Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7110
Peer-review started: August 11, 2017
First decision: August 30, 2017
Revised: September 12, 2017
Accepted: September 26, 2017
Article in press: September 26, 2017
Published online: October 21, 2017
Processing time: 79 Days and 18.6 Hours
To evaluate the short- and long-term results of endoscopic ultrasound-guided transmural drainage (EUS-GTD) for pancreatic fluid collection (PFC) and identify the predictive factors of treatment outcome for walled-off necrosis (WON) managed by EUS-GTD alone.
We investigated 103 consecutive patients with PFC who underwent EUS-GTD between September 1999 and August 2015. Patients were divided into four groups as follows: WON (n = 40), pancreatic pseudocyst (PPC; n = 11), chronic pseudocyst (n = 33), and others (n = 19). We evaluated the short- and long-term outcomes of the treatment. In cases of WON, multiple logistic regression analyses were performed to identify the predictor variables associated with the treatment success. In addition, PFC recurrence was examined in patients followed up for more than 6 mo and internal stent removal after successful EUS-GTD was confirmed.
In this study, the total technical success rate was 96.1%. The treatment success rate of WON, PPC, chronic pseudocyst, and others was 57.5%, 90.9%, 91.0%, and 89.5%, respectively. Contrast-enhanced computed tomography using the multivariate logistic regression analysis revealed that the treatment success rate of WON was significantly lower in patients with more than 50% pancreatic parenchymal necrosis (OR = 17.0; 95%CI: 1.9-150.7; P = 0.011) and in patients with more than 150 mm of PFC (OR = 27.9; 95%CI: 3.4-227.7; P = 0.002).The recurrence of PFC in the long term was 13.3% (median observation time, 38.8 mo). Mean amylase level in the cavity was significantly higher in the recurrence group than in the no recurrence group (P = 0.02).
The reduction of WON by EUS-GTD alone was associated with the proportion of necrotic tissue and extent of the cavity. The amylase level in the cavity may be a predictive factor for recurrence of PFC.
Core tip: It remains unclear that which patients with walled-off necrosis (WON) can be resolved by endoscopic ultrasound-guided transmural drainage (EUS-GTD) alone and which ones should be treated by endoscopic necrosectomy or other additional treatment. In addition, some pancreatic fluid collections (PFCs) develop recurrent fluid collection, and it is also unclear which types of PFCs show recurrence. In this study, we demonstrated that PFC size and proportion of pancreatic parenchymal necrosis were related to the resolution of WON treated by EUS-GTD alone. Regarding long-term follow-up patients, mean amylase level in the cavity was associated with PFC recurrence, suggesting a prolonged stent placement in patients with predicted recurrence.
- Citation: Watanabe Y, Mikata R, Yasui S, Ohyama H, Sugiyama H, Sakai Y, Tsuyuguchi T, Kato N. Short- and long-term results of endoscopic ultrasound-guided transmural drainage for pancreatic pseudocysts and walled-off necrosis. World J Gastroenterol 2017; 23(39): 7110-7118
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7110.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7110
Pancreatic fluid collection (PFC) is a local complication after pancreatitis. Although most PFCs spontaneously improve, some PFCs remain and become infectious, thereby needing therapeutic intervention. In 2012, the revision of the Atlanta classification categorized PFC into the following four types: acute peripancreatic fluid collection, acute necrotic collection, pancreatic pseudocyst (PPC), and walled-off necrosis (WON). According to this revised classification, the development of PPC is considered to be extremely rare in acute pancreatitis, and most PFCs over 4 wk are classified as WON[1]. This suggests that many PFCs after acute pancreatitis that were treated as PPC because of slight debris in the cavity should be addressed as WON, according to the revised classification.
Endoscopic ultrasound-guided transmural drainage (EUS-GTD) is now widely accepted as a minimally invasive method for managing PFC with minimal complications[2]. According to previous reports, most patients with PPC achieved treatment success by EUS-GTD alone[3-6]. Conversely, the treatment success rate of WON by EUS-GTD alone was relatively lower than that of PPC[4,7]. Endoscopic necrosectomy (EN) was performed in patients with no clinical improvement by EUS-GTD. In a recent review of 10 series of EN, the overall treatment success rate was 76%, with 5% procedure-related mortality and 27% morbidity[8]. Although EN is less invasive than surgical necrosectomy, serious complications associated with EN have been reported lately. More recently, novel, fully covered biflanged metal stents have been reported to be effective and feasible for the treatment of WON[9-12]. However, the criteria for WON that should be treated by EN or/and with these metal stents remain unclear. Therefore, it is essential to clarify the cases that can be resolved by EUS-GTD alone and those that should be treated with EN and/or metallic stents in addition to EUS-GTD. To the best of our knowledge, to date, no report has investigated the predictive factors of treatment outcome managed by EUS-GTD alone using the definition of WON in the 2012 Atlanta classification.
Despite initial treatment success, some PFCs develop recurrent fluid collection owing to disconnected pancreatic duct syndrome (DPDS)[13,14]. Although long-term PFC recurrence is unknown, recommendations for permanent stent placement have been reported; however, stent migration or obstruction should be considered[13].
In this study, we classified PFC into the following four groups according to the 2012 Atlanta classification: WON; PPC; chronic pseudocyst; and others, including trauma, pancreatic cancer, and pancreatic fistula (after pancreatic surgery). We evaluated patients characteristics, technical success, treatment success, and complications in these four groups. In particular, we compared the clinical features between the treatment success and failure to identify the factors that affect the treatment outcome in patients with WON managed by EUS-GTD alone. The long-term follow-up results of patients who underwent EUS-GTD were assessed and predictive factors for recurrence of PFC were identified.
We retrospectively investigated 103 consecutive patients with PFC who underwent EUS-GTD between September 1999 and August 2015 at Chiba University Hospital (Chiba, Japan). Mean age of patients was 54.7 years, and a majority of patients were males. PFC caused by acute pancreatitis was classified according to the 2012 Atlanta classification and definition. We distinguished between WON and PPC by contrast-enhanced computed tomography (CT) 1-4 wk after the onset of pancreatitis and evaluated the existence and extent of pancreatic parenchymal necrosis in all patients with PFC. A chronic pseudocyst is defined as a well-demarcated fluid collection without solid debris occurring in the setting of known chronic pancreatitis and the absence of recent severe acute pancreatitis[14]. Indications for EUS-GTD were as follows: (1) infected cases (fever and leukocytosis) despite the administration of intravenous antibiotics; and (2) symptomatic cases, such as abdominal pain or obstruction of the gastric outlet, intestinal system, or biliary system. Informed procedural consents were obtained from all patients.
Before EUS-GTD, a CT scan was obtained from all patients. The standard technique for EUS-GTD involved the following steps. A curved linear array EUS was used to visualize the extent of PFC and to determine the puncture site. Before puncturing, color Doppler was used to identify the regional vessels that needed to be avoided. The cavity was punctured with a 19-gage needle, and then PFC was performed for conducting blood biochemical tests and cultures. A guidewire was inserted through the puncture needle and coiled in PFC under fluoroscopic guidance. The punctured site was dilated by a dilator and a balloon dilator (sometimes an electric dilator was also used). Finally, a 7Fr double pigtail stent and a 7Fr nasocystic drainage catheter were emplaced. The nasocystic drainage catheter was removed after reduction of PFC, and the internal stent was removed within 6 mo after the treatment success.
We assessed the efficacy of EUS-GTD using CT. If the size of the cavity was not reduced after 1-2 wk, we performed additional procedures, such as the multiple transmural gateway technique, percutaneous drainage, or EN.
In this study, we defined technical success as achieving stent and/or nasocystic drainage catheter placement. We defined the treatment success as any reduction in the cavity size to less than 20 mm within 8 wk, as determined by a follow-up CT using EUS-GTD alone, without an additional treatment such as multiple transmural gateway technique, percutaneous drainage, or EN. The treatment success also included the improvement of symptoms. Recurrence was considered to have occurred if the size of the cavity increased to more than 20 mm, regardless of symptoms over 6 mo after EUS-GTD.
The factors associated with the clinical success and recurrence were determined using statistical comparisons. Continuous variables were presented as means (with standard deviations) and medians (with range) and compared using Mann-Whitney U test. Categorical variables were expressed as frequencies and proportions and compared using χ2 tests with Yates’ correction or Fisher’s exact test. In patients with WON, multiple logistic regression analyses were performed to identify the predictor variables associated with the treatment success. The optimal cut-off value of the variables that differentiated between recurrence and no recurrence was determined by the receiver-operating characteristic analysis. In addition, the area under the curve was calculated. The statistical significance was determined as P < 0.05, and datasets were compiled using Microsoft Excel (Microsoft Corporation, Redmond, WA, United States). In addition, the IBM SPSS Statistics software version 20.0 (IBM Corporation, Chicago, IL, United States) was used to perform all the statistical analyses. The statistical methods of this study were reviewed by Kengo Nagashima, PhD from Department of Global Clinical Research, Graduate School of Medicine, Chiba University.
According to the 2012 Atlanta classification and definition for acute pancreatitis, each PFC was classified as WON (n = 40), PPC (n = 11), chronic pseudocyst (n = 33), and others (n = 19) for 103 patients who underwent EUS-GTD. More than 50% of PFCs were located mainly in the pancreatic body or tail. Mean cavity size was 104.9 mm (Table 1).
| characteristics | Value |
| Age, yr | |
| mean (SD) | 54.7 (15.5) |
| Range | 15-89 |
| Median | 56 |
| Gender | |
| Male | 73 (70.9) |
| Female | 30 (29.1) |
| Type of pancreatitis | |
| Acute pancreatitis | 46(44.6) |
| Chronic pancreatitis | 38 (36.9) |
| Other | 19 (18.4) |
| Etiology of PFC | |
| Alcohol | 51 (49.5) |
| Idiopathic | 17 (16.5) |
| Gallstones | 15 (14.5) |
| Trauma | 6 (5.9) |
| Post-surgery (pancreatic fistula) | 7 (6.8) |
| Post-ERCP | 3 (2.9) |
| Pancreatic cancer | 4 (3.9) |
| Category of PFC | |
| WON | 40 (38.8) |
| Pancreatic pseudocyst | 11 (10.7) |
| Chronic pseudocyst | 33 (32.0) |
| Others (cancer/trauma/fistula) | 19 (18.5) |
| Main location of cavity | |
| Head | 19 (18.4) |
| Body or tail | 84 (81.6) |
| Size of cavity, mm (long axis) | |
| mean (SD) | 104.4 (49.0) |
| Range | 30-246 |
| Median | 100 |
Of 103 patients, 4 technically failed, resulting in the technical success rate of 96.1% (95%CI: 90.3%-98.9%). In the technically failed cases, each patient required one of the following additional treatments: surgery, transpapillary drainage, percutaneous drainage, extracorporeal shock wave lithotripsy (ESWL), and observation.
In the WON group (n = 40), a technical failure was reported in 1 patient and treatment success in 23 patients (57.5%; 95%CI: 40.9%-73.0%). The treatment success group comprised 2 patients who underwent percutaneous drainage for PFC distant from the main lesion treated by EUS-GTD. We could not achieve the treatment success by EUS-GTD alone in 16 patients, of whom 2 needed surgical treatment, 11 needed no surgical treatment, and 1 died. In the group treated without surgery, we performed EN in 5 patients, multiple transluminal gateway technique in 2 patients, percutaneous drainage in 5 patients, and continuing conservative treatment until reduction over 8 wk in 3 patients. After additional treatment, 2 patients died. All 3 patients who died after EUS-GTD or EN had a respiratory or renal failure.
In the PPC group (n = 11), there were no technical failures, and we achieved the treatment success in 10 patients (90.9%; 95%CI: 58.4%-99.8%). In the chronic pseudocyst group (n = 33), there were two technical failures, and of 31 patients, we achieved the treatment success in 30 patients (96.8%; 95%CI: 83.8%-99.9%). In the treatment success group of chronic pancreatitis, 3 patients underwent EUS-GTD for PFC distant from the main lesion treated by EUS-GTD. In the others group (n = 19), there was one technical failure, and we achieved the treatment success in 17 patients (94.4%; 95%CI: 73.2%-99.9%).
The procedural complications were encountered in 15 of 103 patients (14.6%), with cases of bleeding (n = 2), stent migration (n = 3), infection (n = 3), pneumoperitoneum (n = 2), localized peritonitis (n = 3), puncture into another organ (n = 1), and mediastinal emphysema (n = 1). All patients were managed conservatively without surgery.
We compared the treatment success group with the treatment failure group. Patients with more than 50% pancreatic parenchymal necrosis (P = 0.004) and a PFC of more than 150 mm (P < 0.001) on CT were significantly associated with treatment failure based on the univariate analysis. However, PFC with infection was not significant (Table 2). Patients with more than 50% pancreatic parenchymal necrosis (OR = 17.0; 95%CI: 1.9-150.7; P = 0.011) and with a PFC of more than 150 mm (OR = 27.9; 95%CI: 3.4-227.7; P = 0.002) on CT were also significantly associated with treatment failure based on the multiple logistic regression analysis (Table 3).
| Treatment success | P value | |||
| Yes (n = 23) | No (n = 16) | |||
| Age, yr | Mean (SD) | 57.8 (18.1) | 55.8 (13.1) | |
| Range | 15-85 | 30-83 | 0.484 | |
| Gender | Male | 16 (69.6) | 14 (87.5) | 0.359 |
| Etiology of pancreatitis | Alcohol | 8 (34.8) | 6 (37.5) | 0.862 |
| Body mass index | Mean (SD) | 23.3 (5.5) | 24.2 (3.6) | 0.203 |
| ASA classification ≥ 3 | Yes | 12 (52.2) | 13 (81.2) | 0.09 |
| Pancreatic parenchymal necrosis ≥ 50% | Yes | 2 (8.7) | 9 (56.3) | 0.004 |
| Duration from onset of pancreatitis to drainage, wk | Mean (SD) | 11.1 (7.8) | 9.4 (10.9) | |
| Range | 3.1-25.3 | 2.0-47.7 | 0.219 | |
| Size of cavity, mm (long axis) | Mean (SD) | 109.9 (35.7) | 156.9 (35.7) | |
| Range | 70-246 | 66-207 | < 0.001 | |
| Size of cavity ≥ 150 mm | Yes | 2 (8.7) | 11 (68.8) | < 0.001 |
| PFC with infection | Yes | 13 (56.5) | 13 (81.3) | 0.203 |
| Follow-up durations, mo | Mean (SD) | 26.9 (30.8) | 28.1 (33.9) | 0.808 |
| Range | 0.7-133.5 | 1.3-128.3 | ||
| Multiple logistic regression | OR | 95%CI | P value |
| Pancreatic parenchymal necrosis (< 50% vs ≥ 50%) | 17.0 | 1.9-150.7 | 0.011 |
| Size of cavity (< 150 mm vs ≥ 150 mm) | 27.9 | 3.4-227.7 | 0.002 |
The treatment success by EUS-GTD alone was not achieved in any patient with WON with more than 50% parenchymal necrosis and a with PFC of more than 150 mm diameter on CT but was achieved in 90% of patients with under 50% pancreatic parenchymal necrosis and with a PFC of less than 150 mm (Table 4). There were two cases of failure with fewer than 50% necrosis and with a PFC of less than 150 mm. In one case with a multilocular type of WON, the necrotic collection remained in the posterior pararenal extraperitoneal space, and we performed percutaneous drainage. In another case, extrapancreatic necrosis without pancreatic parenchymal necrosis extended widely, and EN was conducted.
| Size of cavity < 150 mm | Size of cavity ≥ 150 mm | |
| Pancreatic parenchymal necrosis < 50% | 90.5% (19/21) | 28.6% (2/7) |
| Pancreatic parenchymal necrosis ≥ 50% | 40.0% (2/5) | 0% (0/6) |
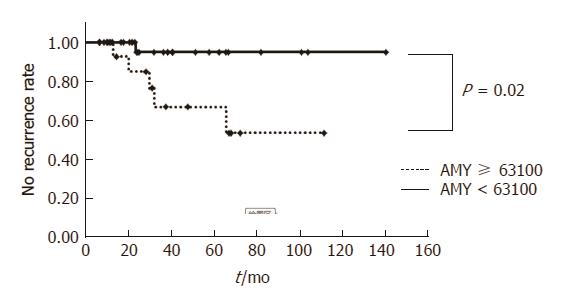
Overall, 75 patients were followed up for more than 6 mo, and the internal stent removal was confirmed. The median observation time was 38.8 mo. Of 75 patients, 10 patients suffered a recurrence, and the overall recurrence rate was 13.3%. The additional treatment for recurrence cases was additional EUS-GTD for 5 patients, transpapillary drainage for one patient, ESWL for one patient, surgery for 2 patients, and observation for one patient. In the recurrence group, the amylase level in the cavity was significantly higher than that in the no recurrence group (P = 0.01; Table 5) based on the univariate analysis. The amylase level at 63,100 in the cavity represented the most sensitive (83.3%) and specific (78.2%) point on the receiver-operating characteristic curve and corresponded to the largest area under the curve (0.820). No recurrence rate of the amylase level in the cavity ≥ 63100 was significantly lower than that of the amylase level in the cavity < 63100 (P = 0.02; Figure 1).
| Recurrence | P value | |||
| Yes (n = 10) | No (n = 65) | |||
| Age, yr | Mean (SD) | 54.4 (12.6) | 55.1 (15.9) | |
| Range | 37-79 | 15-89 | 0.719 | |
| Gender | Male | 9 (90) | 45 (69.2) | 0.266 |
| Type of pancreatitis | Chronic | 6 (60) | 22 (33.8) | 0.162 |
| Main location of cavity | Head | 1 (10) | 16 (24.6) | 0.677 |
| External drainage only | Yes | 2 (20) | 9 (13.8) | 0.634 |
| Duration of internal stent, days (n = 64) | Mean (SD) | 194.9 (106.1) | 243.2 (217.3) | |
| Range | 86-399 | 21-1387 | 0.659 | |
| Spontaneous dislodgement of stent (n = 64) | Yes | 4 (50) | 17 (30.4) | 0.421 |
| Size of cavity, mm (long axis) | Mean (SD) | 91.6 (38.0) | 102.6 (49.3) | |
| Range | 44-167 | 30-230 | 0.612 | |
| Amylase in cavity, IU/L (n = 57) | Mean (SD) | 96930 (55599) | 44719 (53790) | |
| Range | 31380-188000 | 30-273700 | 0.011 | |
| Median | 83075 | 31200 | ||
| PFC with infection | Yes | 2 (20) | 28 (43.1) | 0.298 |
In this study, we revealed the usefulness and feasibility of EUS-GTD for PFC; however, it was less effective in achieving the treatment success for WON as compared with other etiologies of PFC. The PFC size and the proportion of pancreatic parenchymal necrosis were related to the short-term outcomes of WON. In addition, mean amylase level in the cavity was associated with PFC recurrence, suggesting a prolonged stent placement for patients with predicted recurrence.
The treatment success rate of WON by EUS-GTD alone was 61.1%. Some retrospective studies have demonstrated a 45%-63% treatment success rate of EUS-GTD for WON[4,7]. A comparison of these results with our study is difficult because the definition of treatment success was different in each study, which in turn differed from our study, which was based on the 2012 Atlanta classification. A recent report, based on the 2012 Atlanta classification, revealed that the standard EUS-GTD using plastic stents or self-expandable metal stents resolved 70% of sterile WON and 40% of infected WON; however, the rest of WON required EN[15].
After treatment failure by EUS-GTD for WON, EN or other intervention, including surgical treatment, should be considered. A recent randomized controlled trial suggested that the IL-6 level following EN was significantly lower than that following surgical necrosectomy and that major complications or death occurred less frequently after EN compared to those after surgical necrosectomy[16]. Although EN should be considered for WON that cannot be resolved by EUS-GTD, serious complications of EN have been reported. Recently, while three multiple-center trials revealed 75%-91% success rate of EN, the associated mortality and morbidity rated were 5.8%-11% and 26%-33%, respectively[17-19]. In our study, we performed EN on 4 patients, and one patient died due to multiple organ failure. Therefore, if possible, it is better to accomplish the resolution of WON by EUS-GTD alone.
Some factors associated with the failed resolution of WON by EN or standard drainage and EN have been reported, including body mass index > 32[18], American Society of Anesthesiologists physical status classification ≥ 3[19], and multilocular morphology[15]. In this study, we elucidated that the treatment success rate of EUS-GTD alone for WON was significantly lower in patients with more than 50% pancreatic parenchymal necrosis or with a PFC of more than 150 mm on CT. All patients with WON with more than 50% parenchymal necrosis and with a PFC of more than 150 mm needed an additional treatment such as EN, whereas the treatment success was achieved in 90% patients with under 50% pancreatic parenchymal necrosis and within a PFC of 150 mm by EUS-GTD alone (Figure 2). If we can predict the treatment outcome by EUS-GTD with these parameters, we may be able to avoid unnecessary invasive therapy or make an earlier decision to perform additional treatment in a few days.
Instead of EUS-GTD, multiple transmural gateway technique for WON has been reported, and the treatment success rate of multiple transmural gateway technique was significantly better than that of EUS-GTD (94.4% vs 62.1%)[20,21]. More recently, novel, fully covered biflanged metal stents have been reported to be effective for the treatment of WON[9-12]. Regarding the use of these stents, high cost, stent migration, and other potential adverse events have been concerning[22]; therefore, optimal selection for using these stents is needed. According to our study, patients with PFC more than 50% pancreatic parenchymal necrosis or patients with more than 150 mm may be appropriate candidates for these treatments.
The proportion of pancreatic parenchymal necrosis could be associated with the amount of solid debris in the cavity of WON. Reportedly, the morphological findings of WON on EUS have therapeutic implications owing to the large size and more solid debris needing a more aggressive therapeutic method[23]. However, it is often challenging to estimate the necrotic component with the whole observation of PFC on EUS. In addition, even if rich debris seemed to exist in the cavity by EUS, some cases of WON could be resolved by EUS-GTD alone, as shown in Figure 2.
In this study, PPC was relatively rare (10.7%) based on the values in the 2012 Atlanta classification. Notably, 8 of 11 patients categorized as PPC were caused by acute pancreatitis occurring in the setting of known chronic pancreatitis and 3 were atypical etiology of pancreatitis (drug-induced in 2 patients and idiopathic in 1 patient). The treatment success rate of 90.9% for PPC in this study was higher than that of WON. EUS-GTD for chronic pseudocysts was successful. In chronic pancreatitis, PFC often communicates with the main pancreatic duct and stricture of this duct exists. To manage chronic pseudocysts, a combination of EUS-GTD with transpapillary drainage or ESWL for pancreatic stones might be effective.
After successful initial treatment following EUS-GTD for PFC, recurrence of PFC in the long term was higher in patients with higher amylase level in the cavity, indicating the communication of PFC with the pancreatic duct such as chronic pseudocysts or DPDS. DPDS is characterized by the main pancreatic duct cut-off, with an inability to access the upstream pancreatic duct during an ERCP, and CT evidence of viable pancreatic tissue upstream (toward the spleen), in association with a persistent non-healing pancreatic fistula or PFC[24,25]. We routinely removed internal stents following the resolution of PFC almost 6 mo after EUS-GTD. Although a permanent stent placement significantly reduced PFC recurrence in comparison with scheduled stent removal, migrated stents caused bowel obstruction that required surgery[21]. In cases with higher amylase levels in the cavity, transpapillary treatment or prolonged stent placement for more than 6 mo should be considered. However, the timing of stent removal and permanent stent placement remains controversial, and further study is required.
There were some limitations in this study. First, this was a retrospective study conducted at a single tertiary center and the number of patients with WON was relatively less. Second, the study period was long (16 years) and a learning curve might have influenced the results, although the standard technique for EUS-GTD has not changed. Third, there may have been a selection bias because we could not examine all patients with the amylase level in the cavity consecutively.
In conclusion, EUS-GTD is a successful and safe therapeutic technique in a majority of patients with PFC. The cavity size and proportion of pancreatic parenchymal necrosis are predictors for a successful treatment of WON. Higher amylase levels in the cavity might lead to PFC recurrence after stent removal. A prolonged stent placement should be considered in such cases.
The 2012 Atlanta classification categorized PFC into four types. The revised classification suggests that many PFCs after acute pancreatitis that were treated as pancreatic pseudocyst (PPC) because of slight debris in the cavity should be addressed as walled-off necrosis (WON). Most patients with PPC achieved treatment success by EUS-GTD alone. Although endoscopic necrosectomy (EN) was performed in patients with no clinical improvement by EUS-GTD, serious complications of EN have been reported. However, the criteria for WON that should be treated by EN or other additional treatment remained unclear. Therefore, it is crucial to clarify the cases that can be resolved by EUS-GTD alone and those that should be treated with EN or other treatment in addition to EUS-GTD. Some PFCs develop the recurrent fluid collection in long term. Recommendations of permanent stent placement have been reported to reduce recurrence; however, stent migration or obstruction is concerned. A predictive factor of PFC recurrence should also be clarified.
The treatment success rate of WON by EUS-GTD alone was relatively lower than that of PPC. It is unclear that which patients with WON can be treated by EUS-GTD alone and which ones by EN or other additional treatment including metallic stents. Despite initial treatment success, some PFCs develop the recurrent fluid collection in the long term. It is also unclear which types of PFCs show recurrence.
We aimed to evaluate the short- and long-term results of EUS-GTD for PFC following a revision of the PFC framework by the 2012 Atlanta classification and identify the predictive factors of treatment outcome for WON managed by EUS-GTD alone and predictive factors for recurrence of PFC.
The authors retrospectively investigated 103 consecutive patients with PFC who underwent EUS-GTD between September 1999 and August 2015 at Chiba University Hospital. The factors associated with clinical success and recurrence were determined using statistical comparisons. In patients with WON, multiple logistic regression analyses were performed to identify the predictor variables associated with the treatment success. In addition, PFC recurrence was examined in patients followed up over 6 mo and confirmed internal stent removal after successful EUS-GTD. The optimal cut-off value of the variables that differentiated between recurrence and no recurrence was determined by the receiver-operating characteristic analysis. In addition, area under the curve was calculated. The statistical significance was determined as P < 0.05.
The treatment success rate of WON, PPC, chronic pseudocyst, and others was 57.5%, 90.9%, 91.0%, and 89.5%, respectively. The treatment success rate of WON was significantly lower in patients with more than 50% pancreatic parenchymal necrosis (OR = 17.0: 95%CI: 1.9-150.7; P = 0.011) and in patients with more than 150 mm of PFC (OR = 27.9; 95%CI: 3.4-227.7; P = 0.002) on contrast-enhanced computed tomography using the multivariate logistic regression analysis. The recurrence of PFC in the long term was 13.3% (median observation time, 38.8 mo). Mean amylase level in the cavity was significantly higher in the recurrence group than in the no recurrence group (P = 0.02). In cases with higher amylase levels in the cavity, transpapillary treatment or prolonged stent placement for more than 6 mo should be considered. However, the timing of stent removal and permanent stent placement remains controversial, and further study is required.
Reduction of WON by EUS-GTD alone was associated with the proportion of necrotic tissue and the extent of the cavity. Amylase level in the cavity may be a predictive factor for recurrence of PFC.
According to our study, additional treatments, such as EN, should be considered after EUS-GTD in patients with WON with more than 50% parenchymal necrosis and a PFC of more than 150 mm, whereas it may not be needed with under 50% pancreatic parenchymal necrosis and within a PFC of 150 mm.
After successful initial treatment following EUS-GTD for PFC, recurrence of PFC in the long term was higher in patients with higher amylase level in the cavity. This finding indicates the communication of PFC with the pancreatic duct such as chronic pseudocyst or disconnected pancreatic duct syndrome. Therefore, in cases with higher amylase levels in the cavity, transpapillary treatment or prolonged stent placement should be considered.
In this study, we confirmed that the PFC size and the proportion of pancreatic parenchymal necrosis were related to the resolution of WON treated by EUS-GTD alone. If we can predict treatment outcome of WON by EUS-GTD alone, we might be able to avoid unnecessary invasive therapy or make an earlier decision to perform an additional treatment. Moreover, if we can predict recurrence of PFCs by the amylase level in the cavity, we might be able to reduce the recurrence of PFCs with prolonged stent placement or transpapillary treatment. Prospective studies with larger numbers of patients will be needed to confirm the reliability of these predictive factors.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Antonini F, Negoi I, Zerem E S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4337] [Article Influence: 361.4] [Reference Citation Analysis (45)] |
| 2. | van Brunschot S, Bakker OJ, Besselink MG, Bollen TL, Fockens P, Gooszen HG, van Santvoort HC; Dutch Pancreatitis Study Group. Treatment of necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2012;10:1190-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Barthet M, Lamblin G, Gasmi M, Vitton V, Desjeux A, Grimaud JC. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Gardner TB, Chahal P, Papachristou GI, Vege SS, Petersen BT, Gostout CJ, Topazian MD, Takahashi N, Sarr MG, Baron TH. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Haghshenasskashani A, Laurence JM, Kwan V, Johnston E, Hollands MJ, Richardson AJ, Pleass HC, Lam VW. Endoscopic necrosectomy of pancreatic necrosis: a systematic review. Surg Endosc. 2011;25:3724-3730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Itoi T, Nageshwar Reddy D, Yasuda I. New fully-covered self-expandable metal stent for endoscopic ultrasonography-guided intervention in infectious walled-off pancreatic necrosis (with video). J Hepatobiliary Pancreat Sci. 2013;20:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Mukai S, Itoi T, Baron TH, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy. 2015;47:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Yamamoto N, Isayama H, Kawakami H, Sasahira N, Hamada T, Ito Y, Takahara N, Uchino R, Miyabayashi K, Mizuno S. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Sharaiha RZ, Tyberg A, Khashab MA, Kumta NA, Karia K, Nieto J, Siddiqui UD, Waxman I, Joshi V, Benias PC. Endoscopic Therapy With Lumen-apposing Metal Stents Is Safe and Effective for Patients With Pancreatic Walled-off Necrosis. Clin Gastroenterol Hepatol. 2016;14:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, Devière J. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Baron TH, Harewood GC, Morgan DE, Yates MR. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7-17. [PubMed] |
| 15. | Mukai S, Itoi T, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R. Expanding endoscopic interventions for pancreatic pseudocyst and walled-off necrosis. J Gastroenterol. 2015;50:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 17. | Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Gardner TB, Coelho-Prabhu N, Gordon SR, Gelrud A, Maple JT, Papachristou GI, Freeman ML, Topazian MD, Attam R, Mackenzie TA. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Yasuda I, Nakashima M, Iwai T, Isayama H, Itoi T, Hisai H, Inoue H, Kato H, Kanno A, Kubota K. Japanese multicenter experience of endoscopic necrosectomy for infected walled-off pancreatic necrosis: The JENIPaN study. Endoscopy. 2013;45:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Bang JY, Wilcox CM, Trevino J, Ramesh J, Peter S, Hasan M, Hawes RH, Varadarajulu S. Factors impacting treatment outcomes in the endoscopic management of walled-off pancreatic necrosis. J Gastroenterol Hepatol. 2013;28:1725-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Bang JY, Hawes R, Bartolucci A, Varadarajulu S. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc. 2015;27:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Rana SS, Bhasin DK, Sharma RK, Kathiresan J, Gupta R. Do the morphological features of walled off pancreatic necrosis on endoscopic ultrasound determine the outcome of endoscopic transmural drainage? Endosc Ultrasound. 2014;3:118-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Pelaez-Luna M, Vege SS, Petersen BT, Chari ST, Clain JE, Levy MJ, Pearson RK, Topazian MD, Farnell MB, Kendrick ML. Disconnected pancreatic duct syndrome in severe acute pancreatitis: clinical and imaging characteristics and outcomes in a cohort of 31 cases. Gastrointest Endosc. 2008;68:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Tann M, Maglinte D, Howard TJ, Sherman S, Fogel E, Madura JA, Lehman GA. Disconnected pancreatic duct syndrome: imaging findings and therapeutic implications in 26 surgically corrected patients. J Comput Assist Tomogr. 2003;27:577-582. [PubMed] |