Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7888
Peer-review started: May 12, 2017
First decision: June 5, 2017
Revised: September 12, 2017
Accepted: September 26, 2017
Article in press: September 26, 2017
Published online: November 28, 2017
Processing time: 201 Days and 8.6 Hours
To determine the association of circulating miR-125a/b expression with the risk and disease severity of Crohn’s disease (CD), and with inflammatory cytokines.
Plasma samples were collected from patients with active CD (A-CD), or CD in remission (R-CD) and from healthy controls (HCs). The levels of the inflammatory cytokines interleukin-17 (IL-17), tumour necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were measured by enzyme-linked immunosorbent assay. The expression of miR-125a/b was assessed by quantitative polymerase chain reaction (qPCR).
Twenty-nine A-CD patients, 37 R-CD patients, and 37 HCs were included in the study. Plasma miR-125a expression was decreased in A-CD patients compared with that in R-CD patients (P < 0.001) and HCs (P < 0.001). miR-125a expression levels enabled the differentiation of A-CD from R-CD patients [area under curve (AUC) = 0.854] and from HCs (AUC = 0.780), whereas miR-125b expression did not. miR-125a was negatively correlated with C-reaction protein (CRP) (P = 0.017), erythrocyte sedimentation rate (ESR) (P = 0.026), Crohn’s disease activity index (CDAI) (P = 0.003), IL-17 (P = 0.015), and TNF-α (P = 0.004) in A-CD patients. Furthermore, miR-125a was negatively associated with CRP (P = 0.038) and CDAI (P = 0.021) in R-CD patients. Regarding miR-125b, no association with CRP, CDAI, IL-17, TNF-α, or IFN-γ was found in A-CD or in R-CD patients. miR-125a levels gradually increased in A-CD patients who achieved clinical remission (P = 0.009) after 3-mo treatment, whereas they remained unchanged among patients who failed to achieve remission. No changes in miR-125b expression were detected in remission or non-remission patients after treatment.
Circulating miR-125a but not miR-125b is decreased in patients with active disease status and negatively correlates with disease severity and inflammatory cytokines in patients with CD.
Core tip: This study aimed to investigate the association of circulating miR-125a/b expression with the risk and severity of Crohn’s disease (CD) and with inflammatory cytokines. Our results showed that miR-125a but not miR-125b is negatively correlated with the risk of active CD and disease severity and with inflammatory cytokines.
- Citation: Sun CM, Wu J, Zhang H, Shi G, Chen ZT. Circulating miR-125a but not miR-125b is decreased in active disease status and negatively correlates with disease severity as well as inflammatory cytokines in patients with Crohn’s disease. World J Gastroenterol 2017; 23(44): 7888-7898
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7888.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7888
Crohn’s disease (CD), an idiopathic chronic inflammatory disease, is a form of inflammatory bowel disease (IBD) and primarily affects the gastrointestinal tract. This disease causes the development of ulcers and complications, including abscesses and fistulas, affecting all layers of the intestinal wall[1,2]. It is reported that CD has an annual incidence of approximately 24 per 100000 in Europe and 19 per 100000 in North America. However, the incidence rate of CD is still increasing in developing countries, particularly in China due to its considerable increase in gross domestic product (GDP)[3,4] and improvement in quality of life[5].
MicroRNAs (miRNAs) are small endogenous RNAs that can degrade targeted mRNA or inhibit protein synthesis, and increasing evidence shows that miRNAs play a key role in regulating the intestinal immune system[6-9]. For example, miR-29b inhibits transforming growth factor-β (TGF-β)-induced intestinal fibrosis, and let-7 regulates the activity of nuclear factor-κB (NF-κB) and mediates interleukin (IL)-6 down-regulation in CD patients[10,11]. Furthermore, miR-192, miR-142-3p, and miR-21 are notably upregulated in paediatric IBD patients[12], whereas miR-495-5p and miR-19b are down-regulated in the inflamed bowel of CD patients[13,14].
miRNA-125 (miR-125) family, an miRNA family highly conserved throughout evolution, consists of miR-125a and miR-125b[15]. It has been shown that miR-125a inhibits innate macrophage responses by suppressing macrophage differentiation, and the expression level of miR-125a is down-regulated in systemic lupus erythematosus (SLE)[16,17]. miR-125b was correlated with rheumatoid arthritis (RA) disease activity and may serve as a potential biomarker for treatment response in early RA[18]. To date, few studies have investigated the impact of dysregulated miR-125a/b expression in CD patients. Therefore, the aim of this study was to determine the association of circulating miR-125a/b expression with the risk and disease severity of CD and with inflammatory cytokines.
Twenty-nine clinically active CD (A-CD) patients and 37 patients with CD in clinical remission (R-CD) were enrolled in this study from May 2014 to June 2016 at the Department of Gastroenterology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology. The diagnosis of CD was determined according to the Lennard-Jones criteria[19], and active clinical disease was defined as Crohn’s disease activity index (CDAI) above 150 points. Patients with the following conditions were excluded: arthritis with complications or other autoimmune diseases; history of malignant tumour or complications; severe renal and/or kidney dysfunction; and history of serious surgery or severe infection. In the meantime, 37 healthy controls (HCs) age- and gender-matched to the CD patients were enrolled.
The study was approved by the Ethics Committee of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, and all participants provided written informed consent.
Blood samples were collected from all participants into EDTA tubes when they were enrolled in this study and after a 3-mo treatment in the case of A-CD patients. After collection, the blood samples were centrifuged at 1000 g for 15 mins at 4 °C; then, the supernatant (plasma) was removed and stored at -80 °C for further analysis. If red blood cell lysis was discovered, a repeat blood sample was collected from patients or HCs. Total RNA was extracted from the samples with TRIzol reagent (Invitrogen, CA, United States) according to the manufacturer’s instructions.
Total RNA was reverse transcribed using the PrimerScript Real-time Reagent kit (TAKARA BIO Inc. Shiga, Japan). Subsequently, miR-125 a/b expression levels were quantitated with SYBR Premix Ex TaqTM II (TAKARA BIO Inc., Shiga, Japan). The expression level of miR-125a/b was calculated using the 2-ΔΔt method and U6 was used as the internal reference.
IL-17, TNF-α, and IFN-γ measurement by ELISA
The measurement of IL-17, tumour necrosis factor (TNF-α), and interferon (IFN-γ) levels in plasma samples from A-CD and R-CD patients (but not HCs) was performed using commercial enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (all purchased from eBioscience, CA, United States).
C-reaction protein (CRP), erythrocyte sedimentation rate (ESR), and Crohn’s disease activity index (CDAI) were used to assess the severity of CD. In A-CD patients, effective treatments, including immunosuppressive agents and biologics, among others, were used, according to disease status and clinical experience. After 3-mo treatment, the CDAI and blood of patients were assessed again, and changes in miR-125a/b expression were analysed based on clinical remission achievement.
Statistical analyses were performed using SPSS 21.0 software program (IBM, United States) and MS Office 2016 (Microsoft, United States). Data are mainly expressed as mean ± standard deviation, median (1/4-3/4 quarters), or counts. Comparisons among three groups were performed by one-way analysis of variance (ANOVA), Kruskal-Wallis test by ranks, or χ2 test. Comparisons between groups or between each visit in the same group were performed using the Wilcoxon signed-rank test. Receiver operating characteristic (ROC) curve analysis was performed to assess the value of miR-125a/b expression in differentiating A-CD from R-CD patients and patients from HCs. Spearman’s test was used to analyse the correlation of miR-125a/b expression with disease severity and inflammatory cytokines. P < 0.05 was considered significant.
As shown in Table 1, the 29 patients of the A-CD group had a mean age of 31.38 ± 9.51 years, whereas the 37 patients of the R-CD and the 37 HCs had a mean ages of 31.97 ± 8.86 and 31.41 ± 5.69 years, respectively. No difference in age or gender was found between groups (P > 0.05), although significant differences in CRP and ESR (P < 0.001) were observed between groups. Significant differences in inflammatory cytokines IL-17, TNF-α, and IFN and in CDAI were also found between A-CD and R-CD patients (P < 0.001).
| Parameter | A-CD patients (n = 29) | R-CD patients (n = 37) | HCs (n = 37) | P |
| Age (yr) | 31.38 ± 9.51 | 31.97 ± 8.86 | 31.41 ± 5.69 | 0.941a |
| Gender (female/male) | 17/12 | 20/17 | 18/19 | 0.719a |
| CRP (mg/L) | 45.96 (32.27-67.26) | 18.94 (10.78-26.71) | 3.99 (3.33-6.06) | < 0.001a |
| ESR (mm/H) | 43.93 (37.46-57.82) | 16.73 (11.36-20.40) | 13.02 (5.82-15.27) | < 0.001a |
| CDAI score | 206.0 (170.5-253.0) | 95.0 (75.5-112.0) | - | < 0.001c |
| IL-17 (pg/mL) | 46.90 (38.46-60.06) | 18.07 (10.21-20.17) | - | < 0.001c |
| TNF-α (pg/mL) | 72.77 (53.46-83.39) | 22.17 (19.49-30.43) | - | < 0.001c |
| INF-γ (pg/mL) | 33.23 (24.59-39.54) | 11.62 (9.16-15.07) | - | < 0.001c |
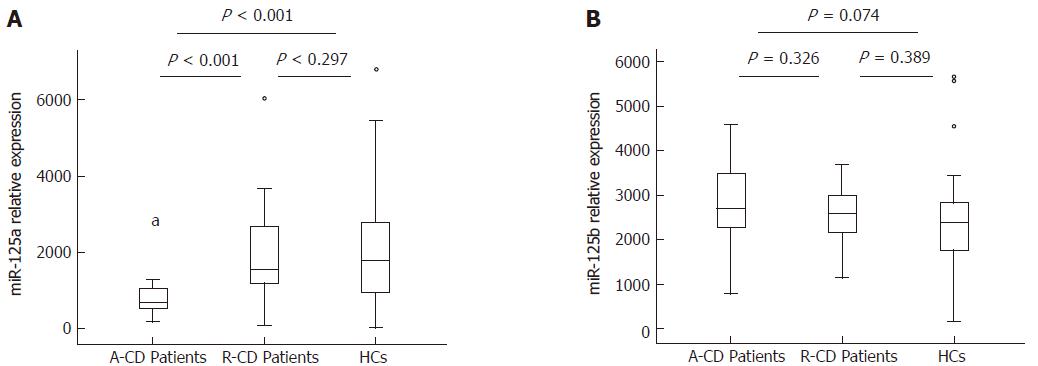
Plasma miR-125a expression was decreased in A-CD patients [0.719 (0.534-1.072)] compared with that of R-CD patients [1.564 (1.159-2.851), P < 0.001] and HCs [1.781 (0.874-2.873), P < 0.001]. However, no significant difference between R-CD patients and HCs (P = 0.297) was found (Figure 1A). No difference in miR-125b expression was observed between A-CD [2.707 (2.168-3.531)] and R-CD patients [2.600 (2.154-3.064)] and between patients and HCs [2.393 (1.705-2.852)], as shown in Figure 1B.
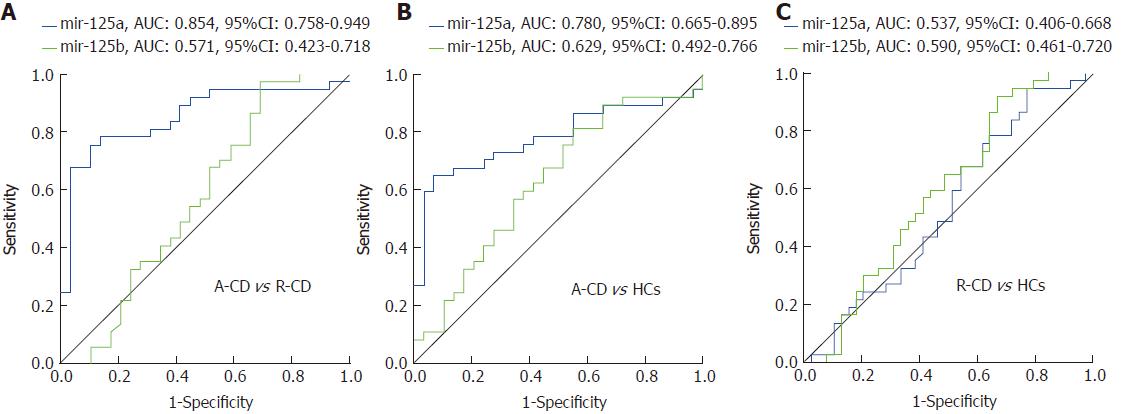
Interestingly, diagnostic value was discovered for miR-125a in differentiating A-CD from R-CD patients [area under ROC curve (AUC) = 0.854, 95%CI: 0.758-0.949) and patients from HCs (AUC = 0.780, 95%CI: 0.665-0.895] (Figure 2A and B). However, miR-125b failed as a predictive factor in differentiating A-CD from R-CD patients (AUC = 0.571, 95%CI: 0.423-0.718) and patients from HCs (AUC = 0.629, 95%CI: 0.492-0.766) (Figure 2A and B). Moreover, neither miR-125a nor miR-125b was able to differentiate R-CD patients from HCs (AUC = 0.537, 95%CI: 0.406-0.668; AUC = 0.590, 95%CI: 0.461-0.720, respectively) (Figure 2C).
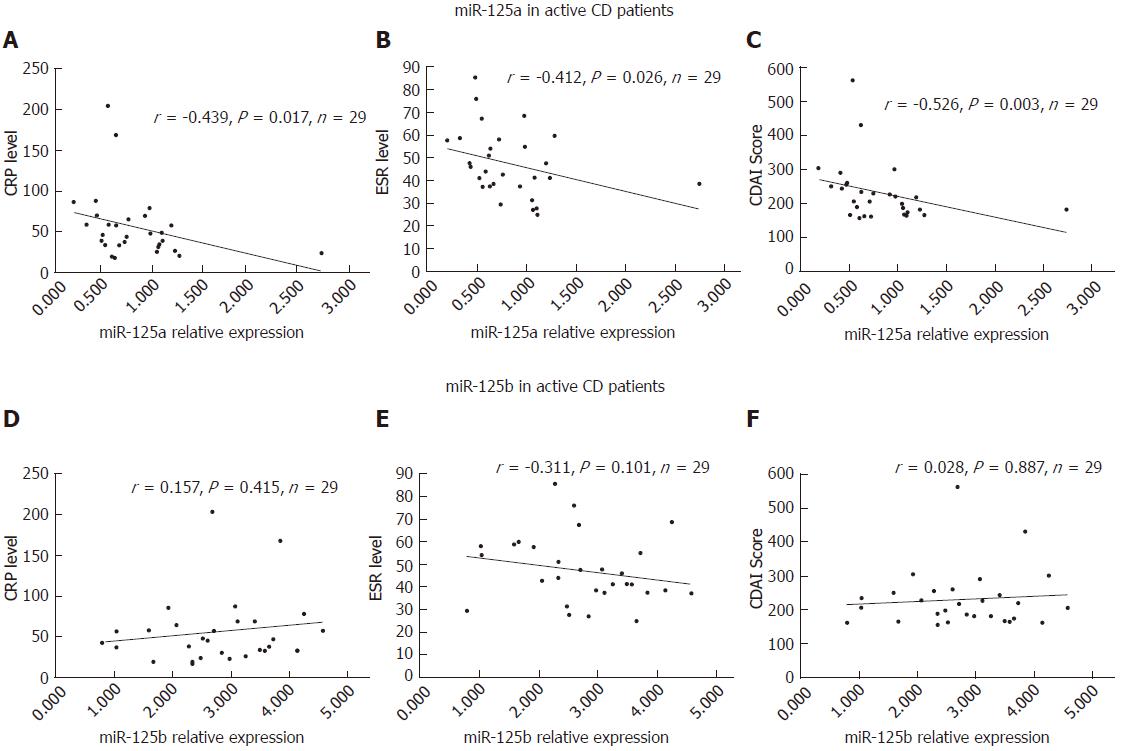
Negative correlations of miR-125a with CRP (r = -0.439, P = 0.017), ESR (r = -0.412, P = 0.026), and CDAI (r = -0.526, P = 0.003) in A-CD patients were observed, as shown in Figure 3A-C. However, no association was found between miR-125b and CRP, ESR, or CDAI in A-CD patients (Figure 3D-F).
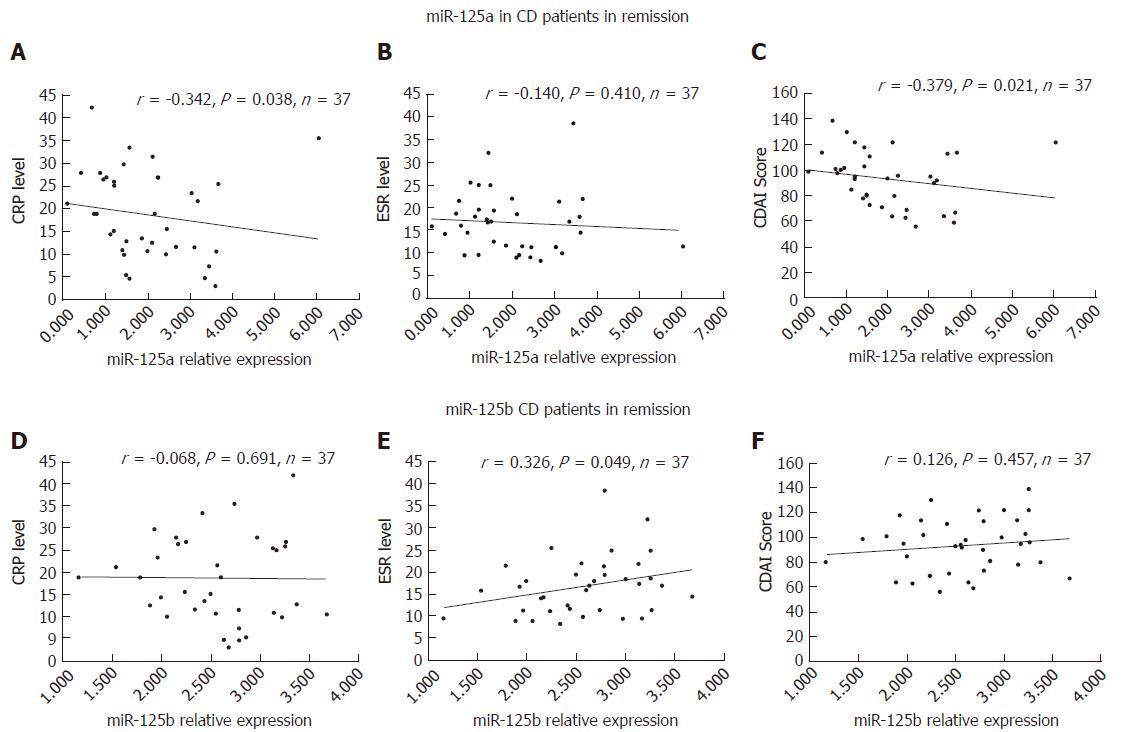
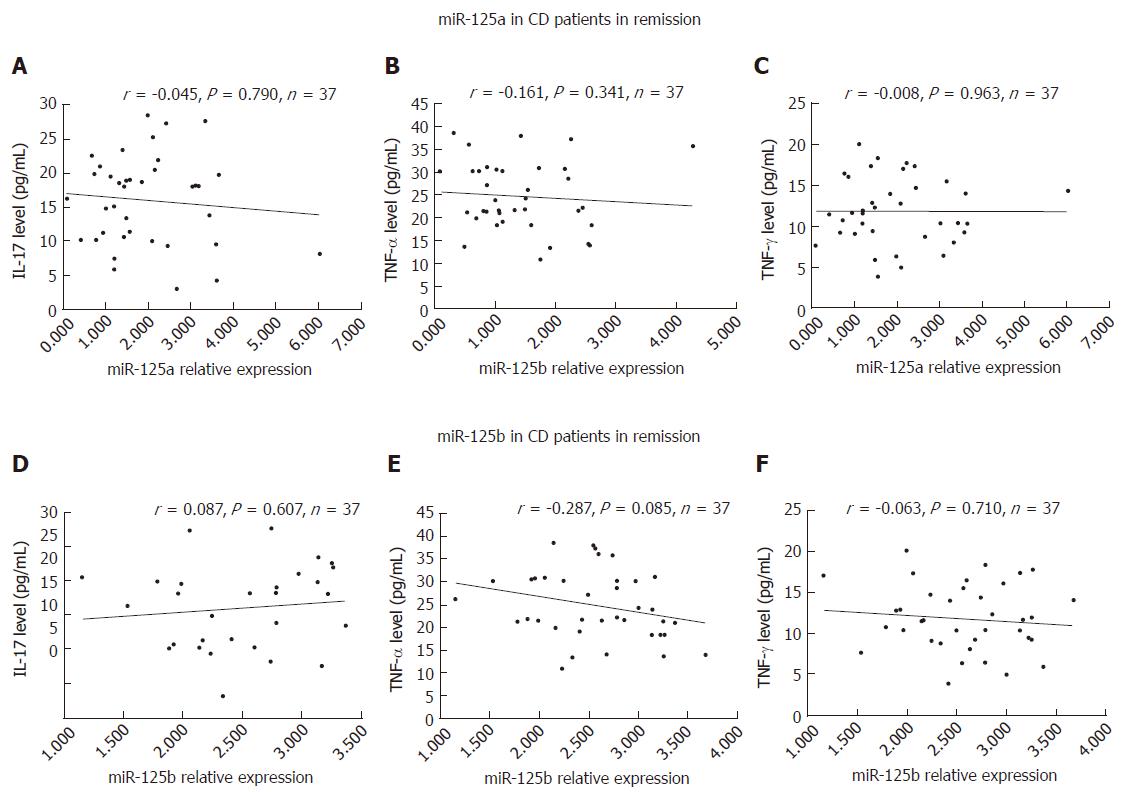
Regarding R-CD patients, we found that the expression of miR-125a was negatively correlated with CRP (r = -0.342, P = 0.038) and CDAI (r = -0.379, P = 0.021), but not ESR (r = -0.140, P = 0.410) (Figure 4A-C). In R-CD patients, miR-125b was significantly associated with ESR (r = 0.326, P = 0.049), but not with CRP (r = -0.068, P = 0.691) or CDAI (r = 0.126, P = 0.457) (Figure 4D-F).
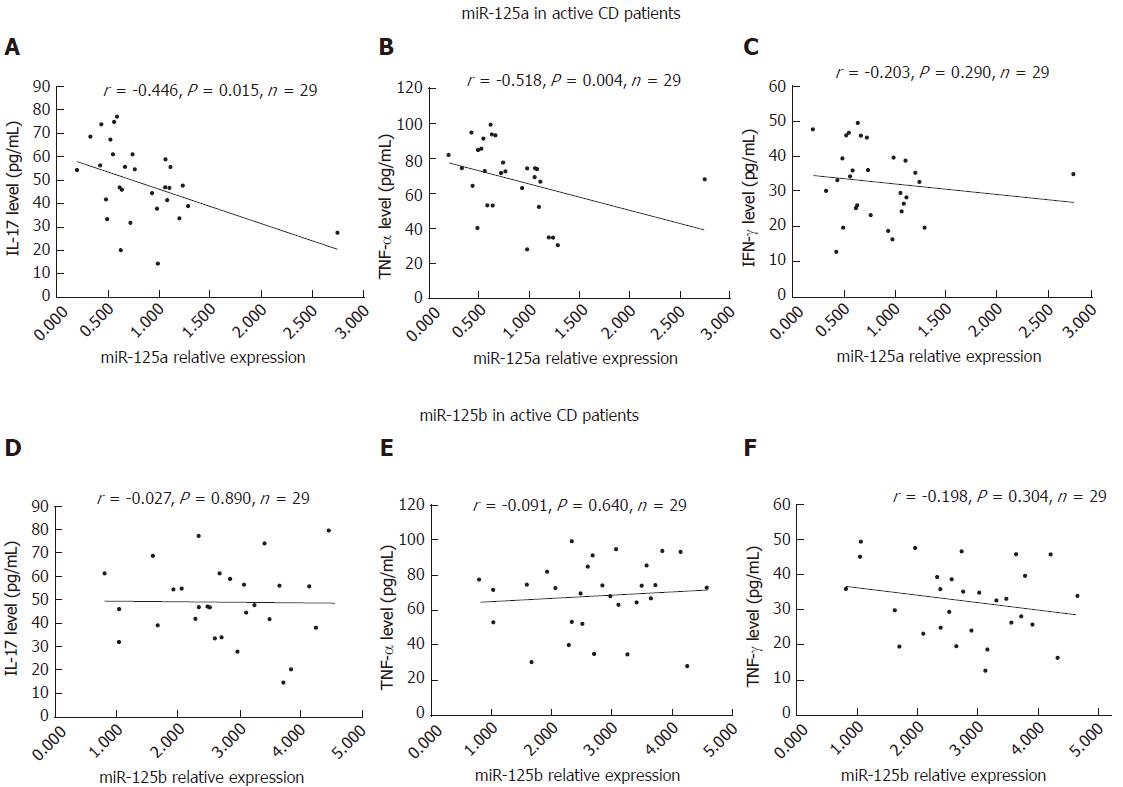
Negative correlations were detected for miR-125a with the inflammatory factors IL-17 (r = -0.446, P = 0.015) and TNF-α (r = -0.518, P = 0.004), but not with IFN-γ (r =- 0.203, P = 0.290), in A-CD patients, as shown in Figure 5A-C. However, no association of miR-125b with the inflammatory factors IL-17 (r = -0.027, P = 0.890), TNF-α (r = 0.091, P = 0.640) and IFN-γ (r = -0.198, P = 0.304) was found in A-CD patients (Figure 5D-F). Regarding R-CD patients, we observed no correlation of miR-125a or miR-125b with the inflammatory factors IL-17, TNF-α or IFN-γ, as shown in Figure 6A-F.
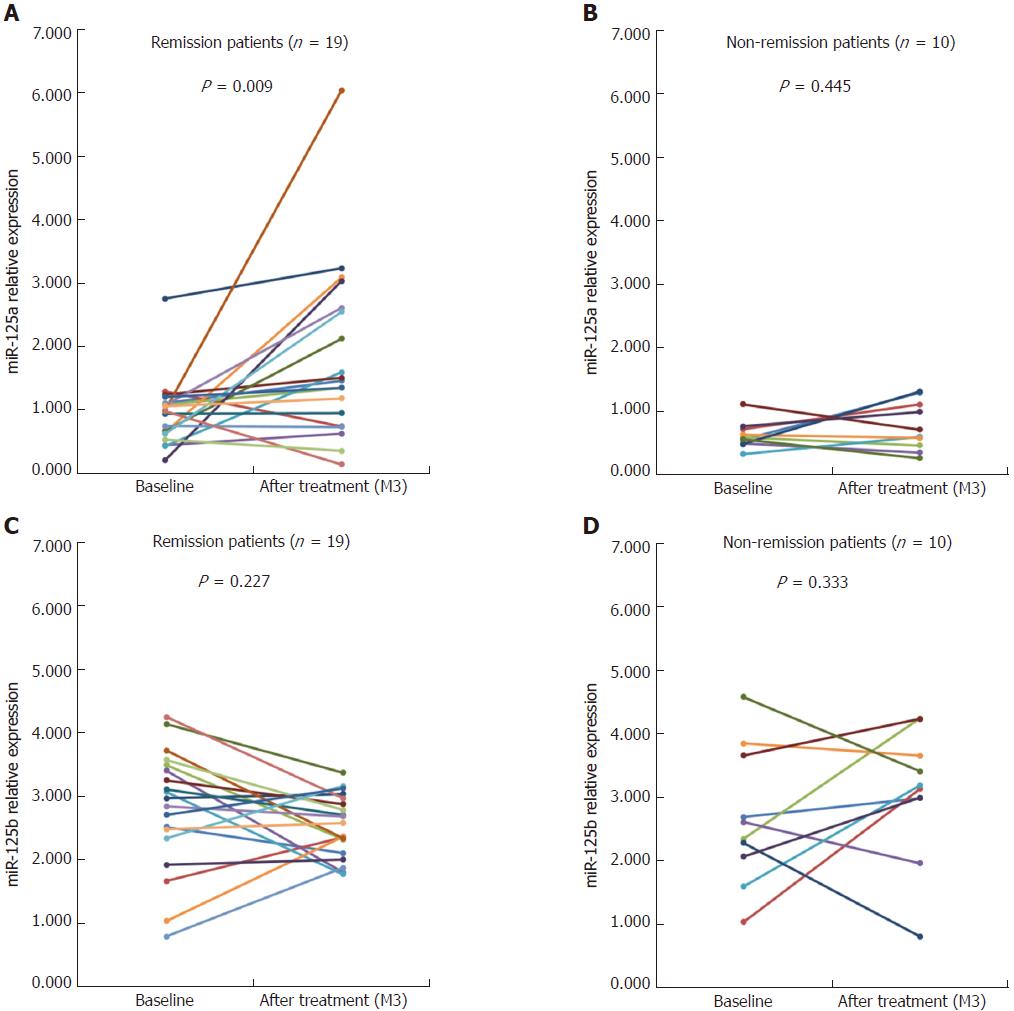
After 3-mo treatment, 19 of 29 A-CD patients achieved clinical remission (CDAI < 150), whereas 10 patients failed to achieve clinical remission. In patients with clinical remission, the miR-125a levels dramatically increased compared with the baseline (P = 0.009); conversely, in non-remission patients, miR-125a levels remained unchanged compared with the baseline. No changes in miR-125b expression were detected, either in remission or non-remission patients after treatment (Figure 7).
CD, a chronic debilitating syndrome, affects all layers of the gastrointestinal tract, causes severe diarrhea, abdominal pain, weight loss, metabolic disorder, and malabsorption, and is a major health concern, leading to huge financial losses of up to $2.2 billion dollars per year in the United States alone[20-23]. Although the pathogenesis of CD is still unclear, accumulating evidence shows that environmental factors, genetics, autoimmunity, and dietary habits may contribute to its development and progression[24,25]. As an important genetic factor, miRNA dysregulation is involved in the aetiological mechanism of several autoimmune diseases, including CD[26]; however, the role of miR-125a/b expression in CD risk and management is largely unstudied[27].
In this prospective study, we observed three notable findings. First, miR-125a expression was decreased in A-CD patients compared with R-CD patients and HCs. The results also showed the ability of miR-125a expression levels to distinguish A-CD from R-CD patients and from HCs, which was not possible using miR-125b expression levels. Second, miR-125a was negatively correlated with disease severity in A-CD patients, and negative correlations of miR-125a with inflammatory cytokines were also found in A-CD patients. Third, miR-125a levels were dramatically increased in A-CD patients who achieved clinical remission after 3-mo treatment, whereas miR-125a levels remained unchanged in non-remission patients. No changes in miR-125b expression were detected in remission or non-remission A-CD patients after treatment.
The miR-125a gene is located on chromosome 19 and in a cluster with miR-99b and miR-7e[28]. In the healthy population, the largest contributor of circulating miR-125a may be germinal center (GC) and hematopoietic stem cells (HSCs). Shaham et al suggest that miR-125a is enriched in HSCs (up to 23-fold more than in total bone marrow), particularly in long-term HSCs (up to 6-fold). Moreover, miR-125a is not restricted to the stem cell population, and its cluster members, miR-99b and let-7e, are preferentially expressed by centroblasts in the GC[16]. Studies show that miR-125a is down-regulated in peripheral CD3+ T cells and negatively correlated with RANTES (also known as CCL5 chemokine) expression by targeting the KLF13 gene in SLE patients[17]. Interestingly, miR-125a expression is also decreased in oral lichen planus, which is a T-cell-mediated autoimmune disease of the oral mucosa[29]. Furthermore, miR-125a is identified as a key regulator of CD4+ T-cell differentiation that prevents autoimmune pathogenesis by controlling the balance between tolerance and autoimmunity[30]. Recently, it was reported that miR-125a participates in immune thrombocytopenic purpura (ITP), by modulating Tregs and Th17[31], which play a key role in CD development and progression and are correlated with CD disease activity[32-34]. These studies indicate that miR-125a is an anti-inflammatory gene that plays a key role in regulating autoimmune diseases, which is consistent with our results showing that miR-125a expression is decreased in A-CD patients and may be used to differentiate A-CD from R-CD patients and from HCs. This might be due to the anti-inflammatory effect of miR-125a, because the levels of inflammatory cytokines were markedly increased in A-CD patients compared with those of R-CD patients and HCs, whereas the extent of inflammation in R-CD patients was similar to that of HCs. The results showing a negative correlation between miR-125a and the inflammatory cytokines IL-17 and TNF-α further confirmed this point of view.
In addition, we found a negative association of miR-125a with disease severity in A-CD patients. Partly in line with our results, Murata et al[35] reported that miR-125a was negatively correlated with some indices of disease activity including CRP in RA, and reduced levels of miR-125a were associated with severe trauma through inflammatory cytokine IL-10 regulation, as shown in polytrauma patients[36], most likely, because of the powerful anti-inflammatory effect of miR-125a. Thus, miR-125a was able to predict the disease severity of CD. Furthermore, we found that the miR-125a levels were dramatically increased in A-CD patients who achieved clinical remission after 3-mo treatment, whereas they remained unchanged in patients who failed to achieve remission. The results proved once again that miR-125a is closely related to inflammation and might be a therapeutic target for CD in the future.
miR125-b has been shown to target the 3’ untranslated region of the TNF-α gene to negatively regulate the inflammatory response[37]. However, another study has shown that miR-125b could promote macrophage mediated inflammation by increasing the expression of co-stimulatory factor[38]. These studies suggest that miR-125b has both anti- and pro-inflammatory effects. We found no correlation of miR-125b with inflammatory factors in A-CD patients, and miR-125b was not able to differentiate A-CD from R-CD patients and from HCs, most likely because miR-125b has both anti- and pro-inflammatory effects, which has been reported in previous studies. Thus, the role of miR-125b in regulating inflammation in CD remains unclear[16,39,40]. Then, we analysed the target genes of miR-125a and miR-125b by Validated Target Module-MicroRNA-gene analysis using the software miRWalk 2.0[41], which showed that miR-125a had 234 reported target genes, whereas miR-125b had 391 reported target genes, and that they shared 110 target genes. Conversely, most miR-125a and miR-125b target genes were different, which may explain the differences between miR-125a and miR-125b in CD.
To the best of our knowledge, this was the first study investigating the correlation between circulating miR-125a/b expression and the risk and disease severity of CD and with inflammatory cytokines. Notwithstanding, there were still some limitations in our study. First, we did not detect the expression of miR-125a/b in the intestinal tract, where miRNA dysregulation might be more frequent than in blood. However, it is difficult to obtain normal intestinal tract tissue from HCs and CD patients who remain remission. Second, the sample size of our study was relatively small, and a larger sample is needed for further studies. Third, IL-17, TNF-α, and IFN-γ expression was not detected in HCs in this study, which would help to further elucidate the role of miR-125a/b in inflammation, which we will investigate in future studies. Finally, the levels of Tregs and Th17 cells, which are essential for CD development and progression, were not determined in the present study. We will also assess these levels in future studies.
In summary, circulating miR-125a but not miR-125b is decreased in active disease status and negatively correlates with disease severity and inflammatory cytokines in patients with Crohn’s disease. Therefore, this study sheds some light on the measurement of circulating miR-125a for the diagnosis and treatment of CD patients.
Crohn’s disease (CD), an idiopathic chronic inflammatory disease, is a form of inflammatory bowel diseases (IBD) and primarily affects the gastrointestinal tract. Few studies have investigated the impact of dysregulated miR-125a/b expression in CD patients.
Accumulating evidence shows that environmental factors, genetics, autoimmunity, and dietary habits may contribute to the development and progression of CD. As an important genetic factor, dysregulated miRNA has been involved in the aetiological mechanism of several autoimmune diseases, including CD; however, the role of miR-125a/b expression in CD risk and management is largely unstudied.
This was the first study that investigated the correlation between circulating miR-125a/b expression and the risk and disease severity of CD and inflammatory cytokines. The authors showed that miR-125a but not miR-125b is negatively associated with the risk for A-CD and disease severity and with inflammatory cytokines.
miR-125a is negatively associated with the risk for A-CD patients and disease severity and with inflammatory cytokines. In the near future, miR-125a may be an important marker for the diagnosis and treatment of CD patients.
Crohn’s disease activity index (CDAI) was used to differentiate A-CD from R-CD patients. Patients with a CDAI above 150 points were defined as A-CD patients, and those with a CDAI below 150 points were defined as R-CD patients.
This work focused on two miRNA molecules and their potential relevance to IBD. It is a well-written and interesting manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Day AS, Yeligar SM S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Smids C, Horjus Talabur Horje CS, Nierkens S, Drylewicz J, Groenen MJM, Wahab PJ, van Lochem EG. Candidate Serum Markers in Early Crohn’s Disease: Predictors of Disease Course. J Crohns Colitis. 2017;11:1090-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Sandborn WJ, Rutgeerts P, Colombel JF, Ghosh S, Petryka R, Sands BE, Mitra P, Luo A. Eldelumab [anti-interferon-γ-inducible protein-10 antibody] Induction Therapy for Active Crohn’s Disease: a Randomised, Double-blind, Placebo-controlled Phase IIa Study. J Crohns Colitis. 2017;11:811-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MN, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJ, Chan FK; Asia–Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 595] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 4. | Wang YF, Ouyang Q, Hu RW. Progression of inflammatory bowel disease in China. J Dig Dis. 2010;11:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3525] [Article Influence: 271.2] [Reference Citation Analysis (5)] |
| 6. | Pua HH, Ansel KM. MicroRNA regulation of allergic inflammation and asthma. Curr Opin Immunol. 2015;36:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14460] [Cited by in RCA: 16068] [Article Influence: 1004.3] [Reference Citation Analysis (2)] |
| 8. | Lodish HF, Zhou B, Liu G, Chen CZ. Micromanagement of the immune system by microRNAs. Nat Rev Immunol. 2008;8:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 356] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 9. | Wu LY, Ma XP, Shi Y, Bao CH, Jin XM, Lu Y, Zhao JM, Zhou CL, Chen D, Liu HR. Alterations in microRNA expression profiles in inflamed and noninflamed ascending colon mucosae of patients with active Crohn’;s disease. J Gastroenterol Hepatol. 2017;32:1706-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Nijhuis A, Biancheri P, Lewis A, Bishop CL, Giuffrida P, Chan C, Feakins R, Poulsom R, Di Sabatino A, Corazza GR. In Crohn’s disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin Sci (. Lond). 2014;127:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1167] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 12. | Zahm AM, Hand NJ, Tsoucas DM, Le Guen CL, Baldassano RN, Friedman JR. Rectal microRNAs are perturbed in pediatric inflammatory bowel disease of the colon. J Crohns Colitis. 2014;8:1108-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Guo Z, Wu R, Gong J, Zhu W, Li Y, Wang Z, Li N, Li J. Altered microRNA expression in inflamed and non-inflamed terminal ileal mucosa of adult patients with active Crohn’s disease. J Gastroenterol Hepatol. 2015;30:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 15. | Yin H, Sun Y, Wang X, Park J, Zhang Y, Li M, Yin J, Liu Q, Wei M. Progress on the relationship between miR-125 family and tumorigenesis. Exp Cell Res. 2015;339:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Lee HM, Kim TS, Jo EK. MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016;49:311-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Zhao X, Tang Y, Qu B, Cui H, Wang S, Wang L, Luo X, Huang X, Li J, Chen S. MicroRNA-125a contributes to elevated inflammatory chemokine RANTES levels via targeting KLF13 in systemic lupus erythematosus. Arthritis Rheum. 2010;62:3425-3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Hruskova V, Jandova R, Vernerova L, Mann H, Pecha O, Prajzlerova K, Pavelka K, Vencovsky J, Filkova M, Senolt L. MicroRNA-125b: association with disease activity and the treatment response of patients with early rheumatoid arthritis. Arthritis Res Ther. 2016;18:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1445] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 20. | Bao X, Feng Z, Yao J, Li T, Yin Y. Roles of Dietary Amino Acids and Their Metabolites in Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2017;2017:6869259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Neovius M, Arkema EV, Blomqvist P, Ekbom A, Smedby KE. Patients with ulcerative colitis miss more days of work than the general population, even following colectomy. Gastroenterology. 2013;144:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 23. | Sarmento A. Editorial: Understanding Crohn’s Disease: Immunity, Genes and Microbes. Front Immunol. 2017;8:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Valenti S, Gallizzi R, De Vivo D, Romano C. Intestinal Behçet and Crohn’s disease: two sides of the same coin. Pediatr Rheumatol Online J. 2017;15:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Burke KE, Boumitri C, Ananthakrishnan AN. Modifiable Environmental Factors in Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2017;19:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Cao B, Zhou X, Ma J, Zhou W, Yang W, Fan D, Hong L. Role of MiRNAs in Inflammatory Bowel Disease. Dig Dis Sci. 2017;62:1426-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Lee JC, Biasci D, Roberts R, Gearry RB, Mansfield JC, Ahmad T, Prescott NJ, Satsangi J, Wilson DC, Jostins L. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat Genet. 2017;49:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 28. | Lehmann TP, Korski K, Ibbs M, Zawierucha P, Grodecka-Gazdecka S, Jagodziński PP. rs12976445 variant in the pri-miR-125a correlates with a lower level of hsa-miR-125a and ERBB2 overexpression in breast cancer patients. Oncol Lett. 2013;5:569-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Hu JY, Zhang J, Cui JL, Liang XY, Lu R, Du GF, Xu XY, Zhou G. Increasing CCL5/CCR5 on CD4+ T cells in peripheral blood of oral lichen planus. Cytokine. 2013;62:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Pan W, Zhu S, Dai D, Liu Z, Li D, Li B, Gagliani N, Zheng Y, Tang Y, Weirauch MT. MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat Commun. 2015;6:7096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Li JQ, Hu SY, Wang ZY, Lin J, Jian S, Dong YC, Wu XF, Dai-Lan , Cao LJ. Long non-coding RNA MEG3 inhibits microRNA-125a-5p expression and induces immune imbalance of Treg/Th17 in immune thrombocytopenic purpura. Biomed Pharmacother. 2016;83:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Siakavellas SI, Bamias G. Role of the IL-23/IL-17 axis in Crohn’s disease. Discov Med. 2012;14:253-262. [PubMed] |
| 33. | Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 519] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 34. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1525] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 35. | Murata K, Furu M, Yoshitomi H, Ishikawa M, Shibuya H, Hashimoto M, Imura Y, Fujii T, Ito H, Mimori T. Comprehensive microRNA analysis identifies miR-24 and miR-125a-5p as plasma biomarkers for rheumatoid arthritis. PLoS One. 2013;8:e69118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Owen HC, Torrance HD, Jones TF, Pearse RM, Hinds CJ, Brohi K, O’Dwyer MJ. Epigenetic regulatory pathways involving microRNAs may modulate the host immune response following major trauma. J Trauma Acute Care Surg. 2015;79:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 1043] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 38. | Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O’Connell RM, Baltimore D. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187:5062-5068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 39. | Sun YM, Lin KY, Chen YQ. Diverse functions of miR-125 family in different cell contexts. J Hematol Oncol. 2013;6:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 40. | Shaham L, Binder V, Gefen N, Borkhardt A, Izraeli S. MiR-125 in normal and malignant hematopoiesis. Leukemia. 2012;26:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 41. | Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1371] [Article Influence: 97.9] [Reference Citation Analysis (0)] |