Published online Dec 21, 2017. doi: 10.3748/wjg.v23.i47.8283
Peer-review started: October 29, 2017
First decision: November 21, 2017
Revised: November 27, 2017
Accepted: December 4, 2017
Article in press: December 4, 2017
Published online: December 21, 2017
Processing time: 52 Days and 10.3 Hours
The measurement of procalcitonin has recently become a mainstay for the diagnosis and therapeutic management of severe bacterial infections, especially those sustained by Gram-negative bacteria. Therefore, the aim of this article is to provide a narrative overview on the potential role of procalcitonin measurement in patients with inflammatory bowel disease (IBD). According to the available scientific literature, the clinical significance of procalcitonin for diagnosing IBD or monitoring disease activity remains elusive, and its association with disease severity is confined to a limited number of case-control studies, with low sample size. Nevertheless, literature data also suggests that a supranormal procalcitonin serum concentration (i.e., > 0.5 ng/mL) may reflect the presence of a number of infective complications in IBD, especially bacterial enterocolitis, bacterial gastroenteritis, intraabdominal abscess, postsurgical infection and sepsis. Rather than for diagnosing or assessing disease activity, the measurement of this biomarker may hence retain practical clinical significance for early prediction, timely diagnosis and therapeutic monitoring of many IBD-associated infections and complications.
Core tip: According to current evidence, the clinical significance of measuring procalcitonin for diagnosing intestinal bowel disease (IBD) or monitoring disease activity remains elusive. Nevertheless, literature data suggests that supranormal procalcitonin concentrations may reflect the presence of a number of infective complications in IBD, including bacterial enterocolitis, bacterial gastroenteritis, intraabdominal abscess, postsurgical infection and sepsis. Rather than for assessing disease activity, the measurement of this biomarker may hence retain clinical significance for predicting or timely diagnosing of many IBD-associated infections and complications.
- Citation: Lippi G, Sanchis-Gomar F. Procalcitonin in inflammatory bowel disease: Drawbacks and opportunities. World J Gastroenterol 2017; 23(47): 8283-8290
- URL: https://www.wjgnet.com/1007-9327/full/v23/i47/8283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i47.8283
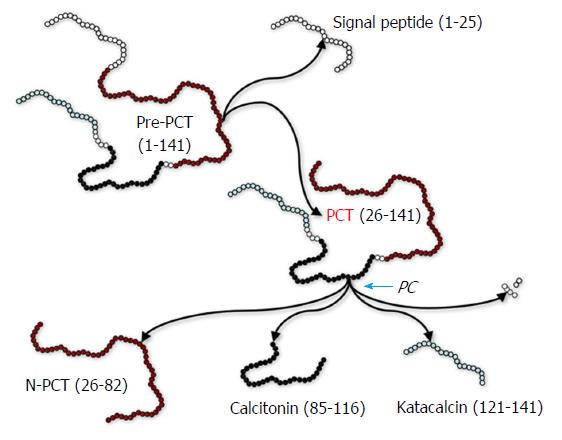
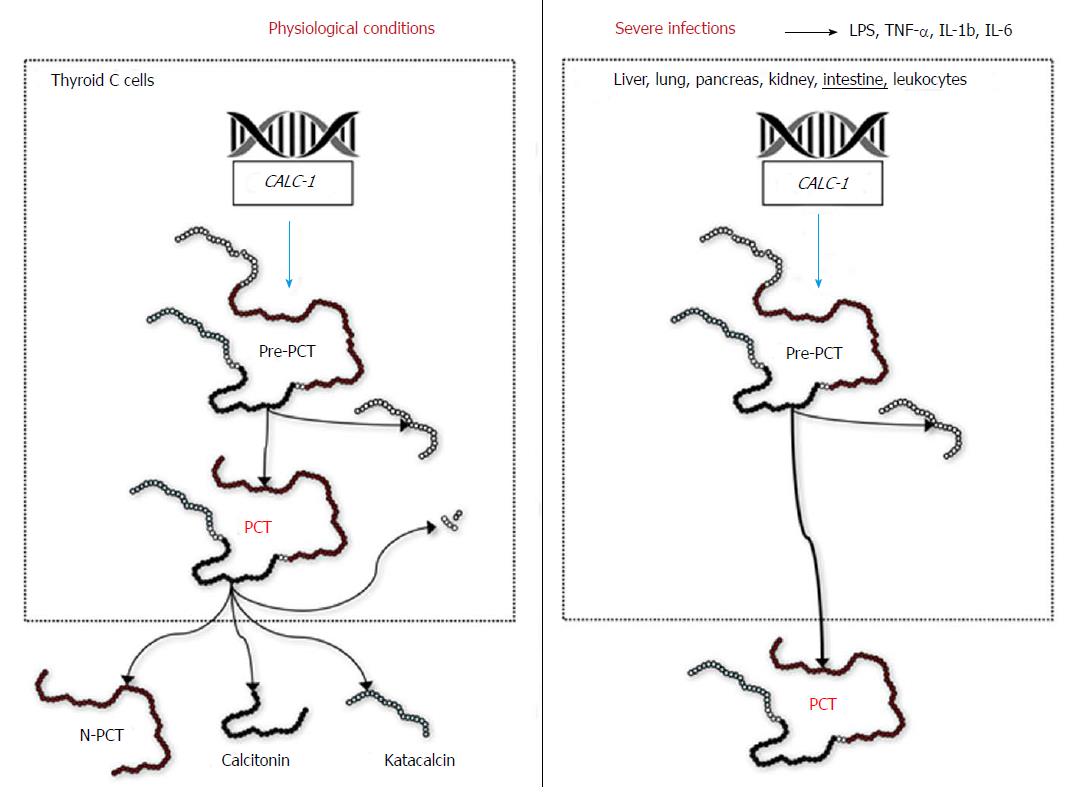
Procalcitonin is the precursor of calcitonin, an essential hormone involved in calcium homeostasis. In physiological conditions, thyroid C cells synthesize pre-procalcitonin, a 141 amino acids precursor of calcitonin, which is then rapidly converted into procalcitonin (116 amino acids) by endopeptidases-catalyzed removal of the 25-amino acid signal sequence[1]. Procalcitonin is then converted into the circulation by the enzyme prohormone convertase (PC) in the mature hormone calcitonin (32 amino acids), N-terminal procalcitonin (57 amino acids) and katacalcin (21 amino acids) (Figure 1)[1]. In physiological conditions, procalcitonin has a very low blood concentration (typically < 0.05 ng/mL) (Figure 2). Nevertheless, in patients with severe bacterial infections, especially in those with systemic infections and sepsis, an extra-thyroid synthesis of procalcitonin occurs in several organs, such as liver, lung, pancreas, kidney and intestine, as well as in leukocytes (Figure 2)[2]. Consequently, its circulating concentration can be enhanced from 100-fold to 10000-fold over. For this reason, finding blood levels of procalcitonin beyond 100 ng/mL is commonplace in patients with sepsis, with the magnitude of such increase often correlating with both the severity of infections and prognosis.
The mechanisms leading to an enhanced extra-thyroid production is prevalently attributable to both direct and indirect bacterial stimulation of the calcitonin gene CALC-1 (directly triggered by endotoxin and other bacterial toxins, or indirectly caused by the metabolic reaction of the organism in response to infection), but is also due to reduced cleavage of the protein into calcitonin, N-terminal procalcitonin and katacalcin (Figure 2)[3]. Notably, procalcitonin synthesis is mostly inhibited (blocked) by interferon-γ in viral infections, so that its concentration remains usually low[4].
In patients with severe bacterial infections, increased synthesis of procalcitonin typically occurs within 2-4 h from the onset of sepsis, reaching peak blood values 6 to 8 h afterward and persisting as long as the inflammatory process continues, regardless of preserved or impaired renal function[5]. The half-life of procalcitonin is usually comprised between 20-24 h. Several lines of evidence suggest that procalcitonin kinetics in the blood may provide more useful clinical information than its absolute value. An increase of serum or plasma procalcitonin values over time is associated with worse prognosis, whilst decreasing levels mirrors improved clinical outcome and/or therapeutic effectiveness.
Procalcitonin was originally identified as a useful marker of severe systemic infections in 1993 by Assicot et al[6], who studied 79 children with suspected infections and showed that procalcitonin value was substantially increased in those with sepsis and procalcitonin serum concentration was also strongly correlated with disease severity and complications. Since then, many other clinical studies and meta-analyses have confirmed the considerable value of this biomarker for early diagnosis, prognostication and even therapeutic management of patients with sepsis and septic shock[7]. Albeit its consolidated role in severe systemic infections, the role of procalcitonin in localized infections has remained less conclusive[8,9]. Nevertheless, recent data suggest that procalcitonin measurement may be clinically useful in patients with bacterial meningitis[10], community-acquired pneumonia[11], erysipelas[12] and arthritis[13].
In all these conditions procalcitonin measurement is now regarded as a first-line screening test for timely identification of bacterial infections and to facilitate rapid establishment of an antibiotic treatment. Notably, the results of the vast majority of microbiological tests cannot be immediately available, so that the severity of the infection may progress unless a final diagnosis can be made. Procalcitonin has many advantages in this respect, since its measurement may allow for identifying infections with minimal host response, is sufficiently specific for discriminating bacterial infections from other severe stimuli that may also induce systemic inflammatory response syndrome, is present early during the course of disease, can be timely and conveniently assayed and, finally, may also provide prognostic information[4,8].
With regards to gastrointestinal infectious disorders, the combination of procalcitonin with symptoms and conventional laboratory tests yielded an improved diagnostic or prognostic accuracy in patients with bacterial pancreatitis[14], acute bacterial appendicitis[15], gastroenteritis[16], ascites[17], intestinal ischemia[18], bacterial peritonitis[19], and other intraabdominal bacterial infections[20]. Controversial evidence has been published about the role of procalcitonin measurement in patients with inflammatory bowel disease (IBD), as thoughtfully discussed in the next section of this narrative review.
An electronic literature search was performed in Embase, MEDLINE (PubMed interface) and Web of Science to identify eligible literature from the earliest available date to October 24, 2017. The following search terms were used: “inflammatory bowel disease” OR “Crohn’s disease” OR “ulcerative colitis” AND “procalcitonin” in title, abstract and keywords, with no language restriction. Review articles, letters to the editor, editorials and original articles were evaluated, and their list of references was also hand-searched to identify additional articles about this topic. The electronic searches returned 29 documents, from which 10 original articles and 1 meeting abstract were finally selected according to their clinical relevance.
The first study assessing the role of procalcitonin in IBD was published by Korczowski et al[21] in 2004. The serum concentration of procalcitonin and C-reactive protein (CRP) was measured in 30 healthy controls and 129 children hospitalized with diarrhea of various origin, which also included 13 children with IBD. Procalcitonin values were found to be higher than the diagnostic cutoff (i.e., 0.5 ng/mL) in 23% children (3/13) with IBD versus 0% of healthy controls (P = 0.019). Moreover, the percentage of children in the overall cohort with bacterial enterocolitis displaying increased serum procalcitonin concentration was as high as 61%.
In the same year, Herrlinger et al[22] published another study including 51 IBD patients [26 with Crohn’s disease (CD), 25 with ulcerative colitis (UC)], along with 25 patients with self-limited enterocolitis. The concentration of procalcitonin was found to be considerably higher in patients with self-limited enterocolitis compared to those with IBD (0.36 ng/mL vs 0.10 ng/mL, P < 0.001). Interestingly, although the procalcitonin concentration was in the normal range in all IBD patients (i.e., < 0.5 ng/mL), those with active disease [i.e., Clinical Disease Activity Index (CDAI) score > 150 or Truelove severity index moderate or severe] had a nearly 40% higher procalcitonin value than those with inactive disease (0.13 ng/mL vs 0.09 ng/mL, P < 0.001).
Thia et al[23] carried out a prospective single-center study, including 81 patients with bacterial gastroenteritis and 71 with IBD (27 with CD, 44 with UC). Procalcitonin displayed good performance for discriminating bacterial gastroenteritis from IBD [area under the curve (AUC), 0.727; P < 0.001], and its serum levels were higher between patients with active or inactive IBD, although such difference did not reach statistical significance (0.052 ng/mL vs 0.003 ng/mL, P = 0.416).
These results were confirmed by Oruç et al[24], who also measured serum procalcitonin in 50 healthy volunteers and 45 patients with IBD (9 with CD, 36 with UC). Significantly higher procalcitonin values were observed in CD patients (0.14 ng/mL; P < 0.05) but not in UC patients (0.10 ng/mL; P = ns) compared to controls (0.06 ng/mL). A procalcitonin threshold of 0.05 ng/mL had modest sensitivity (i.e., 0.67) and very poor specificity (i.e., 0.42) for distinguishing between active and inactive IBD (AUC, 0.57; P = ns).
Oussalah et al[25] carried out a prospective observational study which included 30 patients with CD and 27 with UC. These authors measured serum procalcitonin values and found they were correlated with several demographic and clinical features. The serum concentration of procalcitonin was found to be significantly higher in patients with active IBD than in those with inactive IBD (0.10 ng/mL vs 0.07 ng/mL, P = 0.02). Serum procalcitonin value was also significantly associated with both endoscopic and radiologic indices of activity in CD patients, and with radiologic indices of activity in UC. Interestingly, a serum procalcitonin value > 0.14 ng/mL was found to have optimal diagnostic sensitivity (i.e., 1.00), combined with remarkable diagnostic specificity (i.e., 0.96), for identifying CD patients with more severe disease (AUC, 0.963; P < 0.001). However, its diagnostic accuracy was apparently inadequate for identifying UC patients with more severe disease (AUC, 0.736; P = 0.08).
Koido et al[26] analyzed serum procalcitonin concentrations in 11 healthy volunteers and 18 patients with UC. Disease severity was assessed according to Mayo endoscopic subscore and Truelove and Witts’ severity index. Interestingly, serum procalcitonin values were significantly higher in patients with severe UC (0.096 ng/mL) than in those with mild-to-moderate UC (0.033 ng/mL; P < 0.001) or in healthy controls (0.035 ng/mL; P < 0.001). No difference was found between patients with mild-to-moderate UC and healthy controls (P = 0.311). Notably, a procalcitonin value > 0.055 ng/mL displayed 1.00 sensitivity and 1.00 specificity for identification of severe UC.
Chung et al[27] performed a retrospective study including 58 patients with IBD (38 with CD, 20 with UC) and 71 with intestinal Behçet’s disease. Interestingly, procalcitonin values were not different in patients with active/inactive CD (0.11 ng/mL vs 0.07 ng/mL, P = 0.521) nor in patients with active/inactive UC (0.15 ng/mL vs 0.05 ng/mL, P = 0.553). Nonetheless, the procalcitonin values progressively increased as follows: patients with no infection (0.07 ng/mL), with localized bacterial infection (0.22 ng/mL), and with septic shock or sepsis (3.46 ng/mL; P = 0.001). Overall, procalcitonin displayed 0.83 positive predictive value and 0.84 negative predictive value (AUC, 0.636; P < 0.01) for predicting the infection status.
In a subsequent study, Ge et al[28] studied 80 patients with CD, 16 of whom developed an intraabdominal abscess. The serum concentration of procalcitonin was found to be higher in CD patients with an intraabdominal abscess than in those without (0.505 ng/mL vs 0.112 ng/mL, P < 0.01). A diagnostic threshold of 0.35 ng/mL for procalcitonin displayed 0.81 sensitivity and 0.97 specificity (AUC, 0.954; P < 0.001) for differentiating patients with or without intraabdominal abscess. A significant correlation was also observed between CDAI score and serum procalcitonin value (r = 0.575; P < 0.001).
Nishio et al[29] studied 55 IBD patients (18 with CD, 37 with UC), showing that serum procalcitonin values were significantly correlated with active disease expressed as CDAI index in CD (r = 0.7; P < 0.001), but not with active disease expressed as Mayo score in UC (r = -0.2; P = ns). In particular, patients with severe active to fulminant CD had serum procalcitonin values approximately 3-fold higher than those with non-severe active CD (0.14 ng/mL vs 0.04 ng/mL, P < 0.001).
More recently, Hosomi et al[30] measured serum procalcitonin values in 101 patients with IBD (33 with CD, 68 with UC). No significant correlation was observed between serum procalcitonin values and disease extension, location, perianal involvement, partial Mayo score, and Mayo endoscopic subscore, whilst a weak correlation was observed with Harvey-Bradshaw index (r = 0.353; P = 0.044). In both groups of IBD patients, serum procalcitonin value was not associated with complete mucosal healing and complete clinical remission. Accordingly, the sensitivity and specificity of serum procalcitonin for predicting complete mucosal healing was poor, being 0.86 and 0.35 in CD (cutoff, 0.04 ng/mL; AUC, 0.49), 0.60 and 0.53 in UC (cutoff, 0.03 ng/mL; AUC, 0.57), respectively. The sensitivity and specificity of serum procalcitonin for predicting complete clinical remission was even poorer, being 0.57 and 0.30 in CD (cutoff, 0.03 ng/mL; AUC, 0.35), and 0.21 and 0.46 in UC (cutoff, 0.03 ng/mL; AUC, 0.43), respectively.
Finally, Zielińska-Borkowska et al[31] carried out an observational study including 154 patients undergoing major elective colorectal surgery for cancer (n = 95), IBD (n = 38), and other conditions (n = 21). Overall, 16 patients (10%) developed postsurgical infections due to anastomotic leakage, in whom the frequency of serum procalcitonin concentration > 0.5 ng/mL was significantly higher than those who did not develop complications (31% vs 4%, P < 0.001). A serum procalcitonin value > 1.09 ng/mL displayed 0.87 sensitivity and 0.87 specificity for predicting postsurgical infection (AUC, 0.88; P < 0.01).
Taken together, the available published studies suggest that procalcitonin is probably unwarranted for the diagnosis of IBD and/or assessing disease severity, whilst its measurement in patients with suspected infections may enable a timely diagnosis as well as an effective therapeutic monitoring of infective complications in IBD.
The diagnostic role of procalcitonin for predicting bacterial complications in IBD is supported by reliable biological evidence. It has now been clearly established that bacterial endotoxin and a wide range of cytokines [especially interleukin-1b (IL-1b), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)] are actively released in response to both systemic and localized bacterial infections. This process strongly interplays with CALC1, eliciting abundant extra-thyroid synthesis of pre-procalcitonin, which is in turn rapidly converted to procalcitonin (Figure 2). This extra-thyroid production is magnified in patients with Gram-negative infections, which are associated with the highest circulating values of TNF-α[32].
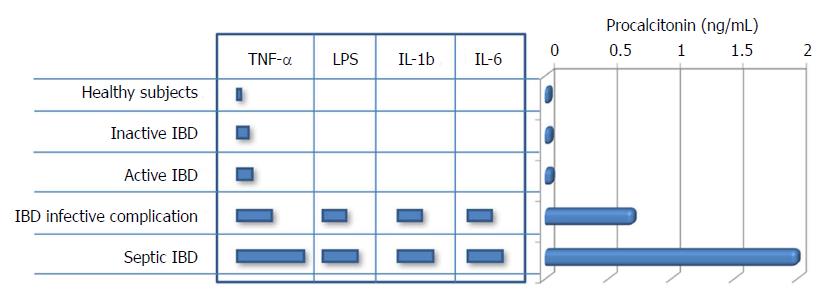
Although the precise mechanism is still unclear, the ensuing PC-mediated cleavage of procalcitonin does not occur efficiently in patients with severe infections, finally contributing to further increase in the circulating concentration of this biomarker[33]. Therefore, it is not surprising that procalcitonin values may be increased in IBD patients with bacterial complications, whilst its concentration remains virtually unchanged in those without infections, irrespective of disease severity (Figure 3). Albeit a modest increase of TNF-α can be frequently observed in patients with IBD (especially in those with CD), which in turn may explain the occasional association with increased values of serum procalcitonin in IBD patients with more active disease (Table 1), its effect on the pathogenesis of IBD seems mostly mediated by altered expression of TNF receptors[34]. On the other hand, TNF-α is more strongly up-regulated by a number of proinflammatory factors, such as endotoxin and other bacterial products. This fact would lead to substantial enhancement of intestinal procalcitonin synthesis and also explains its potential clinical usefulness for predicting bacterial complications in IBD (Figure 3).
| Ref. | Type of study | Study population | PCT | ||
| IBD vs HCs | Active vs inactive IBD | Predicting complications | |||
| Korczowski et al[21], 2003 | Cross-sectional | 129 children with diarrhea and 30 HCs | Non significantly different | Non assessed | PCT predicted bacterial enterocolitis |
| Herrlinger et al[22], 2004 | Cross-sectional | 51 IBD patients (26 with CD and 25 with UC) and 25 patients with self-limited enterocolitis | Nonassessed | PCT ~40% higher in patients with active disease | Nonassessed |
| Thia et al[23], 2008 | Cross-sectional | 71 IBD patients (27 with CD and 44 with UC) and 81 with bacterial gastroenteritis | Nonassessed | PCT non significantly higher in patients with active disease | PCT predicted bacterial gastroenteritis |
| Oruç et al[24], 2009 | Cross-sectional | 45 patients with IBD (9 with CD and 36 with UC) and 50 HCs | PCT higher in CD (but not in UC) than in HCs | PCT nonsignificantly higher in patients with active disease | Nonassessed |
| Oussalah et al[25], 2010 | Prospective observational | 57 IBD patients (30 with CD and 27 with UC) | Nonassessed | PCT ~40% higher in patients with active disease; PCT predicted disease severity in CD but not in UC | Nonassessed |
| Koido et al[26], 2013 | Cross-sectional | 18 patients with UC and 11 HCs | Nonsignificantly different between inactive UC and HCs, higher in active UC than in HCs | PCT ~3-fold higher in patients with active disease | Nonassessed |
| Chung et al[27], 2016 | Cross-sectional | 58 IBD patients (38 with CD and 20 with UC) | Nonassessed | PCT nonsignificantly higher in patients with active disease | PCT predicted bacterial infection and sepsis |
| Ge et al[28], 2016 | Cross-sectional | 80 CD patients (16 with intraabdominal abscess) | Nonassessed | PCT nonsignificantly higher in patients with active disease | PCT predicted intraabdominal abscess |
| Nishio et al[29], 2016 | Cross-sectional | 55 IBD patients (18 with CD and 37 with UV) | Nonassessed | PCT ~3-fold higher in patients with active CD, but not in those with active UC | Nonassessed |
| Hosomi et al[30], 2017 | Cross-sectional | 101 IBD patients (33 with CD and 68 with UC). | Nonassessed | PCT nonsignificantly higher in patients with active disease | Nonassessed |
| Zielińska-Borkowska et al[31], 2017 | Observational | 154 patients undergoing major elective colorectal surgery (38 with IBD) | Nonassessed | Nonassessed | PCT predicted postsurgical infection |
Recent data attest that the rate of Clostridium difficile infection is constantly increasing and is now responsible for a remarkable number of IBD hospitalizations[35]. Unfortunately, the diagnosis of local infective complications is challenging in patients with IBD, since the symptoms are nonspecific or often overlap with those of the underlying pathology. The suggestive endoscopic findings (e.g., pseudomembranous exudates in Clostridium difficile infection) are lacking in the vast majority of IBD patients, whilst stool culture is characterized by long turn-around time (usually around 48 h), high cost, and considerably low specificity[36]. Therefore, the availability of alternative diagnostic biomarkers may be seen as a valuable perspective to follow-up of IBD patients.
According to current evidence in the scientific literature, the clinical significance of measuring procalcitonin for diagnosing and monitoring IBD disease is rather elusive, and its association with disease severity is still confined to a limited number of studies (Table 1). Nevertheless, though procalcitonin values do not seemingly provide clinically useful information as serological marker of disease activity and inflammatory status, literature data suggest that supranormal procalcitonin concentrations may reflect the presence of a number of infective complications in IBD, thus including bacterial enterocolitis, bacterial gastroenteritis, intraabdominal abscess, postsurgical infection and sepsis (Table 1). To conclude, the measurement of this biomarker may retain clinical significance for predicting or timely diagnosing many IBD-associated infections and complications rather than for assessing the disease activity.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sferra TJ, Sivandzadeh GR S- Editor: Chen K L- Editor: Filipodia E- Editor: Huang Y
| 1. | Conlon JM, Grimelius L, Thim L. Structural characterization of a high-molecular-mass form of calcitonin [procalcitonin-(60-116)-peptide] and its corresponding N-terminal flanking peptide [procalcitonin-(1-57)-peptide] in a human medullary thyroid carcinoma. Biochem J. 1988;256:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396-404. [DOI] [Full Text] |
| 3. | Linscheid P, Seboek D, Nylen ES, Langer I, Schlatter M, Becker KL, Keller U, Müller B. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144:5578-5584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013;28:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Meisner M, Schmidt J, Hüttner H, Tschaikowsky K. The natural elimination rate of procalcitonin in patients with normal and impaired renal function. Intensive Care Med. 2000;26 Suppl 2:S212-S216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515-518. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1329] [Cited by in RCA: 1360] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 7. | Lippi G, Montagnana M, Balboni F, Bellone A, Casagranda I, Cavazza M, Da Rin G, Coen D, Giavarina D, Giostra F. Academy of Emergency Medicine and Care-Society of Clinical Biochemistry and Clinical Molecular Biology consensus recommendations for clinical use of sepsis biomarkers in the emergency department. Emerg Care J. 2017;13:6877. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 8. | Christ-Crain M, Müller B. Procalcitonin in bacterial infections--hype, hope, more or less? Swiss Med Wkly. 2005;135:451-460. [PubMed] |
| 9. | Markanday A. Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open Forum Infect Dis. 2015;2:ofv098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Vikse J, Henry BM, Roy J, Ramakrishnan PK, Tomaszewski KA, Walocha JA. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int J Infect Dis. 2015;38:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lippi G, Meschi T, Cervellin G. Inflammatory biomarkers for the diagnosis, monitoring and follow-up of community-acquired pneumonia: clinical evidence and perspectives. Eur J Intern Med. 2011;22:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rast AC, Knobel D, Faessler L, Kutz A, Felder S, Laukemann S, Steiner D, Haubitz S, Fux CA, Huber A. Use of procalcitonin, C-reactive protein and white blood cell count to distinguish between lower limb erysipelas and deep vein thrombosis in the emergency department: A prospective observational study. J Dermatol. 2015;42:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Saeed K, Dryden M, Sitjar A, White G. Measuring synovial fluid procalcitonin levels in distinguishing cases of septic arthritis, including prosthetic joints, from other causes of arthritis and aseptic loosening. Infection. 2013;41:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lippi G, Valentino M, Cervellin G. Laboratory diagnosis of acute pancreatitis: in search of the Holy Grail. Crit Rev Clin Lab Sci. 2012;49:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Acharya A, Markar SR, Ni M, Hanna GB. Biomarkers of acute appendicitis: systematic review and cost-benefit trade-off analysis. Surg Endosc. 2017;31:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Martínez L AB, Marañón P R, Cobo E PV, Tomatis S C, Guerra M L, Peñalba C AC. [Use of procalcitonin as diagnostic marker in acute gastroenteritis]. Rev Chil Pediatr. 2014;85:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Attar BM, Moore CM, George M, Ion-Nedelcu N, Turbay R, Zachariah A, Ramadori G, Fareed J, Van Thiel DH. Procalcitonin, and cytokines document a dynamic inflammatory state in non-infected cirrhotic patients with ascites. World J Gastroenterol. 2014;20:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Cosse C, Sabbagh C, Kamel S, Galmiche A, Regimbeau JM. Procalcitonin and intestinal ischemia: a review of the literature. World J Gastroenterol. 2014;20:17773-17778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 19. | Lippi G, Danese E, Cervellin G, Montagnana M. Laboratory diagnostics of spontaneous bacterial peritonitis. Clin Chim Acta. 2014;430:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Domínguez-Comesaña E, Ballinas-Miranda JR. [Procalcitonin as a marker of intra-abdominal infection]. Cir Cir. 2014;82:231-239. [PubMed] |
| 21. | Korczowski B, Szybist W. Serum procalcitonin and C-reactive protein in children with diarrhoea of various aetiologies. Acta paediatrica (Oslo, Norway : 1992). 2004;93:169-173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Herrlinger KR, Dittmann R, Weitz G, Wehkamp J, Ludwig D, Schwab M, Stange EF, Fellermann K. Serum procalcitonin differentiates inflammatory bowel disease and self-limited colitis. Inflamm Bowel Dis. 2004;10:229-233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Thia KT, Chan ES, Ling KL, Ng WY, Jacob E, Ooi CJ. Role of procalcitonin in infectious gastroenteritis and inflammatory bowel disease. Dig Dis Sci. 2008;53:2960-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Oruç N, Ozütemiz O, Osmanoğlu N, Ilter T. Diagnostic value of serum procalcitonin in determining the activity of inflammatory bowel disease. Turk J Gastroenterol. 2009;20:9-12. [PubMed] |
| 25. | Oussalah A, Laurent V, Bruot O, Guéant JL, Régent D, Bigard MA, Peyrin-Biroulet L. Additional benefit of procalcitonin to C-reactive protein to assess disease activity and severity in Crohn’s disease. Aliment Pharmacol Ther. 2010;32:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Koido S, Ohkusa T, Takakura K, Odahara S, Tsukinaga S, Yukawa T, Mitobe J, Kajihara M, Uchiyama K, Arakawa H. Clinical significance of serum procalcitonin in patients with ulcerative colitis. World J Gastroenterol. 2013;19:8335-8341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Chung SH, Lee HW, Kim SW, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Usefulness of Measuring Serum Procalcitonin Levels in Patients with Inflammatory Bowel Disease. Gut Liver. 2016;10:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ge X, Hu D, Cao Y, Liu Z, Ding C, Tian H, Gong J, Zhu W, Li N, Li J. Procalcitonin in Crohn’s disease with fever episodes, a variable to differentiate intra-abdominal abscess from disease flares. Int J Surg. 2016;36:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Nishio E, Saruta M, Arihiro S, Matsuoka M, Mitsunaga M, Ide D, Iwasaki T, Ogawa M, Sawada R, Nagata Y. The clinical benefit of procalcitonin to assess disease activity and severity in inflammatory bowel disease. Gastroenterology. 2016;150:S995. [DOI] [Full Text] |
| 30. | Hosomi S, Yamagami H, Itani S, Yukawa T, Otani K, Nagami Y, Tanaka F, Taira K, Kamata N, Tanigawa T. Sepsis markers soluble IL-2 receptor and soluble CD14 subtype as potential biomarkers for complete mucosal healing in patients with inflammatory bowel disease. J Crohns Colitis. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Zielińska-Borkowska U, Dib N, Tarnowski W, Skirecki T. Monitoring of procalcitonin but not interleukin-6 is useful for the early prediction of anastomotic leakage after colorectal surgery. Clin Chem Lab Med. 2017;55:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Kim SE. Serum Procalcitonin Is a Candidate Biomarker to Differentiate Bacteremia from Disease Flares in Patients with Inflammatory Bowel Disease. Gut Liver. 2016;10:491-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Bartolovic D, Ignjatovic S, Stankovic S, Nada Majkić S. Procalcitonin and Other Biomarkers of Sepsis in Newborns in the Intensive Care Unit. EJIFCC. 2011;22:24-30. [PubMed] |
| 34. | Ślebioda TJ, Kmieć Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2014;2014:325129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Kucharzik T, Maaser C. Infections and Chronic Inflammatory Bowel Disease. Viszeralmedizin. 2014;30:326-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Navaneethan U, Venkatesh PG, Shen B. Clostridium difficile infection and inflammatory bowel disease: understanding the evolving relationship. World J Gastroenterol. 2010;16:4892-4904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |