,19] confirmed that collagen fibers in gastric tissue were mainly composed of collagen I and collagen III. Type I collagen accounts for approximately 80% of collagen fibers and strong expression of collagen I is found in newborn granulation tissue. Collagen I plays a supportive role in wound healing and excessive deposition can result in scar tissue formation. Collagen III accounts for the remaining 20% and strong expression of collagen III is found in the active capillary proliferation period, which determines the diameter and elasticity of collagen fibers. In contrast to collagen I, deposition of collagen III inhibits scar formation and regulates cell proliferation, growth and differentiation. However, over-deposition of collagen fibers in general leads to scar tissue formation and impaired physiological function. Under normal circumstances, the formation and degradation of collagen fibers are in dynamic equilibrium. Research[20] has confirmed that the ratio of collagen I to collagen III is maintained at 2:1 to 4:1 in normal tissues, which is directly related to the quality of tissue repair. Amino acids in collagen fibers consist mainly of glycine, proline and hydroxyproline[21]. Hydroxyproline is a non-essential amino acid, with just 1%[22,23] distributed in elastic proteins and almost all of the remainder found in collagen. Hydroxyproline accounts for approximately 13.4% of all amino acids in collagen[24]. It is believed that hydroxyproline is unique to collagen fibers, as hydroxyproline content is often used as a quantitative index of collagen deposition in ulcer healing[25].
This study showed that the collagen deposition area, ratio of collagen I to collagen III and hydroxyproline content in the combination therapy group were significantly higher than those in the monotherapy group after treatment. The levels of these factors in the combination therapy group after treatment were similar to those in healthy control tissues. Therefore, hydrotalcite combined with esomeprazole for the treatment of gastric ulcer promotes deposition of collagen fibers, maintains the ratio of collagen I to collagen III, and improves the histological structure of regenerated gastric tissues similar to that in normal mucosa tissues, which is consistent with the findings of Griffin et al[26]. In conclusion, hydrotalcite combined with esomeprazole was superior to esomeprazole alone in improving ulcer healing quality.
Immunohistochemical staining of factor VIII was performed to measure the number of newly generated microvessels[27,28]. We found that the combination therapy group had higher factor VIII expression and the percentage of regular microvascular network was much higher than that in the monotherapy group after treatment, according to NBI magnifying endoscopy. This may be attributed to the fact that hydrotalcite promotes the expression of various growth factors and stimulates angiogenesis, thus increasing oxygen and nutrient supply to the ulcer site to accelerate ulcer healing[4,29].
Studies have shown that the gastric ulcer healing process is regulated by various growth factors and receptors[3,30]. As a multifunctional growth factor, TGF-β1 plays an important role in tissue remodeling, extracellular matrix formation and angiogenesis, ultimately promoting ulcer repair[31,32]. This study demonstrated that the expression level of TGF-β1 in the combination therapy group was significantly higher than that in the monotherapy group after treatment, which was similar to the level in normal control tissues. This may be due to induced expression of TGF-β1 by hydrotalcite, consistent with the study by Chen et al[29].
In conclusion, hydrotalcite combined with esomeprazole is superior to esomeprazole alone in improving the healing quality of gastric ulcer. Specifically, combination therapy improved morphology of the microvascular network, promoted collagen fiber formation, angiogenesis, and the expression of TGF-β1, thereby improving the maturity of regenerated mucosal tissue as well as structure and function of the mucosa. This combination therapy is worthy of wider clinical application. We did not observe the recurrence of gastric ulcer due to our small sample size and short follow-up period. In future, a larger sample size and longer follow-up time will be included to determine whether hydrotalcite combined with esomeprazole can also reduce the recurrence rate of gastric ulcer.
Gastric ulcer is mainly caused by an imbalance in defense mechanisms and injurious factors in the gastric mucosa. The use of Helicobacter pylori eradication therapy and the wide application of proton pump inhibitors have improved the clinical healing rate of gastric ulcer, but its high recurrence rate remains a clinical challenge.
Gastric ulcers represent a public health problem. However, there are very few studies on human gastric ulcer, and most have focused on endoscopic morphology. A research hot spot is to investigate the effects of hydrotalcite combined with esomeprazole on the healing quality of human gastric ulcer in order to understand the background and trends in the treatment of gastric ulcer.
Previous studies of quality of ulcer healing (QOUH) concentrated on endoscopic morphology and not on histologic and ultrastructural changes. In addition, most studies were conducted using rat models. In the present study, the authors determined the effects of hydrotalcite combined with esomeprazole on the healing quality of gastric ulcer by observing morphological changes, and the degree of structure maturity and function of regenerated mucosa. The results showed that hydrotalcite combined with esomeprazole was superior to esomeprazole alone in improving the healing quality of gastric ulcer, specifically, the morphology of the microvascular network, collagen fiber formation, angiogenesis, and high expression of TGF-β1.
The findings of this study suggest that hydrotalcite combined with esomeprazole was superior to esomeprazole alone in improving the healing quality of gastric ulcer. Furthermore, this study also provides readers with important information regarding the best treatment for gastric ulcer. This combination therapy is worthy of wider clinical application.
Gastric ulcer is a common disease in the department of gastroenterology, and is related to acid secretion. Mucosal injury in gastric ulcer is found in the muscularis mucosa layer, which is a characteristic of chronic ulcer formation. Clinical symptoms are mainly a repeated periodic rhythm of upper abdominal pain, accompanied by belching, bloating discomfort, bleeding, perforation, and even cancer, all of which can seriously harm human health.
Available studies on QOUH of human are rare. The authors in this study analyzed the healing quality of gastric ulcer, specifically, the morphology of the microvascular network, collagen fiber formation, angiogenesis, and expression of TGF-β1. This study showed that hydrotalcite combined with esomeprazole was superior to esomeprazole alone in improving the QOUH. The results were meaningful and provided important information concerning the background and trends of treatments for gastric ulcer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bashashati M, Iida T, Lakatos PL S- Editor: Yu J L- Editor: Webster JR E- Editor: Wang CH
| 1. | Tarnawski A, Stachura J, Krause WJ, Douglass TG, Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13 Suppl 1:S42-S47. [PubMed] |
| 2. | Fornai M, Colucci R, Antonioli L, Awwad O, Ugolini C, Tuccori M, Fulceri F, Natale G, Basolo F, Blandizzi C. Effects of esomeprazole on healing of nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers in the presence of a continued NSAID treatment: Characterization of molecular mechanisms. Pharmacol Res. 2011;63:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Kangwan N, Park JM, Kim EH, Hahm KB. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J Gastrointest Pathophysiol. 2014;5:40-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Tarnawski A, Ahluwalia A, Jones MK. Gastric cytoprotection beyond prostaglandins: cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr Pharm Des. 2013;19:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Arakawa T, Watanabe T, Tanigawa T, Tominaga K, Fujiwara Y, Morimoto K. Quality of ulcer healing in gastrointestinal tract: its pathophysiology and clinical relevance. World J Gastroenterol. 2012;18:4811-4822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Slomiany BL, Piotrowski J, Czajkowski A, Yotsumoto F, Slomiany A. Gastric mucosal EGF and PDGF receptor expression with ulcer healing by ebrotidine. Am J Gastroenterol. 1994;89:894-897. [PubMed] |
| 7. | Tarnawski AS, Chai J, Pai R, Chiou SK. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig Dis Sci. 2004;49:202-209. [PubMed] |
| 8. | Nakagawa S, Kato M, Shimizu Y, Nakagawa M, Yamamoto J, Luis PA, Kodaira J, Kawarasaki M, Takeda H, Sugiyama T. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003;58:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Kawamura M, Abe S, Oikawa K, Terai S, Saito M, Shibuya D, Kato K, Shimada T, Uedo N, Masuda T. Topographic differences in gastric micromucosal patterns observed by magnifying endoscopy with narrow band imaging. J Gastroenterol Hepatol. 2011;26:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Cho JH, Chang YW, Jang JY, Shim JJ, Lee CK, Dong SH, Kim HJ, Kim BH, Lee TH, Cho JY. Close observation of gastric mucosal pattern by standard endoscopy can predict Helicobacter pylori infection status. J Gastroenterol Hepatol. 2013;28:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 11. | Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Bi WP, Man HB, Man MQ. Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol. 2014;20:17020-17028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 13. | Takemoto T, Sasaki N, Tada M, Yanai H, Okita K. Evaluation of peptic ulcer healing with a highly magnifying endoscope: potential prognostic and therapeutic implications. J Clin Gastroenterol. 1991;13 Suppl 1:S125-S128. [PubMed] |
| 14. | Sakaki N, Iida Y, Okazaki Y, Kawamura S, Takemoto T. Magnifying endoscopic observation of the gastric mucosa, particularly in patients with atrophic gastritis. Endoscopy. 1978;10:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol. 2013;26:11-22. [PubMed] |
| 16. | Tongtawee T, Kaewpitoon S, Kaewpitoon N, Dechsukhum C, Loyd RA, Matrakool L. Correlation between Gastric Mucosal Morphologic Patterns and Histopathological Severity of Helicobacter pylori Associated Gastritis Using Conventional Narrow Band Imaging Gastroscopy. Biomed Res Int. 2015;2015:808505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Okubo M, Tahara T, Shibata T, Nakamura M, Yoshioka D, Maeda Y, Yonemura J, Ishizuka T, Arisawa T, Hirata I. Changes in gastric mucosal patterns seen by magnifying NBI during H. pylori eradication. J Gastroenterol. 2011;46:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Sidgwick GP, Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol. 2012;26:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Wess TJ. Collagen fibril form and function. Adv Protein Chem. 2005;70:341-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Bonin EA, Campos AC, Coelho JC, Matias JE, Malafaia O, Jonasson TH. Effect of pantoprazole administered subcutaneously on the healing of sutured gastric incisions in rats. Eur Surg Res. 2005;37:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223-4231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 589] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 22. | Heinz A, Schräder CU, Baud S, Keeley FW, Mithieux SM, Weiss AS, Neubert RH, Schmelzer CE. Molecular-level characterization of elastin-like constructs and human aortic elastin. Matrix Biol. 2014;38:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Bochicchio B, Laurita A, Heinz A, Schmelzer CE, Pepe A. Investigating the role of (2S,4R)-4-hydroxyproline in elastin model peptides. Biomacromolecules. 2013;14:4278-4288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45:106-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 457] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 25. | Aita H, Yoneta T, Seto K, Morita H, Hori Y, Takemasa T, Chaki K, Yamada H, Seiki M, Tagashira E. [Studies on the healing promoting action of Z-103 in chronic gastric ulcer models of rats]. Nihon Yakurigaku Zasshi. 1992;99:345-352. [PubMed] |
| 26. | Griffin KJ, Thompson PA, Gottschalk M, Kyllo JH, Rabinovitch A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2014;2:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169-180. [PubMed] |
| 28. | Saenko EL, Ananyeva NM, Tuddenham EG, Kemball-Cook G. Factor VIII - novel insights into form and function. Br J Haematol. 2002;119:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Chen H, Li X, Ge Z, Gao Y, Chen X, Cui Y. Rabeprazole combined with hydrotalcite is effective for patients with bile reflux gastritis after cholecystectomy. Can J Gastroenterol. 2010;24:197-201. [PubMed] |
| 30. | Tarnawski AS, Ahluwalia A. Molecular mechanisms of epithelial regeneration and neovascularization during healing of gastric and esophageal ulcers. Curr Med Chem. 2012;19:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 31. | Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18-28. [PubMed] |
| 32. | Shih SC, Tseng KW, Lin SC, Kao CR, Chou SY, Wang HY, Chang WH, Chu CH, Wang TE, Chien CL. Expression patterns of transforming growth factor-beta and its receptors in gastric mucosa of patients with refractory gastric ulcer. World J Gastroenterol. 2005;11:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |