Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1051
Peer-review started: September 16, 2016
First decision: October 28, 2016
Revised: November 24, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: February 14, 2017
Processing time: 151 Days and 19.7 Hours
AIM
To evaluate the clinical impact of surveillance for head and neck (HN) region with narrow band imaging (NBI) in patients with esophageal squamous cell carcinoma (ESCC).
METHODS
Since 2006, we introduced the surveillance for HN region using NBI for all patients with ESCC before treatment, and each follow-up. The patients with newly diagnosed stage I to III ESCC were enrolled and classified into two groups as follows: Group A (no surveillance for HN region); between 1992 and 2000), and Group B (surveillance for HN region with NBI; between 2006 and 2008). We comparatively evaluated the detection rate of superficial head and neck squamous cell carcinoma (HNSCC), and the serious events due to metachronous advanced HNSCC during the follow-up.
RESULTS
A total 561 patients (group A: 254, group B: 307) were enrolled. Synchronous superficial HNSCC was detected in 1 patient (0.3%) in group A, and in 12 (3.9%) in group B (P = 0.008). During the follow up period, metachronous HNSCC were detected in 10 patients (3.9%) in group A and in 30 patients (9.8%) in group B (P = 0.008). All metachronous lesions in group B were early stage, and 26 patients underwent local resection, however, 6 of 10 patients (60%) in group A lost their laryngeal function and died with metachronous HNSCC.
CONCLUSION
Surveillance for the HN region by using NBI endoscopy increase the detection rate of early HNSCC in patients with ESCC, and led to decrease serious events related to advanced metachronous HNSCC.
Core tip: This is a retrospective study to evaluate the clinical impact of intensive surveillance for head and neck (HN) region by using narrow band imaging (NBI) endoscopy in patients with esophageal squamous cell carcinoma. The detection rate of superficial head and neck squamous cell carcinoma (HNSCC) which could be easily treated with endoscopic resection was dramatically increased after introduction of surveillance for HN region with NBI, and the serious events (loss of laryngeal function, death) due to metachronous advanced HNSCC were led to decrease when comparing with historical control. Surveillance for HN region with NBI might have a clinical impact at the point of reduction of head and neck cancer death in esophageal cancer survivor.
- Citation: Morimoto H, Yano T, Yoda Y, Oono Y, Ikematsu H, Hayashi R, Ohtsu A, Kaneko K. Clinical impact of surveillance for head and neck cancer in patients with esophageal squamous cell carcinoma. World J Gastroenterol 2017; 23(6): 1051-1058
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/1051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.1051
Most of patients with esophageal squamous cell carcinoma (ESCC) have a high prevalence of second primary head and neck squamous cell carcinoma (HNSCC)[1]. Matsubara et al[2] reported an assessment of the risk of a second primary cancer in patients with ESCC undergoing esophagectomy. In that report, HNSCC was the highest risk after esophagectomy and the prognosis after the detection of HNSCC was significantly unfavorable compared to that of other malignancies because of the difficulty in the early detection of HNSCC. An image-enhanced endoscopic technology system, narrow-band imaging (NBI), was reported to be useful for the detection of superficial HNSCC[3-6]. These superficial lesions are depicted clearly as well-demarcated brownish areas without magnification, and increased intraepithelial papillary capillary loops (IPCL) with irregularity are visible as the endoscopic features of superficial HNSCC with magnification[3]. Muto et al[3] reported that both detection rate and diagnostic accuracy of HNSCC were higher in NBI than in white light imaging. Furthermore, several studies have also reported that minimally invasive treatment, such as peroral endoscopic resection (ER) of superficial pharyngeal cancer, was a feasible and effective treatment with curative intent[7-9].
At the beginning of 2006, we introduced intensive surveillance program for the head and neck (HN) region, including the oropharynx, hypopharynx, and larynx, using NBI for all ESCC patients before treatment and at every follow up visit. If early HNSCCs were detected, these lesions were mainly treated with ER after the confirmation of cured esophageal cancer. However, it has not been yet clarified whether prompt detection and intervention for early HNSCC in patients with ESCC would decrease the death rate or the loss of laryngeal function related to metachronous advanced HNSCC. In this study, we compared the detection rate of early HNSCC, and the number of serious adverse events related to metachronous advanced HNSCC, in periods before and after the commencement of NBI surveillance for the head and neck region.
Patients were recruited from our database of patients who have received definitive treatments, such as ER, surgery and chemoradiotherapy (CRT), for ESCC in the National Cancer Center Hospital East. Selection criteria of this study were as follows: (1) initial treatment histologically confirmed ESCC; (2) clinical stage I to III; (3) no prior HNSCC; (4) absence of synchronous advanced cancer containing HNSCC; (5) no recurrence or metastasis of ESCC detected within 6 mo after initial ER or surgery for ESCC; (6) complete response (CR) was achieved and a recurrence after achieving CR or metastasis was not detected within 6 mo of CRT; (7) an observation period longer than a year after treatment for ESCC; and (8) provided written informed consent before all endoscopic evaluation and treatment.
Two groups were classified between the periods before and after commencement of surveillance for the HN region. ESCC patients who were treated between October 1992 and December 2000, with a follow-up until December 2004 were defined as a control group (group A). During this period, the HN region was not intensively observed to detect superficial lesion with endoscopy before treatment or during follow up periods. Group B was defined patients who were treated between January 2006 and December 2008 and followed until December 2014. These patients in group B routinely received intensive surveillance using magnified NBI endoscopy in the HN region.
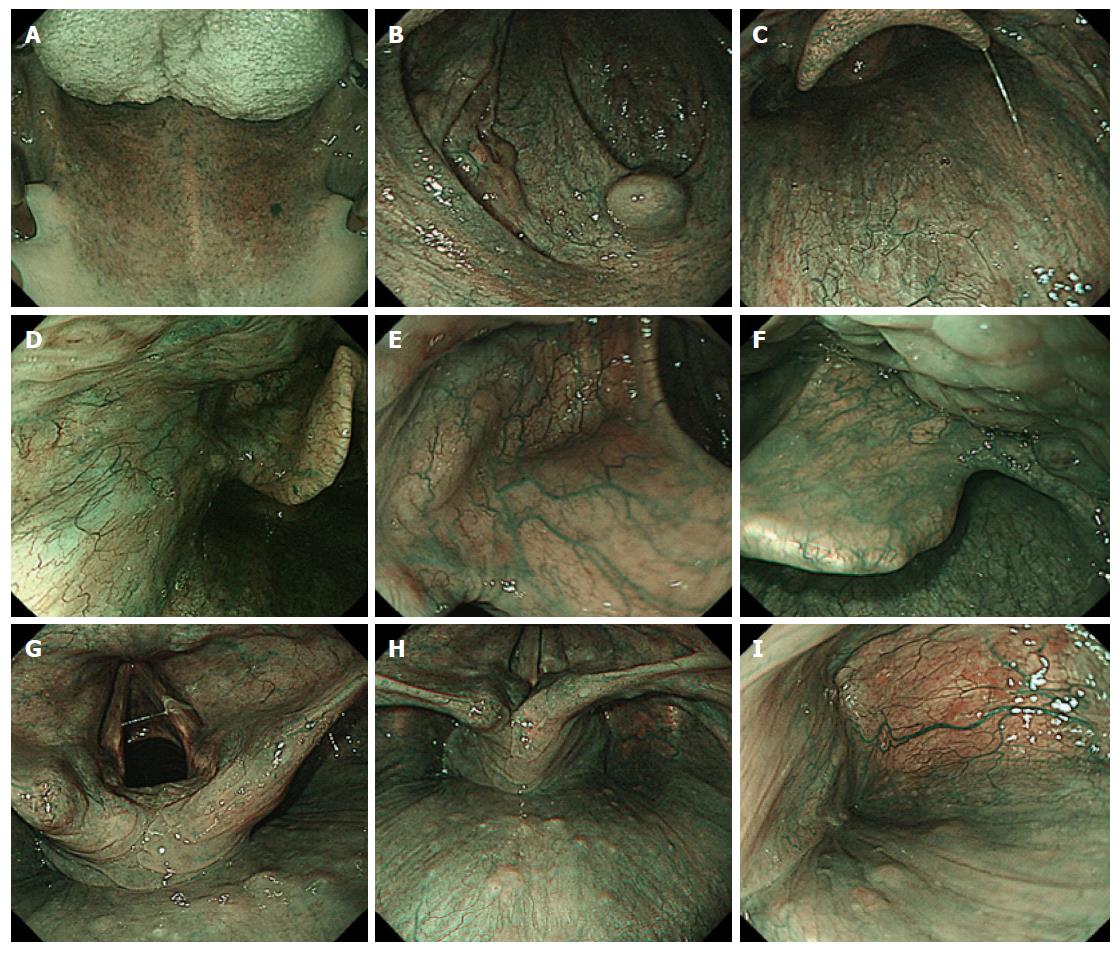
Since the patients in group A underwent conventional endoscopy with white light illumination, intensive surveillance to detect superficial HNSCC was not performed actively. In contrast, the HN regions including the oropharynx, hypopharynx and larynx, were observed using endoscope equipped with a NBI system (Olympus Medical Science, Tokyo, Japan) in group B. The endoscopic observation order in the HN region using NBI endoscopy in our hospital is shown in Figure 1[10].
The follow-up schedule of endoscopic observation after the treatment for esophageal cancer was as follows. Patients who received CRT were evaluated every 6 mo after achieving CR. The initial examination was performed at 3 mo in patients after ER, and every 6 mo thereafter, and an annual examination was performed in patients after esophagectomy.
The histologic diagnosis was made according to criteria proposed by World Health Organization[11]. Clinical staging was determined according to the Japan Society for Head and Neck Cancer, same as the TNM classification 7th edition. Superficial cancers without lymph node or distant metastasis were defined as early cancer and cancers invading muscularis propria and deeper layers were defined as advanced cancer.
Treatment for early HNSCC was provided after initial treatment for ESCC was completed. However, second primary HNSCC was not treated if the ESCC was not cured, because there was few possibility that the treatment for HNSCC affected the prognosis.
Endoscopic resection for HNSCC under general anesthesia was introduced in our hospital at the beginning of 2003. Subsequently, early HNSCC was mainly treated with ER after the confirmation of cured ESCC. When a lesion was small (approximately 10 mm in diameter or less), endoscopic mucosal resection with the cap technique was performed[8,12-14], and endoscopic submucosal dissection (ESD) was performed for larger lesions (over 10 mm in diameter)[7,9]. The procedure of ESD was as follows. A videoendoscope with a water jet system (JIFQ-260J, Olympus, Tokyo, Japan) was used for the entire procedure. And then 7 ml to 10 ml of 2.0% glycerin-free Lugol iodine solution, consisting of 2.0 g potassium iodine and 4.0 g iodine in 100 ml distilled water was sprayed to delineate the margins of the lesion. Markings were placed outside the margin of the lesion with a dual knife (Olympus KD-650L) and an electrosurgical current generator (ICC200, Erbe, Tübingen, Germany) set at 25 W for forced coagulation mode. A saline solution with epinephrine and indigo carmine dye was injected into the subepithelial layer. A circumferential incision around the lesion was performed and then the subepithelial tissue was dissected by the dual knife with 50 W current for forced coagulation mode. After the lesion was resected, a temporary tracheostomy was performed by a head and neck surgeon if laryngeal edema was severe.
The detection rate of superficial HNSCC was evaluated for each group. The incidence rate of metachronous HNSCC and serious events related to metachronous HNSCC, such as death or loss of laryngeal function, were also evaluated.
All information was collected from the medical records or was provided by the patients’ physicians. This retrospective study was approved by the institutional review board of the National Cancer Center in accordance with the Declaration of Helsinki.
SPSS Statistics 22 was used for statistical analysis. The results were expressed as medians. The Fisher’s exact test was used to analyze categorical data to compare proportions. Risks of metachronous HNSCC were estimated by using the Kaplan-Meier method. P value of < 0.05 was considered statistically significant.
A total of 470 patients with stage I to III ESCC were initially treated with definitive treatments (ER: 125, surgery: 119, CRT: 173) between October 1992 and December 2000 as group A, whereas 443 patients with stage I to III ESCC were initially treated (ER: 159, surgery: 161, CRT: 123) between January 2006 and December 2008 as group B. Patients consisting of 254 in group A and 307 in group B were recruited in this study according to the eligibility criteria. The characteristics of these patients are shown in Table 1. The male-to-female ratio, clinical stage of ESCC, and the follow up period were not significantly different between group A and group B, however, median age was significantly higher in patients of group B than in patients of group A. There was a significant difference in a treatment for ESCC in both groups (P = 0.025): the frequency of CRT were higher in group A (group A: 37%, group B: 23%).
| Group A (n = 254) | Group B (n = 307) | P value | |
| Sex | |||
| Male | 214 (84) | 260 (85) | 0.907 |
| Female | 40 (16) | 47 (15) | |
| Age (yr), median (range) | 64 (39-81) | 66 (41-86) | < 0.001 |
| Baseline clinical TNM-stage | |||
| I | 119 (47) | 155 (50) | 0.39 |
| II | 66 (26) | 88 (29) | 0.50 |
| III | 69 (27) | 64 (21) | 0.09 |
| Treatment for primary ESCC | |||
| Surgery | 84 (33) | 124 (40) | 0.079 |
| Endoscopic resection | 77 (30) | 113 (37) | 0.010 |
| Chemoradiotherapy | 93 (37) | 70 (23) | 0.001 |
| Follow-up period | |||
| Median months (range) | 60 (13-145) | 67 (12-107) | 0.150 |
Synchronous superficial HNSCC was detected in only 1 patient (0.3%) in group A. In contrast, the synchronous superficial HNSCC was found in 12 (3.9%) patients in group B (P = 0.008) (Table 2). Among these all 13 patients, 9 patients (69%) were cured of ESCC and 7 of the 9 patients with synchronous HNSCC were treated after the treatment for ESCC. In these 7 patients who were treated for HNSCC, 5 patients underwent organ preserved local resection (ER or surgery). One patient with hypopharyngeal cancer in group A underwent radiotherapy and 1 patient with hypopharyngeal cancer in group B underwent total pharyngo-laryngo-esophagectomy (TPLE) because the tumor was located in a position where treatment to preserve laryngeal function was impossible. The remaining 4 patients did not receive any treatment for synchronous HNSCC because their ESCC was not cured. Most of the patients who were cured of ESCC and received treatment for superficial HNSCC had preserved laryngeal function. No patient died due to synchronous HNSCC in both groups.
| Group A (n = 254) | Group B (n = 307) | P value | |
| Synchronous HNSCC | |||
| No. of patients | 1 (0.3) | 12 (3.9) | 0.008 |
| No. of lesions | 1 | 14 | 0.010 |
| Location of cancer | |||
| Oropharynx | 0 | 5 | |
| Hypopharynx | 1 | 8 | |
| Larynx | 0 | 1 | |
| Treatment for synchronous HNSCC | |||
| ER or surgical local resection | 0 | 7 (58) | |
| TPLE | 0 | 1 (8) | |
| Radiation and/or chemotherapy | 1 (100) | 0 | |
| No treatment | 0 | 4 (33) | |
| Death due to synchronous HNSCC | 0 | 0 |
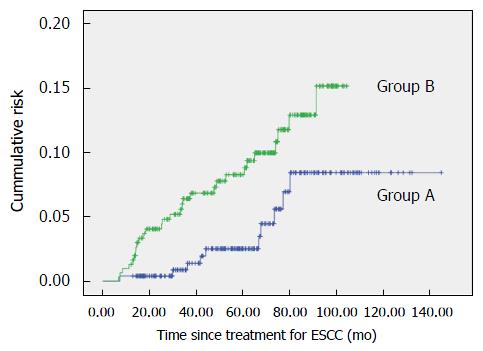
Metachronous HNSCC lesions were detected in 10 patients (3.9%) in group A and in 30 patients (9.8%) in group B (P = 0.008; Table 3). The cumulative risk of metachronous HNSCC after treatment of ESCC is shown in Figure 2. The 5-year cumulative risk of developing metachronous HNSCC after treatment for ESCC was only 2.5% in group A, whereas it was 8.7% in group B (P < 0.001).
| Group A (n = 254) | Group B (n = 307) | P value | |
| Metachronous HNSCC | |||
| No. of patients | 10 (3.9) | 30 (9.8) | 0.008 |
| No. of lesions per patients | 0.404 | ||
| 1 | 9 | 22 | |
| ≥ 2 | 1 | 8 | |
| Total number of cancers | 11 | 53 | 0.007 |
| Location of cancer | |||
| Oropharynx | 3 | 13 | |
| Hypopharynx | 7 | 34 | |
| Larynx | 1 | 6 | |
| Clinical stage | < 0.001 | ||
| I/II | 4 (36) | 53 (100) | |
| III/IV | 7 (64) | 0 | |
| Interval between ESCC and HNSCC | |||
| Median months (range) | 56 (7-80) | 31 (7-107) | 0.130 |
The characteristics of metachronous HNSCCs are shown in Table 3. Eleven metachronous HNSCC lesions were detected in 10 patients in group A, and 53 lesions in 30 patients in group B (P = 0.008). In the clinical stages of metachronous HNSCC, only 4 (36%) lesions were superficial type and stage I/II in group A, however, all 53 lesions were superficial lesions in group B (P < 0.001), and these lesions were stage I/II.
The clinical course of patients with metachronous HNSCC is shown in Table 4. There were no patients in group A who underwent ER as an initial therapy. Of 10 patients in group A, 7 (70%) who were detected metachronous HNSCC had stage III/IV HNSCC at diagnosis. In these 7 patients, only one patient who received radiotherapy achieved a cure for HNSCC.
| Group A, patients (n = 10) | Group B, patients (n = 30) | P value | |
| Metachronous HNSCC | 11 lesions | 53 lesions | < 0.001 |
| I/II | 4 (36) | 53 (100) | |
| III/IV | 7 (64) | 0 (0) | |
| Treatment | |||
| Local resection | 2 (18) | 49 (92) | < 0.001 |
| Endoscopic resection | 0 | 44 | |
| Surgical local resection | 2 | 5 | |
| TPLE | 3 (27) | 0 (0) | 0.001 |
| Radiotherapy alone and/or chemotherapy | 4 (36) | 2 (4) | 0.006 |
| No treatment | 2 (18) | 2 (4) | 0.133 |
| Laryngeal function | < 0.001 | ||
| Maintained | 4 (40) | 30 (100) | |
| Lost | 6 (60) | 0 | |
| Outcome | 0.001 | ||
| Alive | 3 (30) | 26 (87) | |
| Death | 7 (70) | 4 (13) | |
| Death with metachronous HNSCC | 6 (60) | 0 (0) | < 0.001 |
In contrast, metachronous HNSCC was found in 30 patients with 53 lesions in group B (Table 4). Furthermore, all the 53 lesions were superficial cancer alone. ER was performed in 44 of the 53 lesions and only 2 of 44 lesions had local recurrence. One of the 2 patients had re-ER and was cured, and another patient has not receive any active treatments for the superficial cancer. There were no patients who developed lymph node or distant metastasis within the observation period. In addition, 2 patients were received RT alone, and the remaining 2 were not treated for HNSCC because their ESCC recurred after the initial treatment for ESCC.
As serious events, the 7 of 10 patients in group A died due to cancer, and 6 of the 7 patients died due to metachronous HNSCC (group A: 60%, group B: 0%. P < 0.001; Table 4). Furthermore, 6 of the 10 patients (60%) in group A who were detected in metachronous HNSCC lost laryngeal function due to intensive treatment, otherwise none of the 30 patients with the 53 lesions in group B lost laryngeal function (P < 0.001).
Clinical outcome is shown in Table 5. A total of 82 (32%) patients in group A and 53 (17%) patients in group B died during the follow up periods. While there was no significant difference in the frequency of deaths due to the progression of ESCC between both groups [group A vs B: 43 (17%) vs 41 (13%), P = 0.28], the deaths related to metachronous HNSCC were more frequent in group A (group A vs B: 6 (2.4%) vs 0 (0%), P = 0.008). In contrast, other noncancerous diseases or unknown sudden death which might be late toxicity of RT for ESCC were more frequent in group A [27 (11%) vs 8 (2.6%), P < 0.001].
| Group A (n = 254) | Group B (n = 307) | P value | |
| Occurrence of advanced metachronous HNSCC | 7 (2.8) | 0 | 0.003 |
| Loss of laryngeal function | 6 (2.4) | 0 | 0.008 |
| Outcome | < 0.001 | ||
| Alive | 172 (68) | 254 (83) | |
| Dead | 82 (32) | 53 (17) | |
| ESCC | 43 (17) | 41 (13) | 0.284 |
| HNSCC | 6 (2.4) | 0 | 0.018 |
| Other cancer | 6 (2.4) | 4 (1.3) | 0.360 |
| Gastric cancer | 3 (1.2) | 0 | 0.092 |
| Lung cancer | 0 | 2 (0.7) | 0.0503 |
| Lymphoma | 1 (0.4) | 1 (0.3) | > 0.999 |
| HCC | 1 (0.4) | 1 (0.3) | > 0.999 |
| Prostate cancer | 1 (0.4) | 0 | 0.452 |
| Other/unknown | 27 (11) | 8 (2.6) | < 0.0001 |
| Radiation pneumonia | 8 (8.1) | 1 (0.3) | 0.013 |
| Heart failure | 6 (2.4) | 1 (0.3) | 0.050 |
This is the first study to investigate the clinical significance of early detection and intervention to second primary HNSCC in ESCC patients. The innovation of NBI has allowed for the early diagnosis of head and neck cancer. The NBI technique could significantly improve the efficacy of screening and surveillance of HN region, especially the lesions at oropharyngeal and hypopharyngeal mucosal sites. In previous reports, NBI screening was undertaken for the HN region (10%-13%) in ESCC patients[3,4]. In this study, we classified into two groups whether intervention of NBI surveillance was present or not, and detection rate of superficial HNSCC was clarified. Few superficial HNSCCs were detected using conventional endoscopy with white light illumination alone, however, many superficial HNSCCs were detected synchronously (3.9%) and metachronously (9.4%) after commencement of NBI surveillance. Furthermore, multiple metachronous HNSCCs were also detected. One of the main reason of lower HNSCC detection rate is considered that we did not perform NBI surveillance with magnifying endoscopy in all cases. One important point was that almost all of metachronous HNSCC could be detected as superficial cancer by NBI surveillance once from six months to one year.
Furthermore, early detection of second primary HNSCC in ESCC patients brought to minimally invasive treatment, such as peroral ER. In this study, ER was performed in 83% of second primary HNSCCs due to NBI surveillance, and these patients did not lose laryngeal function. In contrast, most of the second primary HNSCCs were detected as advanced cancers in no NBI surveillance from 1992 to 2000, 60% of patients lost laryngeal function due to invasive treatment. Several studies have reported that peroral ER of superficial HNSCC is a feasible and effective treatment with curative intent[9,15,16]. Muto et al[9] reported that local recurrence or distant metastasis after ER or superficial pharyngeal cancer were only 8% and patients who underwent ER had an excellent prognosis, with a 5-year cause-specific survival rate of 97% (95%CI: 93%-100%). We believe development of ER for cancer of oral cavity would progress along with early detection of superficial HNSCC. Loss of laryngeal function is a serious problem, and decreases quality of life in patients with second primary HNSCC. In contrast, we clarified that second primary advanced HNSCC could become the risk of death if superficial HNSCC was not detected. HNSCC was 2.4% of various death factors in no NBI surveillance, however, there was no HNSCC related death after NBI surveillance. In this study, while two groups were different periods (1992-2000 vs 2006-2008), we compared the detection rate of early HNSCC, and the number of serious adverse events related to metachronous advanced HNSCC, in periods before and after the commencement of NBI surveillance for the head and neck region. We suggested that early detection of metachronous HNSCC in ESCC patients led to minimally invasive ER without loss of laryngeal function, and avoided HNSCC related death. Regarding follow-up periods, NBI surveillance was performed in 6 mo for ER and CRT and in 1 year for operation. In our present results, 5 years have passed through NBI surveillance, however, advanced HNSCC was not detected. We believe that 6 mo follow-up periods would be appropriate.
Limitations of this retrospective study are that the data are taken from only a single institution, and historical background, the medical backgrounds of the ESCC patients, are different in each group. Moreover, it is uncertain whether the approximately 5 years of follow up in the present study is long enough to verify the serious events due to metachronous HNSCC. However, it seems impossible to conduct a randomized control study since the usefulness of endoscopic surveillance with NBI has been demonstrated.
In conclusion, endoscopic surveillance using NBI for the HN region improved detection of both synchronous and metachronous superficial HNSCC in patients with ESCC. The early detection and intervention for HNSCC might lead to the reduction of serious adverse events and the risk of death related to HNSCC.
Most of patients with esophageal squamous cell carcinoma (ESCC) have a high prevalence of second primary head and neck squamous cell carcinoma (HNSCC). The innovation of narrow-band imaging (NBI) has allowed for the early diagnosis of head and neck cancer. However, it has not been yet clarified whether prompt detection and intervention for early HNSCC in patients with ESCC would decrease the death rate or the serious events related to metachronous advanced HNSCC. In this study, the authors compared the detection rate of early HNSCC, and the number of serious adverse events related to metachronous advanced HNSCC, in periods before and after the commencement of NBI surveillance for the head and neck region.
NBI is useful to detect the early HNSCC and minimally invasive treatment, such as peroral endoscopic resection (ER) of early HNSCC, is a feasible and effective treatment with curative intent. This results of this study contribute to clarifying the clinical impact of early intervention to metachronous NHSCCs in patient with ESCC.
In this study, many HNSCCs were detected synchronously (3.9%) and metachronously (9.4%) after commencement of NBI surveillance, and all 53 lesions could be detected as early stage. These results are in agreement with previous reports. Minimally invasive treatment (ER) was performed in 83% of these second primary HNSCCs due to NBI surveillance and these patients did not lose laryngeal function or death related to HNSCC. In contrast, most of the second primary HNSCCs were detected as advanced cancers in no NBI surveillance from 1992 to 2000, 60% of patients lost laryngeal function and were died due to invasive treatment.
This study suggested that early intervention for metachronous HNSCC is useful to reduce the serious adverse events and the risk of death related to HNSCC in patient with ESCC.
NBI: A video endoscopic imaging technique that enhances the display of the microstructures and capillaries in the superficial mucosal layer using narrow band filters that change the spectral features of the observation light.
This study investigated the clinical usefulness of surveillance of head and neck cancer in patients with esophageal squamous cell carcinoma. Although the study is retrospectively performed, the results are well analyzed and clearly presented.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Iijima K, Li CJ S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Noguchi T, Kato T, Takeno S, Wada S, Yanagisawa S, Suzuki M. Necessity of screening for multiple primary cancers in patients with esophageal cancer. Ann Thorac Cardiovasc Surg. 2002;8:336-342. [PubMed] |
| 2. | Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:4336-4341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 523] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 4. | Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, Katada N, Masaki T, Nakayama M, Okamoto M. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Nonaka S, Saito Y, Oda I, Kozu T, Saito D. Narrow-band imaging endoscopy with magnification is useful for detecting metachronous superficial pharyngeal cancer in patients with esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2010;25:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Piazza C, Dessouky O, Peretti G, Cocco D, De Benedetto L, Nicolai P. Narrow-band imaging: a new tool for evaluation of head and neck squamous cell carcinomas. Review of the literature. Acta Otorhinolaryngol Ital. 2008;28:49-54. [PubMed] |
| 7. | Iizuka T, Kikuchi D, Hoteya S, Yahagi N, Takeda H. Endoscopic submucosal dissection for treatment of mesopharyngeal and hypopharyngeal carcinomas. Endoscopy. 2009;41:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Suzuki H, Saito Y, Oda I, Nonaka S, Nakanishi Y. Feasibility of endoscopic mucosal resection for superficial pharyngeal cancer: a minimally invasive treatment. Endoscopy. 2010;42:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Muto M, Satake H, Yano T, Minashi K, Hayashi R, Fujii S, Ochiai A, Ohtsu A, Morita S, Horimatsu T. Long-term outcome of transoral organ-preserving pharyngeal endoscopic resection for superficial pharyngeal cancer. Gastrointest Endosc. 2011;74:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Kaneko K, Yano T, Minashi K, Kojima T, Ito M, Satake H, Yajima Y, Yoda Y, Ikematsu H, Oono Y. Treatment strategy for superficial pharyngeal squamous cell carcinoma synchronously combined with esophageal cancer. Oncology. 2013;84:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Barnes D, Eveson JW, Reichart P. World Health Organization Classification of Tumors. Pathology and genetics. Head and Neck tumors. Lyon: IARC Press 2005; . |
| 12. | Tokyo: Kanehara 2005; Japan Society for Head and Neck Cancer, General rules for clinical studies on head and neck cancer. |
| 13. | Inoue H, Endo M, Takeshita K, Yoshino K, Muraoka Y, Yoneshima H. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc. 1992;6:264-265. [PubMed] |
| 14. | Inoue H, Tani M, Nagai K, Kawano T, Takeshita K, Endo M, Iwai T. Treatment of esophageal and gastric tumors. Endoscopy. 1999;31:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Satake H, Yano T, Muto M, Minashi K, Yoda Y, Kojima T, Oono Y, Ikematsu H, Aoyama I, Morita S. Clinical outcome after endoscopic resection for superficial pharyngeal squamous cell carcinoma invading the subepithelial layer. Endoscopy. 2015;47:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |