Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8432
Peer-review started: October 23, 2017
First decision: November 8, 2017
Revised: November 22, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: December 21, 2017
Processing time: 61 Days and 1.7 Hours
Wernicke encephalopathy (WE) is an acute neurological disorder resulting from vitamin B1 deficiency, which is common in chronic alcoholism and is rare in acute liver failure. So far, there are 2 cases of WE reported after liver transplantation. Here, we report a case of a 45-year-old nonalcoholic male patient who developed psychiatric and neurological disturbance 15 d after receiving orthotopic liver transplantation because of hepatitis B-related cirrhosis and portal hypertension. Brain magnetic resonance imaging (MRI) showed symmetric high-signal intensities in the periaqueductal area. The patient was diagnosed with WE and given intravenous high-dose vitamin B1 immediately. His neurological disturbance resolved in 7 d after receiving the vitamin B1. Brain MRI after 5 mo showed nearly complete recovery. Most WE cases may be misdiagnosed in patients after liver transplantation, and we should pay more attention to its onset.
Core tip: Wernicke encephalopathy (WE) is rare in acute liver failure. This is the third case of WE after liver transplantation reported. Most WE may be misdiagnosed in patients after liver transplantation.
- Citation: Xie B, Si ZZ, Tang WT, Qi HZ, Li T. Wernicke encephalopathy in a patient after liver transplantation: A case report. World J Gastroenterol 2017; 23(47): 8432-8436
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8432.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8432
Wernicke encephalopathy (WE) was first reported by Carl Wernicke in 1881[1] and is an acute neurological disorder resulting from vitamin B1 deficiency, which is common in chronic alcoholism. Its typical triad symptoms are ataxia, nystagmus and ophthalmoplegia, and confusion. The prevalence rate of WE has been reported as 0.4%-2.8%[2]. It was overlooked in 68% of alcoholics and 94% of nonalcoholics[3], and the mortality rate reached 20%[4]. With an increasing morbidity, nonalcoholic WE is difficult to diagnose because of its various presentation. Since WE after liver transplantation can be seen in only a few reports[5-7], we present a nonalcoholic patient who developed WE after liver transplantation.
A 45-year-old male patient was admitted to our hospital for liver transplantation because of nausea, abdominal distension for 3 mo, and unconsciousness for 3 d. His preoperative diagnoses were decompensatory cirrhosis, hepatic encephalopathy, portal hypertension and chronic hepatitis B. His hepatic encephalopathy was characterized by changes of behavior, disorientation, confusion and flapping tremor; his Glasgow coma score was 10 (E2V3M5). He underwent successful orthotopic liver transplantation with a model of end-stage liver disease score of 32.3 on January 1, 2017, and received imipenem-cilastin sodium to prevent infection, FK-506 + MMF + pred to prevent acute rejection. Because of his gastrointestinal disorder and malnutrition, parenteral nutrition was given from 3 d before transplantation till 22 d after transplantation. The components were glucose, lipids, amino acids electrolytes and insulin, while the proportion and amount differed in accordance with enteral nutrition status.
The transplanted liver recovered smoothly, without severe complications. The antibiotic therapy was discontinued 10 d after transplantation. On the 15th postoperative day, he became irritable, raving and lethargic, and appeared to gradually develop unclear enunciation, difficulty in grasping objects, and memory loss, without nystagmus or diplopia. He had no paresthesia, muscle tremor or incontinence and no dysfunction in his cardiac, respiratory and urinary system. He had no history of alcohol consumption or psychiatric disorders. At the time, his weight was 56.0 kg, being 54.8 kg before the transplantation; his height was 172 cm and his body mass index was then 18.9 kg/m2. His temperature was 36.8 °C, heart rate was 82/min, respiration was 16/min and blood pressure was 108/68 mmHg. Pupils were equal and reactive to light. In terms of laboratory examinations, blood routine test showed white blood cell count was 8.83 × 109/L, with 87.8% neutrophils and 6.2% lymphocytes, red blood cell count was 2.63 × 1012/L, hemoglobin concentration was 81 g/L, and platelet count was 83 × 109/L. Liver function test showed alanine aminotransferase of 13.3 U/L, aspartate aminotransferase of 23.3 U/L, albumin of 39.8 g/L, and total bilirubin of 17.8 μmol/L. Besides the renal function test, the serum electrolytes test, coagulation function test, blood ammonia test and arterial blood gas were normal. The random blood glucose fluctuated from 4.9 mmol/L to 8.4 mmol/L, and the tacrolimus valley point concentration was 8.1 ng/mL.
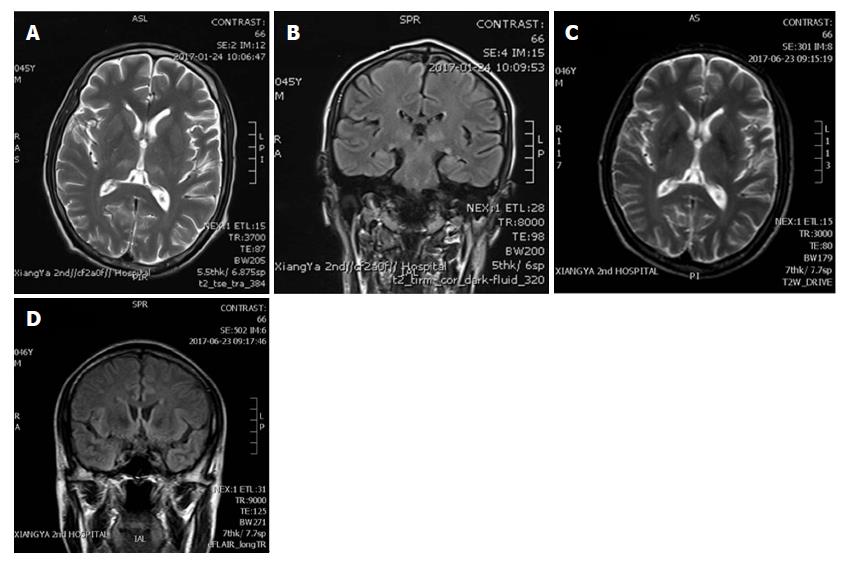
The brain magnetic resonance imaging (MRI) showed symmetrical high T1 and T2 signal intensities in thalamus and pons (Figure 1A) and high signal intensities of T2 Flair in the paraventricular area (Figure 1B). The medical history and brain MRI examination suggested WE, even though the plasma level of thiamine was not tested, and we started intravenous vitamin B1 500 mg daily for 1 wk immediately. His difficulties in speech and grasp resolved within 3 d and other neurological symptoms recovered 7 d after thiamine treatment. Considering the side effect of thiamine, he took 3 compound vitamin B tablets orally for 3 times daily for 3 mo. His general condition was good and he underwent another brain MRI 5 mo after the surgery. It showed significant reduction of previously abnormal signal in thalamus and pons (Figure 1C and D).
WE is caused by vitamin B1 (thiamine) deficiency for many reasons, such as chronic alcoholism, recurrent vomiting, parenteral nutrition, gastrointestinal surgery, cancer, liver diseases and so on. Despite some reports of WE after bone marrow transplantation[8-11], there have been few reports about WE and liver transplantation. In this case, the patient, with a long duration of liver disease, had poor feeding before the surgery due to nausea and abdominal distension, which could be a high-risk factor for WE. It could be the parenteral nutrition given postoperatively without vitamin B1 supplement that led to his WE.
Vitamin B1 is water-soluble, cannot be synthesized by our bodies but can be taken in from food, with no storage in our bodies. Thiamine pyrophosphate, the biologically active form of vitamin B1, is an important coenzyme in the tricarboxylic acid cycle that is involved in energy production[12]. Vitamin B1 deficiency will affect glucose metabolism in the brain, leading to WE because of lactic acid accumulation and acidosis, thereby interfering with neurotransmitter production, release and reuptake[13]. Supplementation of glucose and usage of glucocorticoids can increase the consumption of vitamin B1, which may aggravate WE[2]. The patient in our case received a large amount of glucose for energy since he developed postoperative hyperbilirubinemia and hypernatremia, and was given glucocorticoids to prevent acute rejection. These two therapies increased the consumption of vitamin B1 that led to WE.
The triad symptoms are well known, but only 16% of cases have complete presentations[14]. There are one or two symptoms in the other cases, which lack specificity, making early diagnosis difficult. In WE patients, the thiamine plasma level will decrease, while the blood pyruvate level will increase. But, these tests are not carried out routinely in clinical practice because of multiple interfering factors. The examination of cerebrospinal fluid in WE patients will be normal or with slightly elevated protein, which can help to differentiate it from other diseases. The electroencephalogram (EEG) may be abnormal in unconscious patients, with no specificity. The EEG may have a corresponding change in patients with peripheral neuropathy.
The brain MRI, which is the most important imaging examination, will show symmetric high T1, T2 and T2 Flair signal intensities in mammillary body, medial thalamus, periventricular and periaqueductal regions[15]. It was reported that the sensitivity and specificity of brain MRI for WE diagnosis are 53% and 93%, respectively[16]. At present, the diagnosis of WE is mainly based on the criteria proposed by the 2010 European Union of Neuroscience Association[2], including (1) dietary deficiencies; (2) eye sign; (3) cerebellar dysfunction; and (4) either an altered mental state or mild memory impairment. We can diagnose WE clinically while conforming two of the four elements. In our case, the patient, who had experienced a long period of dietary deficiency, showed gradually deepening unconsciousness and ataxia manifestation-like inappropriate movement and dysarthrosis. His brain MRI supported WE and he recovered rapidly after supplementation of vitamin B1. Above all, we definitively diagnosed WE after liver transplantation. Besides, the differential diagnoses of WE after liver transplantation includes cerebral vascular accident, adverse effects of anti-rejection drugs, hepatic encephalopathy and so on.
WE is a clinical emergency, and once diagnosed, the patient should receive vitamin B1 treatment immediately. The initial treatment requires parenteral routes, either intramuscular or intravenous, to ensure adequate absorption[17]. With a short half-life short, up to 96 min, vitamin B1 should be given three times a day or continuously by intravenous route. Till now, we have not reached a consensus on the dose and course of vitamin B1 therapy. So, the dose of vitamin B1 should be individualized based on the severity[18]. In general, alcoholic WE patients need more vitamin B1 than nonalcoholics. Vitamin B1 should be given before carbohydrates and glucocorticoids because glucose metabolism consumes vitamin B1[2].
In our case, the patient received intravenous vitamin B1 at 500 mg/d, starting immediately upon consideration of the WE diagnosis. His clinical symptoms improved and ataxia disappeared 3 d later, neurological disturbance resolved 7 d later. Then, he took compound vitamin B tablets instead of intravenous vitamin B1. The brain MRI 5 mo later revealed great improvement. The therapy of vitamin B1 supplementation was effective, without adverse reactions.
Vitamin B1 has wide safety range, since it is water-soluble and can excrete easily via the kidney. In order to prevent WE after liver transplantation, patients who had poor nutrition preoperation or needed a long fasting duration postoperation should receive intravenous vitamin B1 at 100 mg daily until returned to their normal diet.
Liver transplantation is a large-scale surgery, and the patients may have poor nutrition preoperation due to abdominal distension or nausea. Many factors increase the consumption of vitamin B1 postoperation, such as surgical stress, hypermetabolism, parenteral nutrition therapy, and use of glucocorticoids. The patients may suffer WE without extra supplementation of vitamin B1. We should consider WE when patients with high-risk factors develop disturbances of consciousness, diplopia or ataxia. Brain MRI can help substantially towards making the diagnosis. The effective treatment of WE is prompt supplementation of vitamin B1. To date, there are few relevant reports of WE after liver transplantation. As transplant doctors, we should improve awareness of this disease to avoid delaying the treatment.
A 45-year-old male patient received liver transplantation due to decompensatory cirrhosis because of hepatitis B and developed Wernicke encephalopathy on the 15th postoperative day.
The patient became irritable, raving and lethargic, and appeared to gradually develop unclear enunciation, difficulty in grasping objects, and memory loss.
Hepatic encephalopathy and adverse effects of anti-rejection drugs.
Thiamine plasma level was not tested, while the other laboratory results were close to normal.
Brain magnetic resonance imaging showed symmetrical high T1 and T2 signal intensities in thalamus and pons and high signal intensities of T2 Flair in the paraventricular area.
No pathological examination was performed.
Intravenous vitamin B1 at 500 mg daily for 1 wk and 3 compound vitamin B tablets orally 3 times daily for 3 mo.
Only 2 other cases of Wernicke encephalopathy after liver transplantation have been reported, and there have been case reports of Wernicke encephalopathy in bone marrow transplantation.
Wernicke encephalopathy is an acute neurological disorder resulting from vitamin B1 deficiency, which is common in chronic alcoholism.
We should pay more attention to Wernicke encephalopathy after liver transplantation to avoid delaying treatment. Patients who had poor nutrition preoperation or who needed a long fasting duration postoperation should receive intravenous vitamin B1 at 100 mg daily until return to their normal diet.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kita K, Salvadori M, Taheri S S- Editor: Chen K L- Editor: Filipodia E- Editor: Huang Y
| 1. | Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 206] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA; EFNS. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 394] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 3. | Day GS, del Campo CM. Wernicke encephalopathy: a medical emergency. CMAJ. 2014;186:E295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Infante MT, Fancellu R, Murialdo A, Barletta L, Castellan L, Serrati C. Challenges in Diagnosis and Treatment of Wernicke Encephalopathy: Report of 2 Cases. Nutr Clin Pract. 2016;31:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Shin NY, Nam HS, Lee SK. Hemorrhagic Wernicke encephalopathy in a patient with liver transplantation. Neurology. 2009;73:1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Benidir AN, Laughlin S, Ng VL. Visual disturbances in total parenteral nutrition dependent liver transplant pediatric patient. Gastroenterology. 2014;146:e10-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Blanco-Múñez O, Suárez-Gauthier A, Martín-García H, Díaz-Konrad V, San Antonio-Román V, Cabello A. [Unusual cortical compromise in a case of Wernicke’s encephalopathy]. Rev Neurol. 2006;42:596-599. [PubMed] |
| 8. | Majolino I, Caponetto A, Scimé R, Vasta S, Fabbiano F, Caronia F. Wernicke-like encephalopathy after autologous bone marrow transplantation. Haematologica. 1990;75:282-284. [PubMed] |
| 9. | Baek JH, Sohn SK, Kim DH, Kim JG, Lee HW, Park SP, Lee KB. Wernicke’s encephalopathy after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35:829-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Bleggi-Torres LF, de Medeiros BC, Ogasawara VS, Loddo G, Zanis Neto J, Pasquini R, de Medeiros CR. Iatrogenic Wernicke’s encephalopathy in allogeneic bone marrow transplantation: a study of eight cases. Bone Marrow Transplant. 1997;20:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, de Medeiros CR. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000;25:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Wijnia JW, Oudman E. Biomarkers of delirium as a clue to diagnosis and pathogenesis of Wernicke-Korsakoff syndrome. Eur J Neurol. 2013;20:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 433] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke’s encephalopathy: a review. Biomed Res Int. 2014;2014:503596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Elefante A, Puoti G, Senese R, Coppola C, Russo C, Tortora F, de Divitiis O, Brunetti A. Non-alcoholic acute Wernicke’s encephalopathy: role of MRI in non typical cases. Eur J Radiol. 2012;81:4099-4104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 742] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 18. | Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |