Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8387
Peer-review started: October 18, 2017
First decision: November 8, 2017
Revised: November 11, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: December 21, 2017
Processing time: 62 Days and 23.9 Hours
To investigate the impact of cigarette smoking on the recurrence rate and recurrence-free survival in patients with hyperlipidemic acute pancreatitis (HLAP).
A total of 863 patients were admitted to our hospital for acute pancreatitis (AP) from January 2013 to March 2016, of whom 88 diagnosed with HLAP were enrolled in this retrospective study. Demographic data, medical history, previous episodes of pancreatitis, consumption of alcohol and cigarettes, as well as biochemical and hematological data were carefully recorded for univariate and multivariate analyses. During follow-up, the information on current smoking status and recurrent AP was gathered. Recurrence-free survival (RFS) was calculated using the Kaplan-Meier method, and the differences between groups were compared using the log-rank test.
No significant differences were observed between the three groups in age or medical history of hyperlipidemia, fatty liver, diabetes mellitus, hypertension, or AP. The current smokers had a remarkably higher recurrence rate and a greater incidence of repeated episodes of AP (50.0% and 77.8%, respectively) than non-smokers (9.8% and 39.0%), and these two percentages were reduced to 9.1% and 36.4% for patients who gave up smoking. The median follow-up time was 13.5 mo and HLAP recurred after hospital discharge in 23 (26.1%) patients. Multivariate analysis identified current smoking (HR = 6.3, P = 0.020) as an independent risk factor contributing to HLAP recurrence. Current smokers had significantly worse RFS than non-smokers (23 mo vs 42 mo), but no significant difference was documented between ex-smokers (34 mo) and non-smokers. The RFS was not significantly different between light and heavy smokers.
Smoking is associated with worse RFS and an increased rate of HLAP recurrence. Continued smoking correlates with a compromised survival and smoking cessation should be recommended.
Core tip: This present study retrospectively enrolled hyperlipidemic acute pancreatitis (HLAP) patients in a large regional central hospital and revealed that cigarette smoking was associated with worse recurrence-free survival and an increased rate of HLAP recurrence. For smokers, continued smoking might be strongly correlated with HLAP recurrence and compromised survival. Therefore, smoking cessation should be strongly recommended.
- Citation: Xiang JX, Hu LS, Liu P, Tian BY, Su Q, Ji YC, Zhang XF, Liu XM, Wu Z, Lv Y. Impact of cigarette smoking on recurrence of hyperlipidemic acute pancreatitis. World J Gastroenterol 2017; 23(47): 8387-8394
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8387
Acute pancreatitis (AP) is a potentially life-threatening acute inflammatory condition of the pancreas with high morbidity and mortality[1]. It is widely believed that the most common etiologies of AP are gallstone disease and alcohol abuse. Currently, 12%-20% of patients with AP have previous hypertriglyceridemia, which has become a well-recognized etiology[2-4]. Hyperlipidemic acute pancreatitis (HLAP) is a form of AP occurring in the presence of severe hypertriglyceridemia and in the absence of other causes[5]. Some researchers even report hypertriglyceridemia as the underlying cause in more than half of all gestational pancreatitis cases[6]. Numerous studies have suggested that compared with other types of pancreatitis, HLAP is associated with more complications, a longer course of disease, and a higher recurrence rate[7].
The exact pathophysiology of HLAP is not entirely certain. At present, it is believed that HLAP is related to injury in the pancreatic tissue (including acinar cells and pancreatic ducts) and microcirculation disturbance caused by free fatty acids (FFAs) that are produced by the pancreatic lipase, which catalyzes decomposition of triglycerides[5]. The resulting ischemia creates an acidic environment, which further triggers FFAs toxicity. In this way, inflammation can be initiated and amplified within the pancreas. Earlier studies found that cigarette smoking was independently associated with pancreatic cancer and chronic pancreatitis (CP) by leading to pancreatic calcification and abnormal secretion of the pancreatic ducts[8-10]. Notably, some recent studies identified that smoking was significantly associated with non-biliary AP instead of biliary AP[11,12], indicating different pathophysiological mechanisms for these subtypes. However, whether cigarette smoking has any long-term impact on HLAP recurrence has not yet been investigated. Because the nicotine in tobacco can cause lipid metabolism disturbance and oxidative stress and further increase blood viscosity and microcirculation dysfunction in the pancreas[13], it is reasonable to hypothesize that smoking may be associated with the high recurrence rate of HLAP.
This present study retrospectively enrolled HLAP patients treated at a large regional central hospital. The impact of cigarette smoking on recurrence rate and recurrence-free survival (RFS) in HLAP patients was investigated.
A total of 863 patients were admitted to our hospital for AP from January 2013 to March 2016, of whom 90 were hospitalized at least twice. Among these patients, those with biliary pancreatitis, alcoholic pancreatitis, and other causes were excluded from the study. The remaining 88 patients identified with HLAP were enrolled. The diagnosis of HLAP was made if patients had AP in the presence of serum total triglyceride (TG) > 11.3 mmol/L (1000 mg/dL), or had a serum TG level of 5.65 to 11.3 mmol/L accompanied by chylous serum after excluding other known risk factors for AP. The diagnosis of AP requires at least two of the following three features: acute upper abdominal pain often radiating through to the back, serum amylase and/or lipase levels ≥ three times the upper limit of normal, and evidence of pancreatitis upon abdominal imaging[14]. The work described was carried out in accordance with The Code of Ethics of the World Medical Association. This research was approved by the Ethics Committees of the First Affiliated Hospital of Medical College, Xi’an Jiaotong University.
Data on tobacco exposure were obtained from the baseline questionnaire and telephone follow-up. All patients were asked if they smoke regularly and, if so, for how many years they had smoked and the average number of cigarettes they smoked per day. Smoking data included smoking pack-years (PY, packages of cigarettes smoked per day multiplied by the number of years for which the individual has smoked). Smoking status was defined as non-smoker (<100 cigarettes during lifetime), ex-smoker, and current smoker. An ex-smoker was defined as one who had quitted smoking for more than 6 mo before the end of follow-up after being discharged from the hospital. A current smoker was defined as one who smoked at least 1 cigarette per day for over 1 year and continued to smoke within 1 year prior to follow-up. Tobacco exposure was characterized as none, light smoker (0 < PY < 10), and heavy smoker (PY ≥ 10)[15,16]. Since alcohol and smoking are often linked behaviors[17], there are questions about the independent influence of smoking. Therefore, exposure of alcohol was also investigated and taken into consideration. In accordance with previous guidelines, a high drinker was defined as one who has drunk at least 40 g/d (20 g/d for female) for over 5 years. Smokers and alcohol abusers were routinely encouraged to give up these bad habits.
All the patients were treated with comprehensive routine therapy, including restriction of oral intake, fluid expansion, parenteral nutrition, analgesia, proton pump inhibitor administration, inhibition of pancreatic enzyme secretion, antibiotics, early enteral nutrition, and plasma exchange, if needed[18].
The information on demographic data, history of hypertriglyceridemia, fatty liver, diabetes, hypertension, previous episodes of pancreatitis, consumption of alcohol and cigarettes, as well as biochemical and hematological data were carefully recorded at admission. During follow-up, we gathered information on current smoking status and recurrent AP. Repeated episodes were defined as patients who were diagnosed with AP more than twice during their lifetime. To avoid recurrence, all patients were counseled to continue dietary fat restriction after treatment. Lipid lowering agents were used for patients when necessary.
Statistical analyses were performed with the SPSS 21.0 software package (SPSS Inc., Chicago, IL, United States). Continuous variables are expressed as the mean ± SE, and comparisons between groups were performed using non-parametric tests, the t-test, or ANOVA, as appropriate. Categorical variables were compared between groups using the χ2 test or Fisher’s exact test. RFS was calculated using the Kaplan-Meier method, and the differences between groups were compared using the log-rank test. Risk factors for the recurrence of HLAP were analyzed by univariate analysis first, and those with P < 0.20 and possible clinical effect were included in multivariate analysis using a Cox proportional hazards model. A P value < 0.05 was considered statistically significant. The statistical methods and results of this study were reviewed by a biostatistician (Qian Li, PhD) at Xi’an Jiaotong University.
Of the 88 patients with HLAP who were enrolled and analyzed (66 men and 22 women with a mean age of 40.9 ± 1.1 year), 36 and 11 patients were documented as current smokers and ex-smokers, respectively, while 41 patients had no cigarette smoking history. The characteristics of the patients in the three groups are shown in Table 1. No significant differences were observed between the three groups in age or medical history of hyperlipemia, fatty liver, diabetes mellitus, hypertension, or AP. Of the current smokers, 55.6% had a history of AP, which was significantly higher than that of non-smokers (31.7%). Cigarette smoking patients were more likely to have concomitant alcohol abuse than non-smokers (P = 0.037), and more male patients than females tended to smoke. Biochemical tests of serum TG, cholesterol (CHOL), amylase, lipase, and calcium levels were not significantly different between these groups, and nor were the leukocyte and platelet counts. Patients who currently or previously smoked had higher systemic inflammatory response syndrome (SIRS) proportions and bedside index for severity in acute pancreatitis (BISAP) scores than non-smokers, but the differences were not significant.
| Clinical | Non-smokers | Current-smokers | Ex-smokers | P value |
| parameter | No. or mean | No. or mean | No. or mean | |
| N | 41 | 36 | 11 | |
| Age (yr) | 42.7 ± 2.0 | 39.1 ± 1.2 | 40.0 ± 2.2 | 0.446 |
| Gender (M/F) | 20/21 | 36/0 | 10/1 | < 0.001a |
| Heavy alcohol drinking | 4 | 12 | 2 | 0.037a |
| Smoking pack-years | 0 | 13.5 ± 2.2 | 7.8 ± 2.9 | < 0.001a |
| History of hyperlipemia | 39 | 30 | 8 | 0.084 |
| History of fatty liver | 16 | 15 | 4 | 0.943 |
| History of diabetes mellitus | 10 | 14 | 5 | 0.258 |
| History of hypertension | 8 | 3 | 2 | 0.364 |
| History of AP | 13 | 20 | 3 | 0.065a |
| Leukocyte (× 109/L) | 12.5 ± 0.5 | 13.1 ± 0.7 | 12.3 ± 1.1 | 0.506 |
| Platelet count (× 109/L) | 185.2 ± 11.8 | 171.7 ± 8.2 | 175.6 ± 20.1 | 0.680 |
| Serum TG (mmol/L) | 18.3 ± 1.7 | 15.1 ±1.5 | 17.1 ± 2.9 | 0.358 |
| Serum CHOL (mmol/L) | 9.0 ± 0.6 | 8.4 ± 0.6 | 8.0 ± 0.8 | 0.316 |
| Serum amylase (U/L) | 569.3 ± 104.5 | 507.7 ± 111.4 | 629.8 ± 121.9 | 0.270 |
| Serum lipase (U/L) | 1297.1 ± 228.1 | 874.1 ± 170.2 | 1214.4 ± 582.0 | 0.406 |
| Serum calcium (mmol/L) | 2.0 ± 0.04 | 2.1 ± 0.04 | 2.0 ± 0.08 | 0.197 |
| SIRS | 17 | 17 | 8 | 0.182 |
| BISAP score | 0.8 ± 0.1 | 0.8 ± 0.2 | 1.4 ± 0.3 | 0.224 |
| Hospitalization (d)1 | 10.9 ± 0.8 | 8.6 ± 0.7 | 11.9 ± 1.6 | 0.015a |
| Recurrence of AP | 4 | 18 | 1 | < 0.001a |
| Repeated episodes of AP | 16 | 28 | 4 | 0.001a |
In addition, current smokers had a remarkably higher recurrence rate and a greater incidence of repeated episodes of AP (50.0% and 77.8%, respectively) than non-smokers (9.8% and 39.0%, respectively). It is worth noting that these two percentages were reduced to 9.1% and 36.4% for patients who gave up smoking after being discharged from the hospital.
The median follow-up time was 13.5 mo (2-42 mo) by September 2016. HLAP recurred after discharge from the hospital in 23 (26.1%) patients. To investigate the risk factors contributing HLAP recurrence, we examined 19 potential variables and analyzed them by univariate analysis, as shown in Table 2. Univariate analysis identified that smoking history and smoking pack-years were risk factors associated with higher HLAP recurrence. Specifically, current smoking was a risk factor relative to non-smoking (HR = 5.1, 95%CI: 1.7-15.2, P = 0.003). Smoking PY < 10 was a protective factor relative to PY ≥ 10 (HR = 0.4, 95%CI: 0.2-0.9, P = 0.035). Additionally, the following three variables had a P value < 0.20: gender (P = 0.168), history of hyperlipemia (P = 0.141), and history of AP (P = 0.117). When introducing the five variables with P value < 0.20 in univariate analysis into multivariate analysis using a Cox proportional hazards model, we identified current smoking (HR = 6.3, 95%CI: 1.3-29.8, P = 0.020) as an independent risk factor contributing to HLAP recurrence (Table 3).
| Variable | n | Number of recurrence | HR (95%CI) | P value |
| Age (≤ 40/> 40, yr) | 46/42 | 14/9 | 0.7 (0.3-1.6) | 0.417 |
| Gender (M/F) | 66/22 | 20/3 | 2.4 (0.7-7.9) | 0.168 |
| Heavy alcohol drinking (Y/N) | 18/70 | 6/17 | 1.4 (0.5-3.5) | 0.507 |
| Smoking history (Y/N) | 47/41 | 19/4 | 4.3 (1.4-12.5) | 0.009 |
| Non-smokers | 41 | 4 | 1 (ref.) | 1 (ref.) |
| Current-smokers | 36 | 18 | 5.1 (1.7-15.2) | 0.003 |
| Ex-smokers | 11 | 1 | 1.1 (0.1-9.5) | 0.959 |
| Smoking pack-years (> 0 but < 10/≥ 10) | 64/24 | 11/12 | 0.4 (0.2-0.9) | 0.035 |
| 0 | 41 | 4 | 1 (ref.) | 1 (ref.) |
| 0 < PY < 10 | 23 | 7 | 3.8 (1.1-13.1) | 0.032 |
| ≥ 10 | 24 | 12 | 4.6 (1.5-14.2) | 0.009 |
| History of hyperlipemia (Y/N) | 77/11 | 18/5 | 0.5 (0.2-1.3) | 0.141 |
| History of fatty liver (Y/N) | 35/53 | 9/14 | 1.0 (0.4-2.2) | 0.917 |
| History of diabetes mellitus (Y/N) | 29/59 | 10/13 | 1.7 (0.7-3.9) | 0.208 |
| History of hypertension (Y/N) | 13/75 | 2/21 | 0.7 (0.2-2.9) | 0.585 |
| History of AP (Y/N) | 36/52 | 13/10 | 1.9 (0.8-4.4) | 0.117 |
| Leukocyte (< 10/≥ 10, × 109/L) | 23/65 | 5/18 | 0.6 (0.2-1.7) | 0.379 |
| Platelet count (< 100/≥ 100, × 109/L) | 4/84 | 1/22 | 0.8 (0.1-5.7) | 0.790 |
| Serum TG (≤ 11.3/> 11.3, mmol/L) | 32/56 | 7/16 | 0.9 (0.4-2.2) | 0.813 |
| Serum CHOL (≤ 5.5/> 5.5, mmol/L) | 16/72 | 4/19 | 1.0 (0.3-2.8) | 0.934 |
| Serum amylase (< 540/≥ 540, U/L) | 62/26 | 18/5 | 1.6 (0.6-4.4) | 0.335 |
| Serum lipase (< 600/≥ 600, U/L)1 | 31/34 | 8/9 | 0.6 (0.2-1.5) | 0.273 |
| Serum calcium (< 2.0/≥ 2.0, mmol/L) | 28/60 | 8/15 | 1.0 (0.4-2.3) | 0.918 |
| SIRS (Y/N) | 42/46 | 11/12 | 1.1 (0.5-2.4) | 0.858 |
| BISAP score (< 2/≥ 2) | 69/19 | 19/4 | 1.3 (0.5-3.9) | 0.610 |
| Variable | Hazard ratio | 95%CI | P value |
| Gender (Male) | 0.5 | 0.1-3.0 | 0.449 |
| Smoking history (Current) | 6.3 | 1.3-29.8 | 0.020 |
| Smoking pack-years (≥ 10) | 1.0 | 0.4-2.7 | 0.969 |
| History of hyperlipemia (Yes) | 0.7 | 0.2-1.8 | 0.435 |
| History of AP (Yes) | 1.4 | 0.6-3.3 | 0.422 |
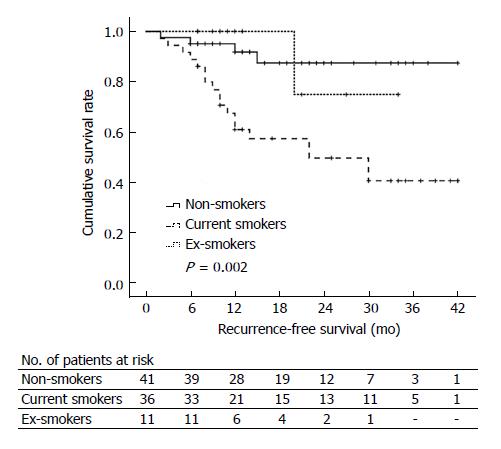
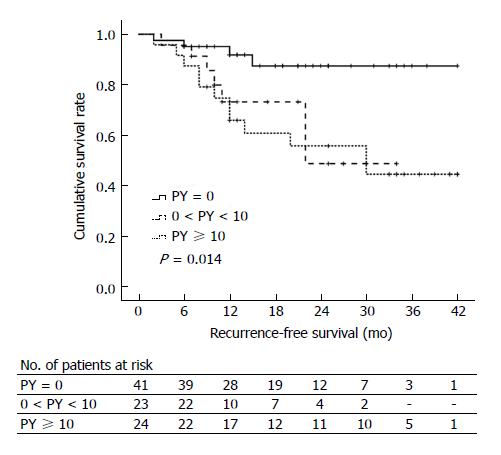
During follow-up, four non-smoker patients, one ex-smoker patient, and 18 current smoker patients experienced AP recurrence with different RFS time. Figure 1 shows the Kaplan-Meier curves for RFS of non-, ex-, and current smokers. The median RFS time in non-smokers, ex-smokers, and current smokers was 42 mo, 34 mo, and 23 mo, respectively (P = 0.002). Current smokers had significantly worse RFS than non-smokers (P = 0.001), but no significant difference in RFS was documented between ex-smokers and non-smokers (P = 0.962). Kaplan-Meier curves for RFS in non-smokers (PY = 0), light smokers (0 < PY < 10), and heavy smokers (PY ≥ 10) are shown in Figure 2. The median RFS of non-smokers, light smokers, and heavy smokers was 42 mo, 23 mo, and 30 mo, respectively, indicating that the RFS was significantly worse in smokers than in non-smokers (P = 0.014). However, the RFS was not significantly different between light and heavy smokers (P = 0.749).
In the present study, univariate and multivariate analyses identified that cigarette smoking status is an independent risk factor contributing to the recurrence of HLAP[19]. To the best of our knowledge, this is the first study evaluating the influence of cigarette smoking on HLAP recurrence. A recent comprehensive study analyzed 2810 patients (1065 with gallstone-related AP, 1222 with non-gallstone related AP, and 523 with recurrent AP/CP) and demonstrated that cigarette smoking was associated with non-gallstone-related AP and recurrent AP/CP[12]. Unfortunately, this study did not evaluate any correlation between cigarette smoking and HLAP recurrence. As non-gallstone-related AP was a broad category including various causes and pathogeneses, it was difficult to analyze the pathophysiologic mechanism of cigarette smoking leading to recurrent HLAP. The most significant difference between the study by Setiawan et al[12] and ours is that our study takes HLAP into consideration as a specific disease to evaluate the effects of cigarette smoking on HLAP recurrence.
Notably, current smokers appeared to be associated with a higher recurrence rate and a greater incidence of repeated episodes of AP. In addition, the RFS was significantly worse in smokers than in non-smokers. However, the RFS was not significantly different between light and heavy smokers. This finding implied that cigarette smoking might contribute to HLAP recurrence but not in a dose- or duration-dependent manner; however, this could not be fully demonstrated in the present study due to short follow-up period and the small number of patients enrolled. Rebours et al[9] demonstrated in their study that alcoholic CP occurred earlier at a 20-pack-year threshold, and that patients had more calcifications. Similar results were observed in other research[8,11,20] with regard to CP or AP. Therefore, the importance of smoking in the recurrence of different AP cases might differ. Whether the level of tobacco exposure influences HLAP recurrence and RFS needs to be further evaluated.
Interestingly, ex-smokers who had quitted smoking for at least 6 mo after discharge from the hospital had a significant advantage with regard to the recurrence rate and RFS compared to current smokers in the present study. Univariate and multivariate analyses also demonstrated that current smoking was an independent risk factor contributing to HLAP recurrence. Therefore, lifestyle modifications are a key feature in the long-term management of HLAP[21,22]. Due to a possible increase in recurrence rate related to smoking, doctors should always strongly suggest that the patient quit smoking once HLAP has been diagnosed. Moreover, current smokers had a higher proportion of concomitant alcohol abuse than non- & ex-smokers in our cohort. We also entered ‘‘high alcohol drinking’’ as a potential variable influencing HLAP recurrence[23]. Although there were no data indicating a potential influence of high alcohol consumption on HLAP recurrence, it was recognized as a synergistic factor with smoking for AP occurrence and a negative prognostic factor for AP survival[3,24,25]. However, only a small sample of alcohol drinkers were enrolled in our study since alcohol drinking is not as common among the Chinese population as in Western populations, which might contribute to bias in this data analysis.
Several studies have shown unique molecular characteristics and behavior patterns of pancreatic cancer related to tobacco use. However, the mechanism by which cigarette smoking promotes HLAP recurrence remains unknown. Some experimental and clinical studies indicated that cigarette smoke aggravates pancreatic acinar cell injury and pancreatic calcification by increasing oxidative stress and the production of pro-inflammatory cytokines[8,20,26,27]. Nicotine, the main poisonous element of tobacco, might accumulate significantly in the pancreas and participate in regulating lipid peroxidation, resulting in HLAP and HLAP recurrence.
There are several limitations in our present study. First, measurement error in self-reported tobacco use is possible and may have led to some degrees of nondifferential misclassification of exposure. Second, more-detailed classification is needed to clarify the potential dose- and duration-dependent correlation between cigarette smoking and HLAP. Finally, the number of cases within our exposure categories by pancreatitis type is small, and long-term follow-up is needed.
In the present study, we found that cigarette smoking was associated with worse RFS and an increased recurrence rate of HLAP. For smokers, continued smoking might be strongly correlated with HLAP recurrence and compromised survival. Therefore, smoking cessation should be strongly recommended. Future studies are needed to clarify possible changes in the metabolic and molecular characteristics of HLAP related to tobacco use and to determine whether these changes contribute to disease recurrence.
Hyperlipidemic acute pancreatitis (HLAP) is a form of AP occurring in the presence of severe hypertriglyceridemia and in the absence of other causes. The exact pathophysiology of HLAP is not entirely certain. It is believed that HLAP is related to pancreatic tissue injury and microcirculation disturbance caused by free fatty acids. Some recent studies identified that smoking was significantly associated with non-biliary AP instead of biliary AP, but whether cigarette smoking has any long-term impact on HLAP recurrence has not yet been investigated. This is the first study evaluating the influence of cigarette smoking on HLAP recurrence.
Authors performed this study to better understand the relationship between cigarette smoking and HLAP recurrence, as well as the pathophysiologic mechanism of recurrent HLAP.
The main objective of this study was to investigate the impact of cigarette smoking on the recurrence rate and recurrence-free survival in HLAP. The authors found that cigarette smoking was associated with worse RFS and an increased recurrence rate of HLAP. These findings provide references for further clarifying the mechanism of HLAP.
A total of 88 patients diagnosed with HLAP were enrolled in this retrospective study. Demographic data, medical history, previous episodes of pancreatitis, consumption of alcohol and cigarettes, as well as biochemical and hematological data were carefully recorded for univariate and multivariate analyses. During follow-up, the information on current smoking status and recurrent AP was gathered. Recurrence-free survival was calculated using the Kaplan-Meier method, and the differences between groups were compared using the log-rank test.
Current smokers had a remarkably higher recurrence rate and a greater incidence of repeated episodes of AP than non-smokers, and these two percentages were reduced to 9.1% and 36.4% for patients who gave up smoking. The median follow-up time was 13.5 mo. Multivariate analysis identified current smoking as an independent risk factor contributing to HLAP recurrence. Current smokers had significantly worse RFS than non-smokers, but no significant difference was documented between ex-smokers and non-smokers.
In the present study, the authors found that cigarette smoking was associated with worse RFS and an increased recurrence rate of HLAP. For smokers, continued smoking might be strongly correlated with HLAP recurrence and compromised survival. Smoking cessation for at least 6 mo would lead to a significant advantage in recurrence rate and RFS compared to current smokers.
The study revealed that smoking is associated with worse RFS and higher recurrence rate of HLAP. Besides, smoking cessation for at least 6 mo would lead to a significant advantage in recurrence rate and RFS compared to current smokers. For the future research, more detailed classification is needed to clarify the potential dose- and duration-dependent correlation between cigarette smoking and HLAP. Besides, expanding the number of cases and long-term follow-up are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bradley EL, Gonzalez-Ojeda AG S- Editor: Chen K L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 518] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 2. | Chang MC, Su CH, Sun MS, Huang SC, Chiu CT, Chen MC, Lee KT, Lin CC, Lin JT. Etiology of acute pancreatitis--a multi-center study in Taiwan. Hepatogastroenterology. 2003;50:1655-1657. [PubMed] |
| 3. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1344] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 4. | Yin G, Cang X, Yu G, Hu G, Ni J, Xiong J, Hu Y, Xing M, Chen C, Huang Y. Different Clinical Presentations of Hyperlipidemic Acute Pancreatitis: A Retrospective Study. Pancreas. 2015;44:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 6. | Papadakis EP, Sarigianni M, Mikhailidis DP, Mamopoulos A, Karagiannis V. Acute pancreatitis in pregnancy: an overview. Eur J Obstet Gynecol Reprod Biol. 2011;159:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Qiu L, Sun RQ, Jia RR, Ma XY, Cheng L, Tang MC, Zhao Y. Comparison of Existing Clinical Scoring Systems in Predicting Severity and Prognoses of Hyperlipidemic Acute Pancreatitis in Chinese Patients: A Retrospective Study. Medicine (Baltimore). 2015;94:e957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Law R, Parsi M, Lopez R, Zuccaro G, Stevens T. Cigarette smoking is independently associated with chronic pancreatitis. Pancreatology. 2010;10:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Rebours V, Vullierme MP, Hentic O, Maire F, Hammel P, Ruszniewski P, Lévy P. Smoking and the course of recurrent acute and chronic alcoholic pancreatitis: a dose-dependent relationship. Pancreas. 2012;41:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Maisonneuve P, Lowenfels AB, Müllhaupt B, Cavallini G, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L, Frulloni L. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Sadr-Azodi O, Andrén-Sandberg Å, Orsini N, Wolk A. Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut. 2012;61:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Setiawan VW, Pandol SJ, Porcel J, Wilkens LR, Le Marchand L, Pike MC, Monroe KR. Prospective Study of Alcohol Drinking, Smoking, and Pancreatitis: The Multiethnic Cohort. Pancreas. 2016;45:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Chowdhury P, Walker A. A cell-based approach to study changes in the pancreas following nicotine exposure in an animal model of injury. Langenbecks Arch Surg. 2008;393:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4323] [Article Influence: 360.3] [Reference Citation Analysis (45)] |
| 15. | Shih WL, Chang HC, Liaw YF, Lin SM, Lee SD, Chen PJ, Liu CJ, Lin CL, Yu MW. Influences of tobacco and alcohol use on hepatocellular carcinoma survival. Int J Cancer. 2012;131:2612-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Zhang XF, Wei T, Liu XM, Liu C, Lv Y. Impact of cigarette smoking on outcome of hepatocellular carcinoma after surgery in patients with hepatitis B. PLoS One. 2014;9:e85077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Di Leo M, Leandro G, Singh SK, Mariani A, Bianco M, Zuppardo RA, Goni E, Rogger TM, Di Mario F, Guslandi M. Low Alcohol and Cigarette Use Is Associated to the Risk of Developing Chronic Pancreatitis. Pancreas. 2017;46:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Yin G, Hu G, Cang X, Yu G, Hu Y, Xing M, Chen C, Huang Y, Tang M, Zhao Y. C-reactive protein: rethinking its role in evaluating the severity of hyperlipidemic acute pancreatitis. Pancreas. 2014;43:1323-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Sun X, Huang X, Zhao R, Chen B, Xie Q. Meta-analysis: Tobacco smoking may enhance the risk of acute pancreatitis. Pancreatology. 2015;15:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Coté GA, Yadav D, Slivka A, Hawes RH, Anderson MA, Burton FR, Brand RE, Banks PA, Lewis MD, Disario JA. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266-273; quiz e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. Am J Gastroenterol. 2004;99:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Lindkvist B, Appelros S, Manjer J, Berglund G, Borgstrom A. A prospective cohort study of smoking in acute pancreatitis. Pancreatology. 2008;8:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Munigala S, Conwell DL, Gelrud A, Agarwal B. Heavy Smoking Is Associated With Lower Age at First Episode of Acute Pancreatitis and a Higher Risk of Recurrence. Pancreas. 2015;44:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Kristiansen L, Grønbaek M, Becker U, Tolstrup JS. Risk of pancreatitis according to alcohol drinking habits: a population-based cohort study. Am J Epidemiol. 2008;168:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 26. | Chowdhury P, Bose C, Udupa KB. Nicotine-induced proliferation of isolated rat pancreatic acinar cells: effect on cell signalling and function. Cell Prolif. 2007;40:125-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Greer JB, Thrower E, Yadav D. Epidemiologic and Mechanistic Associations Between Smoking and Pancreatitis. Curr Treat Options Gastroenterol. 2015;13:332-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |