Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.7047
Peer-review started: June 14, 2017
First decision: July 17, 2017
Revised: August 18, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: October 14, 2017
Processing time: 124 Days and 19.5 Hours
Herein, we present a case of gastric adenocarcinoma of fundic gland type (GA-FG) spreading to heterotopic gastric glands (HGG) in the submucosa. A 58-year-old man with epigastric pain was referred to our hospital and underwent an esophagogastroduodenoscopy. A Borrmann type II gastric cancer at the antrum and a 10 mm submucosal tumor-like lesion in the lesser curvature of the upper third of the stomach were detected. Histological examination of the biopsy specimens obtained from the submucosal tumor-like lesion suggested a GA-FG. Therefore, endoscopic submucosal dissection was performed as excisional biopsy, and histopathological examination of the resected specimen confirmed a GA-FG and HGG proximal to the GA-FG. Although the GA-FG invaded the submucosal layer slightly, the submucosal lesion of the GA-FG had a poor stromal reaction and was located just above the HGG in the submucosa. Therefore, we finally diagnosed the lesion as a GA-FG invading the submucosal layer by spreading to HGG.
Core tip: A 58-year-old man had a 10mm submucosal tumor-like lesion in the lesser curvature of the upper third of the stomach. Histological examination of the biopsy specimens suggested a gastric adenocarcinoma of fundic gland type (GA-FG), therefore, endoscopic submucosal dissection was performed as excisional biopsy. Histopathological examination of the resected specimen confirmed a GA-FG invading the submucosal layer slightly, and heterotopic gastric glands (HGG) proximal to the GA-FG. A GA-FG spreading to HGG in the submucosa was diagnosed because the submucosal lesion of the GA-FG had a poor stromal reaction and was located just above the HGG.
- Citation: Manabe S, Mukaisho KI, Yasuoka T, Usui F, Matsuyama T, Hirata I, Boku Y, Takahashi S. Gastric adenocarcinoma of fundic gland type spreading to heterotopic gastric glands. World J Gastroenterol 2017; 23(38): 7047-7053
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/7047.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.7047
Gastric adenocarcinoma of fundic gland type (GA-FG) is a new histological type of gastric cancer proposed by Ueyama et al[1] in 2010. As the concept of GA-FG has spread since then, new findings have been reported[2]. GA-FG tends to invade the submucosal layer while it is still small because it arises from the deep layer of the lamina propria mucosae[1]. On the other hand, heterotopic gastric glands (HGG) are regarded as paracancerous lesions[3,4], and gastric cancers associated with HGG have been reported[5,6]. However, HGG are not considered to contribute to the development of GA-FG because the pathogenesis of GA-FG is different from that of the regular histological type of gastric cancer. We encountered a case of GA-FG suggested to have invaded the submucosal layer by spreading to HGG, which coincidentally existed proximal to the GA-FG. In addition to a GA-FG with a very rare mode of submucosal invasion, this case demonstrated the possibility of GA-FG arising from the gastric mucosa with atrophic change, provided there are remnant gastric fundic glands. Herein, we report this case with a brief review of the literature.
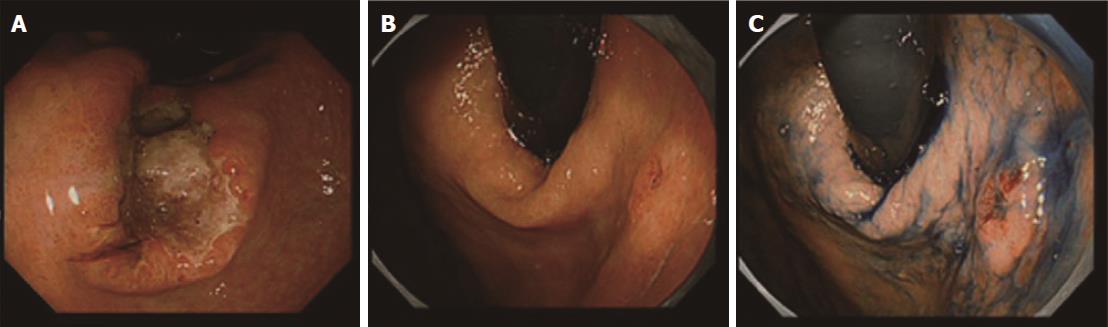
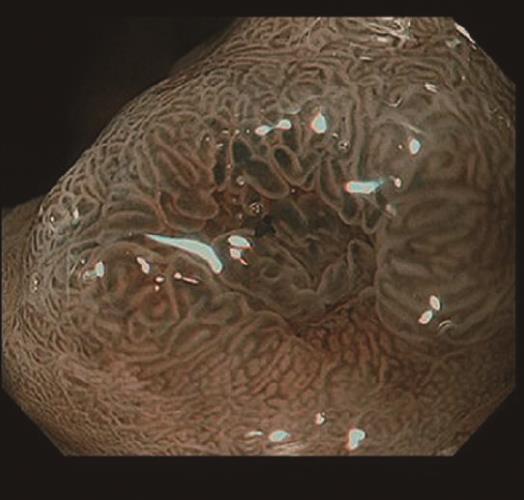
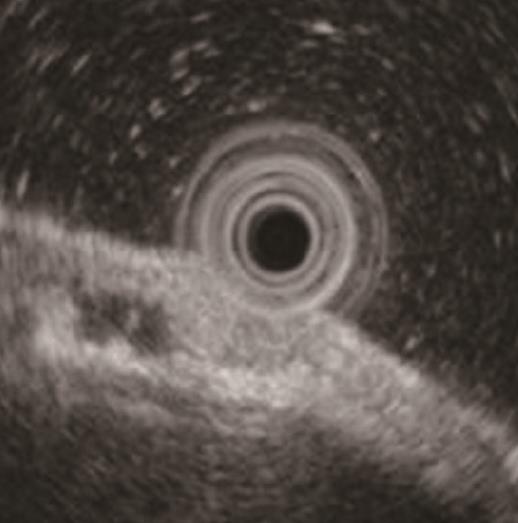
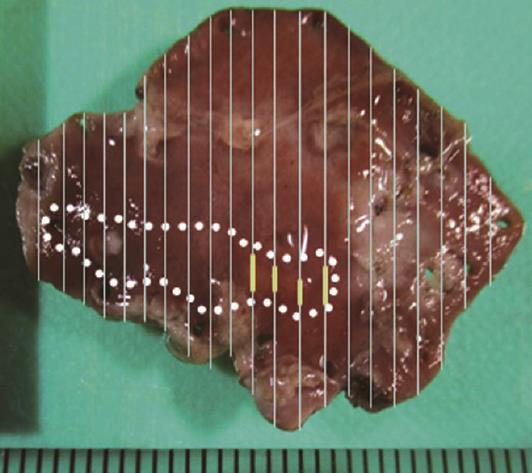
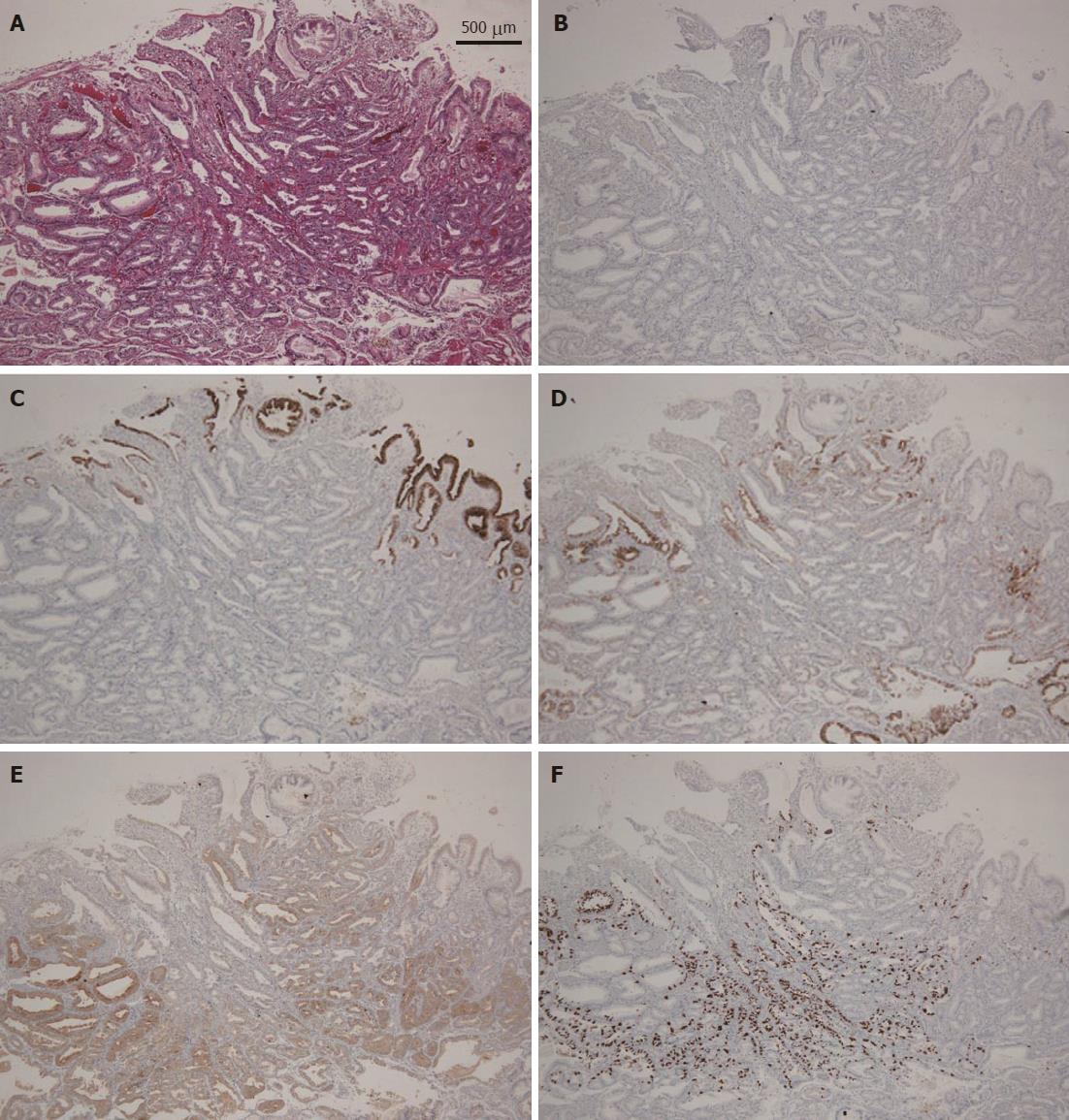
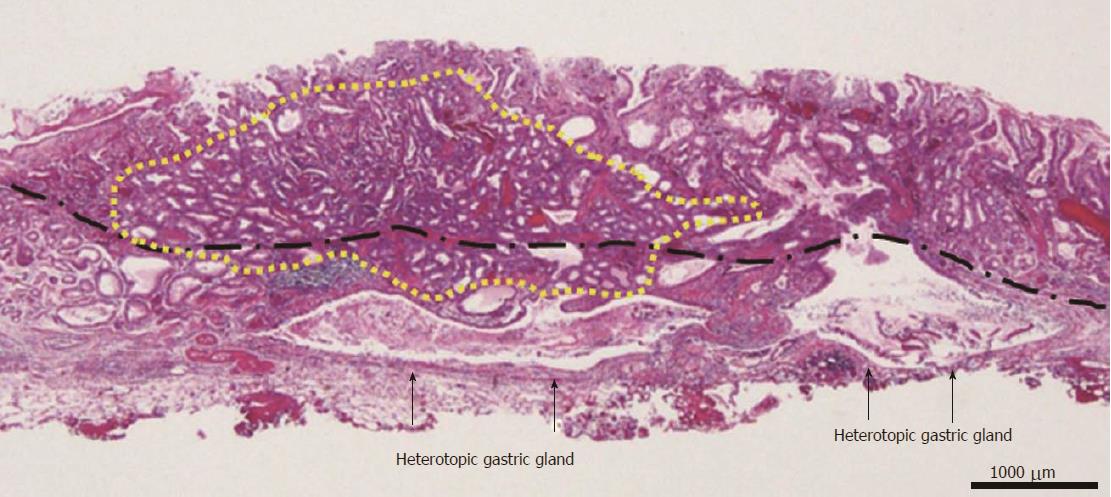
A 58-year-old man with epigastric pain was referred to our hospital. Esophagogastroduodenoscopy (EGD) revealed a Borrmann type II gastric cancer at the antrum (Figure 1A) and a 10 mm submucosal tumor-like lesion in the lesser curvature of the upper third of the stomach (Figure 1B and C). Atrophy of the gastric mucosa was classified as O-1 (according to the Kimura and Takemoto classification[7]), although the patient had undergone Helicobacter pylori (H. pylori) eradication therapy 4 years ago, and has not taken any proton pump inhibitors since then. Histological examination of the biopsy specimens obtained from the submucosal tumor-like lesion showed that the superficial area retained the normal foveolar epithelium. However, atypical cells mimicking fundic gland cells with mildly enlarged nuclei were observed in the deep layer of the lamina propria mucosae. These findings suggested a GA-FG. Narrow-band imaging with magnifying endoscopy (NBI-ME) performed later showed a regular microvascular pattern and a regular microsurface pattern according to the vessel plus surface classification system[8] with a concomitant depressed area at the center of the submucosal tumor-like lesion expected to be a biopsy scar (Figure 2). Endoscopic ultrasonography (EUS) findings revealed the tumor slightly invading the third layer and a hypoechoic mass located in the third layer near the tumor (Figure 3). Blood test findings revealed carcinoembryonic antigen level of 2.2 ng/mL and carbohydrate antigen 19-9 level of 14.3 U/mL, indicating that the tumor markers were within the normal range. Although gastric wall thickening due to the advanced gastric cancer at the antrum was seen on the abdominal CT scan, nodal or distant metastasis was absent. Based on the above findings, a partial gastrectomy was required for gastric cancer at the antrum. The submucosal tumor-like lesion in the lesser curvature of the upper third of the stomach was suspected to be a GA-FG; hence, we decided to perform endoscopic submucosal dissection (ESD) as excisional biopsy. Since histopathological findings of the ESD-resected specimen may indicate the need for an additional surgery, ESD was performed before surgery for the gastric cancer at the antrum, and en bloc resection was achieved. The ESD-resected specimen was 30 mm in diameter, whilst a 7 mm × 4 mm slightly elevated lesion was identified (Figure 4). Similar to the histological findings of the biopsy specimens, atypical cells mimicking fundic gland cells, mainly chief cells and partially parietal cells, with mildly enlarged nuclei were seen mainly in the deep layer of the lamina propria mucosae. The mucosal surface was covered completely with non-neoplastic foveolar epithelium; thus, the tumor was not exposed to the mucosal surface (Figure 5A). Upon immunohistochemical examination, the tumor showed positivity for MUC6 and negativity for MUC2 and MUC5AC, indicating a gastric phenotype (Figure 5B-D). Moreover, diffuse positivity for pepsinogen-I and scattered positivity for H/K-ATPase was found, indicating a differentiation dominantly toward chief cells and focally toward parietal cells (Figure 5E and F). Although the lesion was located mainly in the deep layer of the lamina propria mucosae, it had partially invaded the submucosal layer up to 450 μm. Furthermore, HGG were observed in the submucosal layer, proximal to the tumor. The submucosal lesion of the tumor had a poor stromal reaction and was located just above the HGG in the submucosa (Figure 6). Based on these findings, the final diagnosis was: U, Less, 30 × 20 mm, type 0-IIa, 7 × 4 mm, adenocarcinoma of fundic gland type, pT1b1 (450 μm), UL (-), ly (-), v (-), pHM0, pVM0 (according to the Japanese classification of gastric carcinoma[9]). In addition, we have concluded that the GA-FG had invaded the submucosal layer by spreading to the HGG. After obtaining these results, we performed EUS again to examine the presence of other HGG. Thus, several hypoechoic masses considered to be HGG were seen in the entire stomach, and we diagnosed diffuse type HGG. A partial gastrectomy was required for the Borrmann type II gastric cancer at the antrum. Moreover, due to the high risk of cancer arising from the gastric remnant because of the diffuse type HGG, we performed a total gastrectomy upon the patient’s request. After the total gastrectomy, the gastric cancer at the antrum was diagnosed as: L, Less, type 2, 40 × 35 mm, por1, pT3 (SS), intermediate type (int), INFa, ly2, v2, pN0, pPM0, pDM0 (according to the Japanese classification of gastric carcinoma[9]), and multiple HGG in the entire stomach were confirmed. However, we could not find evidence of this cancer related to diffuse type HGG. In addition, considering the risk of multiple gastric cancers associated with diffuse type HGG, the entire stomach was examined histologically. In doing so, we detected an intramucosal cancer in the lesser curvature of the lower gastric body, which was not previously detected by endoscopy before surgery. Although there was no remnant GA-FG near the ESD ulcer scar, a distribution of HGG was seen.
GA-FG is a new histological type of gastric cancer[1]. According to Ueyama et al[10], endoscopic properties of GA-FG include no intestinal metaplasia or atrophic change in the surrounding mucosa, faded or whitish color, submucosal tumor shape with a soft appearance, and dilated vessels with branching architecture. Although the pathogenesis of GA-FG is unclear, GA-FG tends to arise from the normal gastric mucosa of the fundic gland region without atrophic change or intestinal metaplasia[2]. Therefore, H. pylori infection is not considered to contribute to the development of GA-FG, unlike for the regular histological type of gastric cancer.
On the other hand, HGG are gastric glands that are observed in the submucosa and are naturally seen in the lamina propria mucosae[3,4]. Clinically, HGG are regarded as paracancerous lesions and are associated with gastric cancer, particularly, multiple gastric cancers[3,4]. HGG are classified into 4 types based on their number and range of distribution: solitary type with 3 sites or less, localized type with 4-9 sites at the focal area, broad type with 4-9 sites in the broad area, and diffuse type with at least 10 sites which exist in the entire stomach[4]. Of these, the diffuse type is seen in 98% of gastric cancer complications, and the rate of complication with multiple gastric cancers is reported to be 32%[3]. The association between HGG and gastric cancer is unclear. However, one dominant hypothesis is that both HGG and gastric cancer develop because of repeated erosion and regeneration of the mucosa, suggesting that HGG are paracancerous lesions[3,4]. Caution is required when performing EGD for patients with HGG, particularly diffuse type HGG, since there is the possibility that micro-gastric cancers that are difficult to detect endoscopically may be present. Indeed, as in our case, after total gastrectomy, histopathological examination revealed a micro-intramucosal cancer that was not detected endoscopically before surgery. There is no consensus on the treatment of HGG. As mentioned previously, because the rate of complication of gastric cancer is very high, particularly with diffuse type HGG, EGD must be conducted periodically with caution. In our case, in addition to the high risk of gastric cancer arising due to diffuse type HGG, a partial gastrectomy was required for the Borrmann type II gastric cancer at the antrum. Therefore, a total gastrectomy was performed upon the patient’s request.
The GA-FG observed in our case presented a submucosal tumor shape, which corresponds to the endoscopic properties reported by Ueyama et al[10]. A possible reason the GA-FG presented a submucosal tumor shape is that, in addition to the proliferation of tumor cells in the deep mucosal layer, the HGG were also present in the submucosa. Retrospectively, the hypoechoic mass near the GA-FG that was seen on EUS before ESD was suggested to be HGG. The ESD-resected specimen showed no tumor exposed to the mucosal surface; thus, NBI-ME showed a regular microvascular pattern and a regular microsurface pattern. Since GA-FG arises from the deep layer of the lamina propria mucosa, there is a high rate of submucosal invasion while the tumor diameter is small[1]. In our case also, the GA-FG invaded the submucosal layer up to 450 μm. However, the submucosal lesion of the GA-FG had a poor stromal reaction and was located just above the HGG in the submucosa. Therefore, the GA-FG was considered to have invaded the submucosal layer by spreading to the HGG. There are a few case reports of regular histological type of gastric cancer spreading to HGG[11]. However, to the best of our knowledge, this is the first case of GA-FG spreading to HGG. Currently, in the Japanese classification of gastric carcinoma, there is no definition concerning the depth of tumor invasion that spreads to HGG in the submucosa[9]. However, since similarly, esophageal cancer with intraductal spreading is handled as intramucosal cancer as long as it is retained in the esophageal proper glands[12], gastric cancer spreading to HGG should likely be handled as intramucosal cancer[5,6].
As mentioned earlier, atrophic gastritis related to H. pylori infection is not considered to be involved in the development of GA-FG. On the other hand, gastric cancer associated with HGG is considered to result from chronic inflammation due to H. pylori infection. Therefore, it is thought that there is no correlation between GA-FG and HGG. In our case, the GA-FG and HGG were considered to be coincidentally present in the vicinity. Furthermore, it is thought that HGG resulted from chronic inflammation due to H. pylori infection before eradication therapy, and pseudopyloric gland metaplasia in the fundic gland region progressed. The GA-FG may have occurred from the remnant gastric fundic glands despite the progression of pseudopyloric gland metaplasia and spread continuous to the proximally located HGG.
Until recently, GA-FG has been recognized as cancer arising from gastric mucosa without atrophic change[2]. However, cases of GA-FG arising from gastric mucosa with atrophic change have now been reported[13]. Together with these cases, our case suggests that GA-FG could occur even from the gastric mucosa with atrophic change, where HGG could be present, provided the remnant gastric fundic glands exist. This is an important case demonstrating that when performing endoscopy, one needs to keep in mind the possibility of GA-FG presenting on the gastric mucosa with atrophic change, chronic gastritis, or intestinal metaplasia.
A 58-year-old man with epigastric pain but no other symptoms.
A gastric adenocarcinoma of fundic gland type (GA-FG) was suspected based on the histological examination of the biopsy specimens obtained from the submucosal tumor-like lesion that revealed atypical cells mimicking fundic gland cells with mildly enlarged nuclei.
Gastric submucosal tumors, including leiomyoma, leiomyosarcoma, gastrointestinal stromal tumor, and lipoma.
Initial laboratory findings were within the normal limits.
Esophagogastroduodenoscopy revealed a 10 mm submucosal tumor-like lesion in the lesser curvature of the upper third of the stomach, which had a regular microvascular pattern and a regular microsurface pattern based on narrow- band imaging with magnifying endoscopy.
An endoscopic submucosal dissection (ESD)-resected specimen revealed GA-FG spreading to heterotopic gastric glands (HGG) in the submucosa because the submucosal lesion of the GA-FG had a poor stromal reaction and was located just above the HGG.
ESD as excisional biopsy and total gastrectomy.
Although there are a few case reports of regular histological type of gastric cancer spreading to HGG, this is the first case of GA-FG spreading to HGG.
GA-FG, which differentiates into chief cells, is a new histological type of gastric cancer proposed by Ueyama et al in 2010. HGG, which are observed in the submucosa, are considered to be paracancerous lesions.
We need to keep in mind the possibility of GA-FG presenting on the gastric mucosa with atrophic change, chronic gastritis, or intestinal metaplasia.
Dear Editor, this is well done case report with literature review. Authors described the case extensively and compare it against the literature. Also they provided excellent documentation and provided all necessary explanations.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Amedei A, Hauser G, Barone M S- Editor: Qi Y
L- Editor: A E- Editor: Ma YJ
| 1. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22:10523-10531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Iwanaga T, Koyama H, Takahashi Y, Taniguchi H, Wada A. Diffuse submucosal cysts and carcinoma of the stomach. Cancer. 1975;36:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Iwanaga T, Furukawa Y, Ishiguro S. Histological observation of 102 cases with submucosal diffuse heteropathia of the stomach. Saishinigaku. 1986;41:2418-2426. |
| 5. | Kosugi S, Kanda T, Hatakeyama K. Adenocarcinoma arising from heterotopic gastric mucosa in the stomach. J Gastroenterol Hepatol. 2006;21:483-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kim DH, Kim KM, Oh SJ, Oh JA, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Early gastric cancer arising from heterotopic gastric mucosa in the gastric submucosa. J Korean Surg Soc. 2011;80 Suppl 1:S6-S11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. |
| 8. | Yao K, Takaki Y, Matsui T, Iwashita A, Anagnostopoulos GK, Kaye P, Ragunath K. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am. 2008;18:415-433, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 10. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Tanaka H, Baba Y, Isono Y, Matsusaki S, Sase T, Okano H, Saito T, Mukai K, Ito T, Watanabe G. [Gastric intramucosal cancer spreading to submucosal heterotopic gastric glands]. Nihon Shokakibyo Gakkai Zasshi. 2015;112:1657-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 13. | Isono Y, Baba Y, Tanaka H, Mukai K, Sase T, Saito T, Okano H, Matsusaki S, Kumazawa H, Watanabe G. Long-term follow-up of a gastric adenocarcinoma of the fundic gland type. Gastroenterol Endosc. 2015;57:2639-2644. [DOI] [Full Text] |