Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.7989
Peer-review started: July 24, 2017
First decision: August 30, 2017
Revised: October 6, 2017
Accepted: October 17, 2017
Article in press: October 17, 2017
Published online: December 7, 2017
Processing time: 136 Days and 4.3 Hours
To investigate a safer way to set up the disease model of cystic echinococcosis without contamination risk and develop a novel experimental murine model of hepatic cystic echinococcosis.
C57B/6 mice were injected with human protoscolices of three different concentrations via the portal vein. The mice were followed for 10 mo by ultrasound, gross anatomy, and pathological and immunological examinations. The protoscolex migration in the portal vein, hydatid cyst growth, host immune reaction, and hepatic histopathology were examined periodically.
The infection rates in the mice in the high, medium, and low concentration groups were 90%, 100%, and 63.6%, respectively. The protoscolices migrated in the portal vein with blood flow, settled in the liver, and developed into orthotopic hepatic hydatid cysts, resembling the natural infection route and course.
We have established an improved experimental model of hepatic cystic echinococcosis with low biohazard risk but stable growing dynamics and immune reaction. It is especially useful for new anti-parasite medication trials against hydatid disease.
Core tip: In this experimental study, we developed a novel murine model of cystic echinococcosis. This orthotopic model resembles primary infection route and natural infectious course with low biohazard risk and high efficiency.
- Citation: Zhang RQ, Chen XH, Wen H. Improved experimental model of hepatic cystic hydatid disease resembling natural infection route with stable growing dynamics and immune reaction. World J Gastroenterol 2017; 23(45): 7989-7999
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/7989.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.7989
Hydatid disease caused by Echinococcus granulosus is a worldwide zoonosis. It is highly prevalent in Xinjiang, China[1]. Humans are accidentally infected by Echinococcus granulosus egg-contaminated areas. Infection causes serious economic, medical, veterinary, and public health impact[2]. Animal model plays an important role in the study for novel drugs, surgical approaches, and vaccine development. An ideal experimental model should orthotopically induce hydatid disease in the most affected organ, i.e., the liver. The model should resemble the natural infection route and course with a stable and predictable growth pattern. However, traditional animal models exhibit a biohazard risk when feeding animals with parasitic eggs and induce the parasite cyst in the abdomen cavity as the secondary infection[3]. In humans, hydatid disease demonstrates a chronic infectious course and it takes decades for the parasite to settle and grow in the liver[2]. The life cycle includes six stages: (1) adult Echinococcus granulosus, which is about 3-6 mm in length, resides in the bowel of its definite host; (2) gravid proglottids release eggs that are passed in the feces; (3) these eggs are then ingested by a suitable intermediate host, including sheep, goat, pigs, cattle, horses, and camels. The eggs then hatch in the bowel and release oncospheres that penetrate the intestinal wall. These oncospheres then migrate through the circulatory system to various organs of the host; (4) at the organ site, the oncosphere develops into a hydatid cyst. The cyst enlarges gradually, producing protoscolices and daughter cysts that fill the cyst interior; (5) the cyst-containing organs are then ingested by the definite host, causing infection. After ingestion, the protoscolices evaginate, producing protoscolexes; and (6) the scolexes of the organisms attach to the intestine of the definite host and develop into adults in 32-80 d. After invading into the gastrointestinal tract, its life cycle then continues in humans. The eggs then release oncospheres in the small intestine. In the liver, oncospheres migrate through the circulatory system and produce hydatid cysts.
To develop such an experimental animal model in order to mimic the natural life circle is expensive and time-consuming. In addition to the time and cost, the biohazard risk also cannot be ignored. Oral feeding with parasitic eggs can cause high infection risk for researchers and requires a high-level biohazard lab to perform the studies[4-7]. Thus, the development of a highly accurate animal model with low contamination risk to interpret short-term research results would be beneficial. In this study, we established a mouse model by injecting mice with protoscolices obtained from human hydatid cysts via the portal vein. Ultrasound studies detected cysts within 4 mo. The protoscolex migration, hydatid cyst formation, growing dynamics, pathological development, and immune reactions were followed until 10 mo.
This study proposes a way to circumvent many problems linked to an animal model for hydatid cyst closer to natural infection, i.e., ingestion of oncospheres. Feeding animals Echinococcus eggs in the lab is risky because of biohazard for the lab personnel that can accidentally ingest or inhale the eggs. For this reason, most experimental work was done on the peritoneal injections with protoscolices, which does not reproduce the natural route of infection (ingestion of oncospheres) but the natural route of secondary echinococcosis (which is what happens when the contents of cysts are spilled into the peritoneal cavity). In addition, the disease model also has the following benefits: (1) small rodents were used so that the experiment can save labor and cost on big animals; (2) injection was performed via the portal vein instead of feeding from mouth so that biohazard of collecting parasite eggs can be avoided; and (3) the model bypassed hatching in the small intestine so that time can be shortened and evacuation contamination be avoided. Using this model, we further proved that injected parasite can steadily grow up into hydatid cysts in the liver and stimulate host immune reaction.
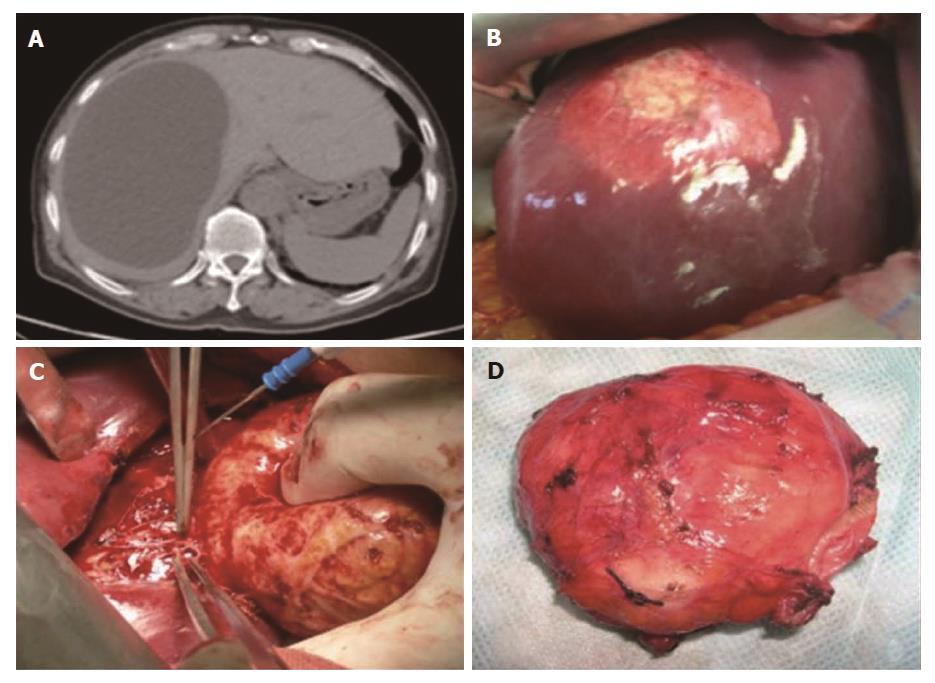
The protoscolices in this study were collected from hydatid cysts in naturally infected patients during an open surgery in the First Affiliated Hospital of Xinjiang Medical University (Figure 1). Written informed consent and an image release agreement were obtained in advance from all subjects. The number of protoscolices was adjusted in 0.9% NaCl solution with a 95% viability rate.
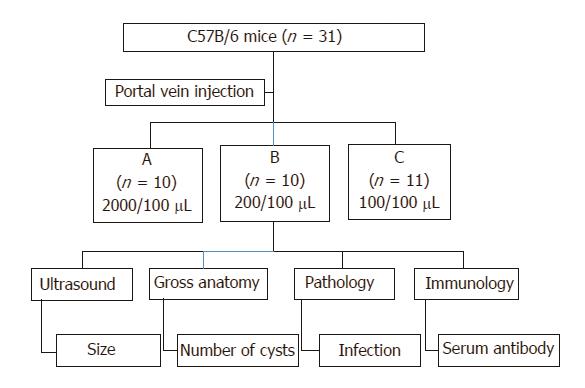
Three different concentrations were prepared for a parallel experiment design with long-term follow-up (Figure 2): group A (2000 protoscolices/100 μL), group B (200 protoscolices/100 μL), and group C (100 protoscolices/100 μL).
Viability was confirmed using the 1% eosin exclusion test to determine the viability of the protoscolices. The viable protoscolices could exclude the eosin such that they were colorless and mobile, while dead protoscolices stained red. The viability was calculated by the number of viable cells divided by the total number of protoscolices. The protoscolices used for injection had more than 95% viability.
Eight-week-old female C57B/6 mice were purchased from the Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China). The mouse weight varied from 20 to 24 g. They were maintained in an SPF level Experimental Animal Center of the First Affiliated Hospital, Xinjiang Medical University and acclimatized in the animal facility for one week before injection.
The animals were shaved, scrubbed, and then moved to a sterile surgical area. The animals were anesthetized with chloral hydrate (300 mg/kg) and remained anesthetized during the operation. A 1.5-cm incision was made from the bladder up to the level of the xiphoid. The skin and muscle layers were retraced by tissue retractors to hold them on the left and right sides. The intestines were carefully moved to one side with sterile gauze to expose the portal vein. The portal vein was located under the pancreas. The needle connected to the syringe filled with protoscolices was inserted into the portal vein and the protoscolex solution was released. After injection, the needle was slowly retracted and a piece of gauze was pressed on the puncture site to prevent backflow of blood for 5 min. The intestines were placed back into the abdomen and the abdominal wall was closed. Mice were maintained on the heating pad for recovery with frequent inspection, and no occurrence of bleeding or infection was found.
In total, 31 mice were randomly divided into three groups (Figure 2): group A: 2000 protoscolices in 100 μL saline, n = 10; group B: 200 protoscolices in 100 μL saline, n = 10; group C: 100 protoscolices in 100 μL saline, n = 11 mice.
After injection, the mice were observed regularly by non-invasive animal ultrasound to measure the cyst formation, location, distribution, and size. One mouse from each group was euthanized every month and examined for the presence of cysts. The liver tissue and hydatid cysts were examined microscopically to record the morphological and pathological changes. The liver and hydatid cyst wall were examined histologically by H&E staining to track the migration path of the protoscolices from the portal vein to the liver. Blood samples were collected to detect IgG production. The experiment grouping and follow-up design are illustrated using a flowchart in Figure 2.
Livers and hydatid cysts were fixed in 10% formalin, embedded in paraffin, cut into 5-μm sections, and stained with haematoxylin-eosin, and images were obtained using light microscopy to evaluate the tissue structure and pathological changes.
Blood samples were collected at different time points for detection of IgG antibodies using a nephelometric technique (Beckman Array 360; Beckman Coulter Instruments, Brea, United States)
All experimental protocols were approved by the Ethical Committee of the First Affiliated Hospital of Xinjiang Medical University (Approved project number: 20141217003). Informed consent was obtained from all subjects. All methods were performed according to the relevant guidelines and regulations of the Declaration of Helsinki and National Institutes of Health Guide for Care and Use of Laboratory Animals.
SPSS Software 17 for Windows (SPSS Inc., Chicago, United States) was used for statistical analyses. The differences between groups were determined using t-tests, and P-values less than 0.05 were considered significant. A standard score was used to evaluate the normal distribution of cyst formation efficiency among the three groups.
In natural infection cycle, the adult parasite worms release eggs from feces and contaminate the environment. Eggs can survival for a year even in the drought and freezing environment and accidentally infect human residence via feces-oral route. In human digestive tract, the parasite eggs hatch and release oncospheres that penetrate the intestinal mucosa. They migrate passively through blood in the portal vein to reach the liver for final settlement. One oncosphere develops into a hydatid cyst. The hydatid cyst grows up with cyst fluid and infective protoscolices. In our experimental model, by injecting the protoscolices into the portal vein directly, we bypassed the contractable egg hatch stage in the intestine, and obtained the primary hydatid cyst in the liver. The final number of the developed cysts in fact depends on many factors, e.g., space in the liver and the viability of the protoscolex.
After the mice were injected with protoscolices at three different concentrations (2000/100 μL in group A, 200/100 μL in group B, and 100/100 μL in group C), the hydatid disease infection rates in the mice in the three groups were 90% (9/10 in group A), 100% (10/10 in group B), and 63.6% (7/11 in group C), respectively (Table 1). There was no significant difference in the infection rates among the three groups (P < 0.05).
| Group | Concentration of injected protoscolices | Number of infected mice | Number of non-infected mice | Total | Infection rate (%)a |
| Group A | 2000/100 μL | 9 | 1 | 10 | 90.0 |
| Group B | 200/100 μL | 10 | 0 | 10 | 100.0 |
| Group C | 100/100 μL | 7 | 4 | 11 | 63.6 |
| Total | 26 | 5 | 31 | 84.55 |
Four weeks after portal vein injection, visual lesions on the liver could be found. After 4 mo, the hydatid cysts presented significant growth. Table 2 presents the anatomical locations of the hydatid cysts in the mouse liver (Table 2). Hydatid cysts occurred in any part of the liver and there was no significant preference in any of the liver lobes.
| Mouse ID | Group A | Group B | Group C |
| 1 | Middle lobe: 2 | Middle lobe: 2 | Right lobe: 3 |
| Upper right lobe: 1 | Lower right lobe: 2 | Lateral left lobe: 1 | |
| Lower right lobe: 1 | Lateral left lobe: 3 | ||
| 2 | Middle lobe: 6 | Middle lobe: 1 | Lateral left lobe: 2 |
| Upper right lobe: 5 | Lateral left lobe: 1 | ||
| 3 | Multiple cysts all over the liver | Lower right lobe: 1 | None |
| 4 | Middle lobe: 6 | Upper right lobe: 2 | Middle lobe: 3 |
| Upper right lobe: 2 | Lateral left lobe: 3 | ||
| Lower right lobe: 2 | |||
| Lateral left lobe: 2 | |||
| 5 | Multiple cysts all over the liver | Middle lobe: 3 | Lower right lobe: 1 |
| Upper right lobe: 1 | Lateral left lobe: 3 | ||
| 6 | None | Lower right lobe: 1 | None |
| 7 | Multiple cysts all over the liver | Upper right lobe: 1 | Middle lobe: 1 |
| 8 | Middle lobe: 1 | Lateral left lobe: 4 | Middle lobe: 2 |
| Upper lobe: 1 | Lateral left lobe: 3 | ||
| 9 | Multiple cysts all over the liver | Lateral left lobe: 1 | Middle lobe: 1 |
| 10 | Multiple cysts all over the liver | Upper right lobe: 1 | None |
| Lower right lobe: 2 | |||
| 11 | - | - | None |
The fundamental structure of the four major liver lobes of rat and mouse livers and the segmentation of human liver according to Couinaud are similar and the fundamental structure is comparable. These findings allow the previous use of rodent models in experimental hepatobiliary surgery. The murine and human livers are comparable due to the similarity of the fundamental structures. These findings allow the use of mice to develop experimental models of hydatid disease.
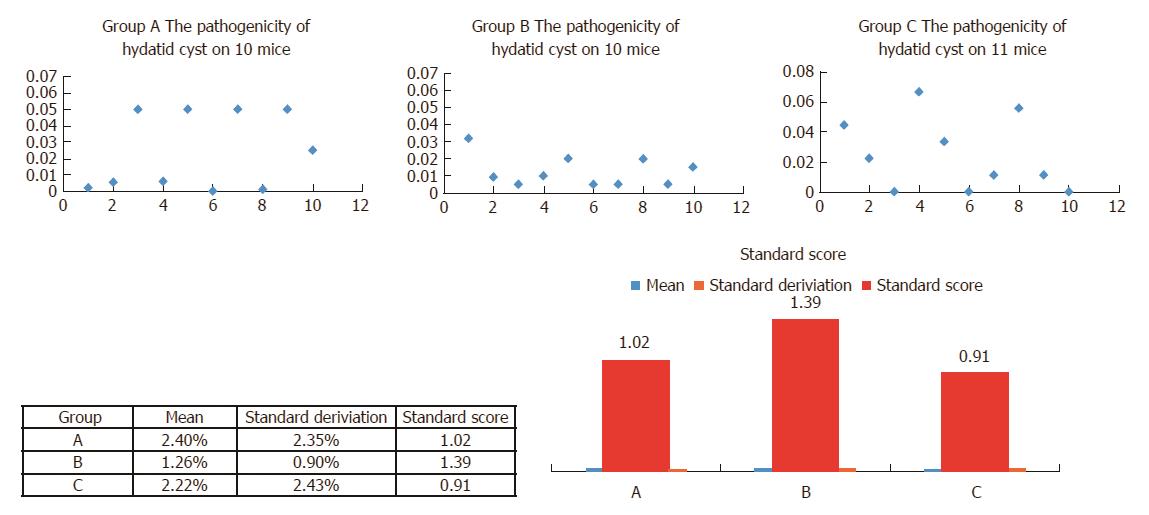
After 6 mo, when the hydatid cysts were fully developed in the mouse liver, the ratio of developed cysts/number of protoscolices was evaluated using two markers: pathogenicity (number of cysts per protoscolex) and number of hydatid cysts per mouse. The gross anatomy and column illustration are shown in Figure 3. Cyst abundance in each mouse reflects protoscolex immune reaction, which stimulates the host immune system to produce IgG against the parasite. Although group A had the highest parthenogenesis (2.395 ± 0.7424) and cyst abundance (47.90 ± 14.848), the condensed lesion made observation of the individual cyst impossible.
During the 10-mo long follow-up period, no mouse died due to portal vein bleeding, surgery related infection, or cachexia, unless a mouse was euthanized during the monthly routine examination. In terms of the hydatid disease model success rate, there was no significant difference among the three groups. However, the experimental model on hydatid requires a more reliable normal distribution. Thus, the standard score was used in this study to compare the reliability and efficiency of the animal models (Figure 4).
The standard score was used in this study to compare the reliability and efficiency of animal models. Standard score was calculated as (raw score - mean)/SD (Figure 4). This value indicates how well the model reflected the normal distribution compared to other models (the normal distribution of groups A, B, and C is shown in Figure 4, upper panel). This value allows comparisons to be made between the three models with different distribution characteristics, i.e., mean and SD. Thus, a score of 1.39 in group B indicates that its performance was better compared with groups A (1.02) and C (0.91).
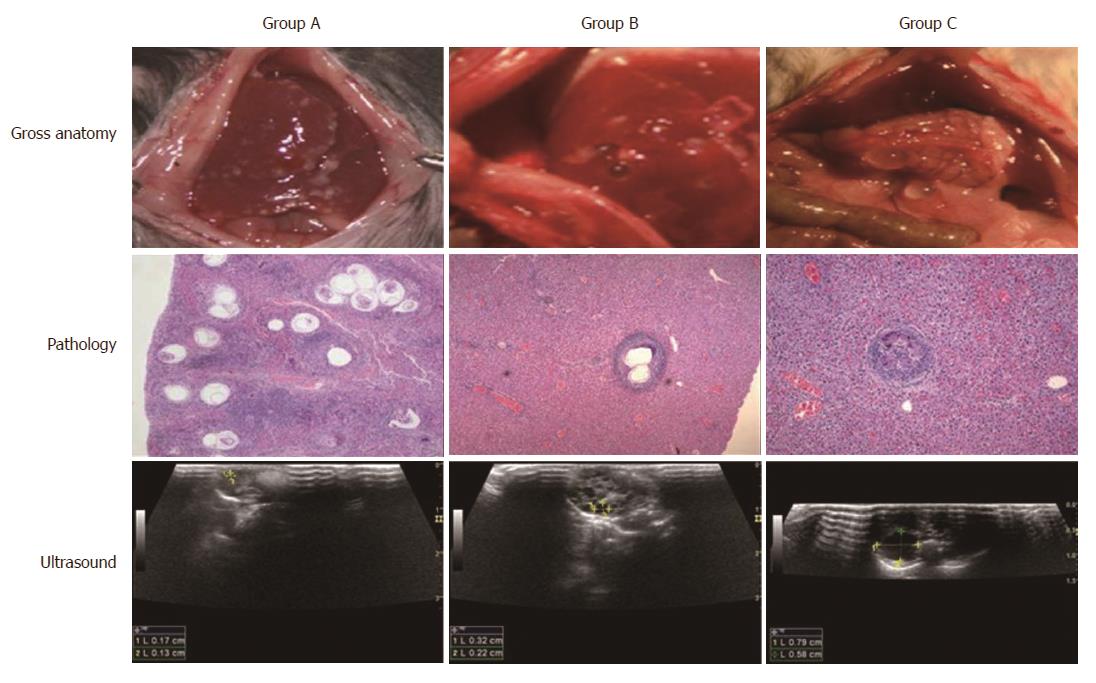
The path and course of the protoscolex migration from the portal vein to the liver were tracked by open examination, pathology, and ultrasound. On the day of portal vein inoculation with human Echinococcus granulosus protoscolices, the branches of the portal vein diameter increased. With congestion of condensed protoscolices (Figure 5, middle panel), 1 d after inoculation, the inflammatory cell migration was incarcerated; 3 d after inoculation, a significant inflammatory reaction zone formed; 3 wk later the protoscolex developed into vesicles (Figure 5, middle panel); and 6 wk after inoculation, none of the protoscolex could be found but visible vesicular structures of hydatid cyst were observed (Figure 5, middle panel). The open examination showed the distribution and cyst abundance in the livers of groups A, B, and C (Figure 5, upper panel). After 4 mo, ultrasound detected spherical, fibrous rimmed cysts with surrounding host reaction. After 6 mo, an even larger parent cyst with satellite daughter cysts within or outside the parent cyst was found (Figure 5, lower panel). The rodents have the four major liver lobes similar to human hepatic Couinaud segments. Murine and human livers are comparable due to the similarity of the fundamental structures. These findings allow the use of mice to develop the experimental hydatid disease model.
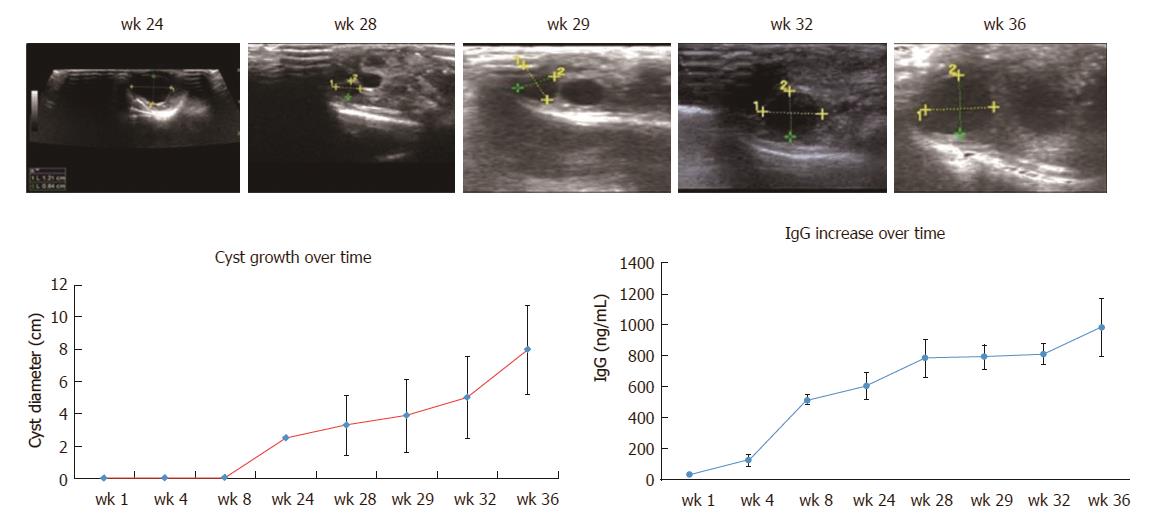
After 6 mo, ultrasound could detect a stable increase in the number of hydatid cysts (Figure 6). The average cyst diameters in group B on the 24th, 28th, 29th, 32nd, and 36th weeks were 2.48 ± 0.91 mm, 3.29 ± 1.86 mm, 3.87 ± 2.26 mm, 5.00 ± 2.57 mm, and 7.98 ± 2.75 mm, respectively (Table 3), indicating a significant increase in diameter over time after 6 mo (P < 0.001).
| Week 24 | Week 28 | Week 29 | Week 32 | Week 36 | P | |
| Cyst diameter (mm) | 2.48 ± 0.91 | 3.29 ± 1.86 | 3.87 ± 2.26 | 5.00 ± 2.57 | 7.98 ± 2.75 | 0.001 |
When the protoscolices migrated in the portal vein, the host had a low level of IgG (40 ng/mL at week 1). After approximately 6 mo, the hydatid cyst became fully developed, and the cyst began to release different antigens to modulate the host immune surveillance. IgG increased in parallel with the hydatid cyst volume (500-800 ng/mL during weeks 24-32). Parasitic antigens stimulated a series of complex host immune responses, which may benefit both the host and parasite for a symbiotic relationship (800-900 ng/mL at week 36) (Figure 6).
Microscopic examination of the mouse liver revealed parasitism related pathological changes. After injection, the protoscolices congested the portal vein. They caused dilatation of the vessel sinusoids. Dead protoscolices resulted in focal degeneration and necrosis, and the mouse liver reacted with an increased diameter of central veins. The mouse liver also showed protective immune reactions, such as lymph cell infiltration and fibrosis capsules (Figure 7).
Hydatid disease is defined as a zoonotic disease or neglected tropical disease. It is a public health problem worldwide. As one of the most serious endemic diseases, it is extremely hazardous in Xinjiang, China due to poor health education and a lack of effective medication[8,9]. Thus, an animal model is needed as the basis for the development of new medication against hydatid disease[10].
To establish a mouse model of echinococcosis granulosus, different infection routes have been investigated: orally, intraperitoneally, or intravenously. Different parasitic stages have been employed, such as parenterally with eggs, hatched eggs, or activated oncospheres. In addition to the low infection rate, the generation of experimental animals orally with eggs might pose potential contamination risks to the laboratory personnel who are exposed to feces of the infected mouse and the liver with hydatid cysts[3,5-7].
In this study, an effective animal model was established to mimic the natural infection route and course of echinococcosis in humans. This animal model showed the following specific advantages.
Echinococcus granulosus poses the greatest risk because it is the most common and widely distributed species. Accidental ingestion of infective eggs is the primary laboratory hazard. A single infective egg from the feces of the definitive host could potentially result in serious infection. Handling parasites requires special care and a special lab facility[4].
Cystic echinococcosis is considered an occupational infection. Certain people, e.g., shepherds, slaughters, stockbreeders, and farmers, are at higher risk of the disease because their career makes them to work closely with animals. When producing a disease model, the researchers are exposed to the risk due to the oral feeding of parasite eggs to animals.
In this study, an improved protocol without feeding high risk eggs orally into the gut, but instead, with injecting protoscolices via the portal vein, reduced the occurrence of laboratory-acquired infection in the laboratory and is safe for animal care personnel.
Hydatid disease can use many other wild herbivores as an animal model, such as sheep, goats, cattle, camels, buffalos, pigs, and kangaroos, but small rodents show high feasibility in the general animal center. A mouse model is easy for performing biochemical examinations with mouse-derived antibodies. In this study, the animal model was established using the most popular intermediate host mice. Good susceptibility to human protoscolices and a high yield of hydatid cysts were observed in the liver.
The hydatid cysts formed by the metacestode (larval stage) migrate from the intestine to the liver via the portal vein and finally develop into hydatid cysts most often in the liver[1,2]. C57B/6 mice were injected via the portal vein with protoscolices from humans. This animal model mimics the natural infectious route. Protoscolices migrated from the portal vein into the liver lobe, forming hydatid cysts on the orthotopic and primary infection organ.
Small cystic larvae were observed macroscopically in the liver 3 wk post-injection. The laminated layer was found 6 wk post-injection. At four months post-injection, larger larval cysts were found in the orthotopic liver. A laminated layer with mature protoscolices was observed to be surrounded by lymphocytes.
Mouse and human liver anatomies are similar except that the human liver has a larger right lobe and a large right portal vein compared with the left side. When the human superior mesenteric vein and splenic vein are confluent in the portal vein, the majority of blood flow goes into the right portal vein, thus carrying more parasites in the flow into the right lobe. The majority of human hepatic hydatid cysts (60%-80%) were found in the right lobe. However, in the mouse model, the liver lobes showed no significant difference in this proportion. The middle lobe exhibited the highest volume percentage (approximately 30% of the total liver volume). Thus, the lesion location in the mouse liver showed no lobe preference and no lobe appeared in the liver. Mice injected with 2000 protoscolices produced more than 100 vesicles by the end of the study (9 mo). Mice injected with 200 protoscolices yielded 1-4 vesicles. Technically, the location of the hydatid lesions could be controlled by fixing the right lobe by selectively blocking the portal vein. To decrease the confounding factors, selective blockade of the portal vein was avoided in this study but it was technically possible in specific circumstances.
In human beings, the final fate of chronic hydatid cysts in the liver are quite different. Some cysts keep on expanding slowly in decades of years without obvious symptoms. Some cysts grow up to certain volume (e.g., when the cyst diameter is larger than 5 cm) and become symptomatic. Some cysts rupture spontaneously and the spillage of parasite tissue causes the secondary echinococcosis. Some cysts have necrotic processes leading to a solidification and/or calcification of the cysts. The cysts collapse and gradually disappear. According to ultrasonographic features of the hydatid cyst, the WHO classified cystic echinococcosis from CE1-CE5: CE1 and CE2 are active, CE3 is transitional, and CE4 and CE5 are inactive. In this study, the hydatid cysts can form in months (vs many years in humans) and the ultrasonography showed the cysts formed in murine livers are CE1, the most active type, making the model a reliable animal model for any further study.
The mice produced the host immune protection following primary injection of protoscolices. Accordingly, the protoscolices developed cyst membranes and capsules that are highly effective in protecting the parasite from host immune destruction. IgG is a marker that reflects the host-parasite immune reaction. When a hydatid cyst develops in the liver, host IgG in serum is significantly elevated and can be used as an indirect marker for a coarse estimate of hydatid cyst volume and parasitic burden[11].
Group B was optimally injected with a concentration of 200 protoscolices, and the cyst number (2.60 ± 0.618) left sufficient space for intervention and further follow-up observation. In group B, the number of cysts and protoscolices was proportional to the volume of the cysts. The radius of the individual cyst gradually increased accordingly over time (not linearly). In group B, 100% of the mice developed hydatid cysts with ultrasound detectable lesions. The hydatid cysts became distended and palpable in 4 mo. Group B was superior for research due to its low dose of infection and predictable cyst development as well as better normal distribution. It will benefit experimenters to observe the in vivo efficacy of new treatments against hydatid without the need for sacrificing the mouse[12-15].
In summary, we have developed a model of hydatid disease not on sheep, dogs, or humans, but on small rodents so that the experiment can save labor and cost, and avoid ethic issue on sheep or humans. Injection via the portal vein instead of feeding from mouth can avoid collecting parasite eggs with bio-hazard risk. This model can bypass the hatching stage in the intestine so that it saves time and avoid contamination. Using the animal model, we further showed the animal model can steadily grow up into hydatid cysts in the liver and steadily stimulate host’s specific immune reaction. The proper cyst density and anatomical localization enable accurate monitoring. In this study, larval Eechinococcus granulosus infection was induced in mice, the most popular experimental intermediate host. Using this experimental model, the parasite cyst growth and immune reaction proportional to the cyst volume can be examined.
Hydatid disease is caused by Echinococcus granulosus. It is a worldwide zoonosis. It is highly prevalent in Xinjiang, China. The animal disease model is of great significance for the drug development against parasite disease.
Echinococcosis is mostly caused by close contact with infected dogs or occupational exposures. The researchers producing hydatid disease model have the high risk of infection because they handle parasite eggs to feed animals through oral route.
To avoid contamination risk of handling parasite eggs, this study investigated a safer way for developing an experimental murine model of cystic echinococcosis in the liver.
Bypassing the oral feeding of contaminant parasite eggs, human protoscolices were injected via the portal vein. Using this method, the tapeworm eggs that may contaminate lab and consequently enable transmission to human beings are avoided.
The pathological results confirmed that protoscolices kept alive and moved from the portal vein into different liver segments and lobes, and the three different protoscolice injection concentrations led to different infection rates of 90%, 100%, and 63.6%, respectively.
By sterile injection with human protoscolices via the portal vein, a novel murine model was developed with echinococcosis vacuoles formed in the liver. Without contamination risk to researchers, this disease model is suitable for anti-hydatid treatment trials.
The good experience that can be learnt from this study is the portal vein injection, which can bypass the oral feeding with parasite eggs. The protoscolices migrate in the portal vein with blood flow, settle in the liver, and develop into orthotopic hepatic hydatid cysts, resembling the natural infection route and course. The lesson that can be learnt from this study is protoscolex collection. Only the fresh protoscolex can result in success parasite growth.
With this model, the further anti-hydatid medicines and interventional treatment can be tried. With the quantitative immune results, the effects can be monitored by blood test.
Using standard score calculated as (raw score - mean)/SD, the best injection method has been screened. This value allows comparisons to be made between the three models with different distribution characteristics. The portal vein injection at 200 protoscolices in 100 μL saline is the best method for the future model.
The authors would like to thank Dr. Jian-Hua Wang and Dr. Xin-Yu Duan from the First Affiliated Hospital of Xinjiang Medical University for their kind technical support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sugimura H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Possenti A, Manzano-Román R, Sánchez-Ovejero C, Boufana B, La Torre G, Siles-Lucas M, Casulli A. Potential Risk Factors Associated with Human Cystic Echinococcosis: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10:e0005114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Mandal S, Mandal MD. Human cystic echinococcosis: epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac J Trop Med. 2012;5:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Shaikenov BS, Rysmukhambetova AT, Massenov B, Deplazes P, Mathis A, Torgerson PR. Short report: the use of a polymerase chain reaction to detect Echinococcus granulosus (G1 strain) eggs in soil samples. Am J Trop Med Hyg. 2004;71:441-443. [PubMed] |
| 4. | Gemmell MA. Safe handling of infected definitive hosts and eggs of Echinococcus spp. Bull World Health Organ. 1968;39:122-125. [PubMed] |
| 5. | Youssefi MR, Mirshafiei S, Moshfegh Z, Soleymani N, Rahimi MT. Cystic echinococcosis is an occupational disease? J Parasit Dis. 2016;40:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Farahmand M, Yadollahi M. Echinococcosis: an occupational disease. Int J Occup Environ Med. 2010;1:88-91. [PubMed] |
| 7. | Craig PS, Woods ML, Boufana B, O’Loughlin B, Gimpel J, San Lett W, McManus DP. Cystic echinococcosis in a fox-hound hunt worker, UK. Pathog Glob Health. 2012;106:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Yang S, Wu W, Tian T, Zhao J, Chen K, Wang Q, Feng Z. Prevalence of Cystic Echinococcosis in Slaughtered Sheep as an Indicator to Assess Control Progress in Emin County, Xinjiang, China. Korean J Parasitol. 2015;53:355-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Qingling M, Guanglei W, Jun Q, Xinquan Z, Tianli L, Xuemei S, Jinsheng Z, Huisheng W, Kuojun C, Chuangfu C. Prevalence of hydatid cysts in livestock animals in Xinjiang, China. Korean J Parasitol. 2014;52:331-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Wang S, Yang T, Zhang X, Xia J, Guo J, Wang X, Hou J, Zhang H, Chen X, Wu X. Construction of In Vivo Fluorescent Imaging of Echinococcus granulosus in a Mouse Model. Korean J Parasitol. 2016;54:291-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Díaz A, Casaravilla C, Barrios AA, Ferreira AM. Parasite molecules and host responses in cystic echinococcosis. Parasite Immunol. 2016;38:193-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Durgun Yetim T, Basoglu A, Taslak Sengul A, Yetim I, Serdar Bekdemir O, Hokelek M. Comparison of the protoscolocidal effectiveness of hypertonic saline, povidone-iodine and albendazole solutions in an experimental lung hydatid cyst model. J Int Med Res. 2011;39:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Gorgas D, Marreros N, Rufener R, Hemphill A, Lundström-Stadelmann B. To see or not to see: non-invasive imaging for improved readout of drug treatment trials in the murine model of secondary alveolar echinococcosis. Parasitology. 2017;144:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Joekel DE, Deplazes P. Optimized dexamethasone immunosuppression enables Echinococcus multilocularis liver establishment after oral egg inoculation in a rat model. Exp Parasitol. 2017;180:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Wang S, Yang T, Zhang X, Xia J, Guo J, Wang X, Hou J, Zhang H, Chen X, Wu X. Construction of In Vivo Fluorescent Imaging of Echinococcus granulosus in a Mouse Model. Korean J Parasitol. 2016;54:291-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |