Published online Oct 7, 2017. doi: 10.3748/wjg.v23.i37.6894
Peer-review started: June 28, 2017
First decision: July 27, 2017
Revised: August 12, 2017
Accepted: September 6, 2017
Article in press: September 5, 2017
Published online: October 7, 2017
Processing time: 92 Days and 20.8 Hours
To examine usefulness of virtual biopsy using endocytoscopy by comparing the in vivo endocytoscopic and histopathological images of gastric cancers.
Endocytoscopy was performed in 30 patients with early gastric cancer. Of these, 26 patients showed well differentiated adenocarcinomas, while 4 patients showed poorly differentiated adenocarcinomas (including one signet ring cell carcinoma). Cancerous and non-cancerous areas were observed after double staining with 0.05% crystal violet and 0.1% methylene blue. The endocytoscopic images obtained were evaluated by an expert endoscopist and an expert pathologist without knowledge of patient clinical data, and endocytoscopic and histopathological diagnoses were compared.
The endocytoscopic images of the cancerous area were assessed as evaluable in 25 (83.3%) and 27 (90%) patients by endoscopist A and pathologist B, respectively, and those of the non-cancerous area as evaluable in 28 (93.3%) and 23 (76.7%) patients by the endoscopist and pathologist, respectively. The sensitivity, specificity, and diagnostic accuracy of gastric cancer diagnosis using evaluable endocytoscopic images were 88.0% and 92.9%, and 90.6% by endoscopist A, and 88.9% and 91.3%, and 90.0% by pathologist B, respectively. Evaluation of the diagnostic concordance rate between the endoscopist and the pathologist by inter-observer agreement calculation revealed no significant difference between the two observers. The inter-observer agreement (κ-value) for endocytoscopic diagnosis was 0.745.
Endocytoscopy is useful for the differentiation of cancerous from non-cancerous gastric mucosa, making it a promising tool for virtual biopsy.
Core tip: Endocytoscopy, which allows for ultra-high-magnifying observation of the gastrointestinal mucosa, has recently been developed, enabling the visualization of the gastrointestinal mucosa at resolutions approaching those of histology. Although the usefulness of virtual biopsy using endocytoscopy in the diagnosis of esophageal cancer, colorectal cancer, lung cancer and ulcerative colitis has been reported, few studies are available on the diagnosis of gastric cancer. This study showed the usefulness of virtual biopsy using endocytoscopy by comparing the in vivo endocytoscopic and histopathological images of gastric cancers.
- Citation: Tsurudome I, Miyahara R, Funasaka K, Furukawa K, Matsushita M, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Watanabe O, Nakaguro M, Satou A, Hirooka Y, Goto H. In vivo histological diagnosis for gastric cancer using endocytoscopy. World J Gastroenterol 2017; 23(37): 6894-6901
- URL: https://www.wjgnet.com/1007-9327/full/v23/i37/6894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i37.6894
The incidence of gastric cancer has been decreasing worldwide, but gastric cancer still remains a major cause of cancer-related death. The annual number of deaths from this disease is second only to that from lung cancer in Japan. A global analysis of gastric cancer-related mortality reveals higher mortality rates in East Asia (Japan, China, and South Korea) compared to those in Europeans and Caucasian populations of the United States.
Early detection, accurate staging, and appropriate treatment administration are important to improve survival outcomes and reduce mortality rates in gastric cancer. Recent advances in endoscopic technology have been remarkable, and high-resolution endoscopy has made it possible to detect endoscopically-treatable early gastric cancer more frequently. Magnifying endoscopy and image-enhanced endoscopy have been developed, and NBI-magnifying endoscopy has facilitated the differential diagnosis and histological typing of gastric cancer[1-3]. However, the gold standard for the diagnosis of malignant gastrointestinal tumors is histopathological examination, and definitive diagnosis requires endoscopic biopsy. However, diagnostic biopsy is time-consuming, increasing the risk of bleeding in patients taking antithrombotic agents, in addition to causing submucosal fibrosis, which potentially hampers endoscopic treatment.
Two endoscopic technologies (confocal endomicroscopy and endocytoscopy) have recently been developed, making it possible to observe living cells in vivo. To date, we have reported that confocal endomicroscopy can distinguish gastric cancer from normal gastric mucosa[4], and determine the mucin phenotype of gastric cancer by visualizing brush borders and goblet cells[5]. Fluorescent dyes used for confocal laser endomicroscopy stain cells, enabling the evaluation of structural atypia, but not that of nuclear atypia, because they do not stain nuclei. However, dye spraying during endocytoscopy results in the staining of cells and nuclei, thereby producing in vivo images similar to those on the histological study of biopsies (the current gold standard for pathological diagnosis). As endocytoscopy provides real-time microscopic images in vivo, its application to histological diagnosis by virtual biopsy (without the need to take biopsies) is promising. Although the usefulness of virtual biopsy using endocytoscopy in the diagnosis of esophageal cancer, colorectal cancer, lung cancer, and ulcerative colitis has been reported[6-9], few studies to date have evaluated its usefulness in the in vivo diagnosis of gastric cancer[10-12].
In this study, we retrospectively examined the usefulness of virtual biopsy using endocytoscopy, by comparing in vivo endocytoscopic and histopathological images of gastric cancers.
This pilot study was performed at the Nagoya University Hospital between January 2011 and October 2012. Thirty patients with early gastric cancer were enrolled (Table 1). Of these, 26 patients showed well differentiated, and 4 patients showed poorly differentiated adenocarcinomas (including one signet ring cell carcinoma). This study was approved by the Ethics Committee of Nagoya University Hospital (approval no. 911), and informed consent was obtained from all patients.
| Variable | |
| Patients | 30 |
| Sex (male/female) | 24/16 |
| Mean age, yr (range) | 72.2 (48-87) |
| Histological type of gastric cancer | |
| Well differentiated adenocarcinoma | 26 |
| Poorly differentiated adenocarcinoma (including signet ring cell carcinoma) | 4 |
| Histological type of non-cancerous mucosa | |
| Normal mucosa | 10 |
| Intestinal metaplastic mucosa | 20 |
| Mean tumor size, mm, (range) | 17 (3-70) |
| Macroscopic type | |
| 0-I | 1 |
| 0-IIa | 12 |
| 0-IIc | 17 |
| Location | |
| Upper | 6 |
| Middle | 9 |
| Lower | 15 |
| Infiltration depth | |
| Mucosal | 25 |
| Submucosal | 5 |
| H. pylori infection | |
| Eradicated | 11 |
| Persistent infection | 14 |
| Negative | 5 |
An integrated-type endocytoscope (GIF-Y0002, Olympus Co., Tokyo, Japan) was used in the present study. As with a conventional magnification endoscope, the magnification was adjustable by pulling a lever to perform continuous observation, from conventional to ultra-high magnification. The maximum magnification was × 380, the microscopic field area 700 μm × 600 μm, and the depth of observation was 50 μm. The image quality of the endocytoscope was comparable to that of GIF-Q260 (Olympus, Japan), and was sufficient to make an accurate diagnosis.
Patients were pretreated with an oral dose of 20000 units of pronase and 1 g of sodium bicarbonate, followed by an intramuscular injection of 20 mg of butylscopolamine or 1 mg of glucagon. Under intravenous conscious sedation, the patients underwent endocytoscopy. Conventional observation was performed. Then, the lesion was washed sufficiently using saline water, and sprayed with approximately 5 mL of 0.05% crystal violet and 0.1% methylene blue. One minute later, the excess dye solution was washed away, and ultra-high-magnifying observation was performed. If cells and nuclei were poorly stained, the lesion was sprayed again with the dyes. Both the lesion and the surrounding non-cancerous mucosa were observed. All examinations were performed by a single expert endoscopist (Tsurudome I), qualified as Board certified fellow of the Japan Gastroenterological Endoscopy Society.
Lesions observed by endocytoscopy underwent endoscopic biopsy or endoscopic resection. Biopsies and resected specimens were fixed, embedded in paraffin, and cut into thin sections horizontal to the mucosal surface. They were stained with hematoxylin and eosin (H&E), and examined and classified by expert pathologists according to the revised Vienna classification[13]. Lesions classified as category 4 in the Vienna classification were defined as gastric cancer, but those classified as category 3 were defined as gastric adenoma, and excluded from the category of gastric cancer.
Definitions of endocytoscopic findings compared to histopathological images
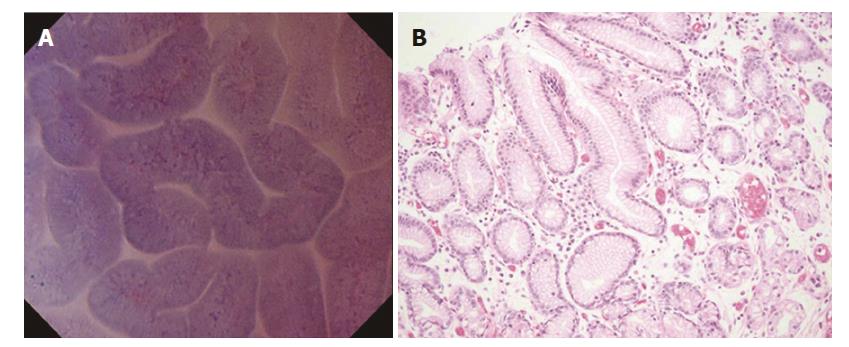
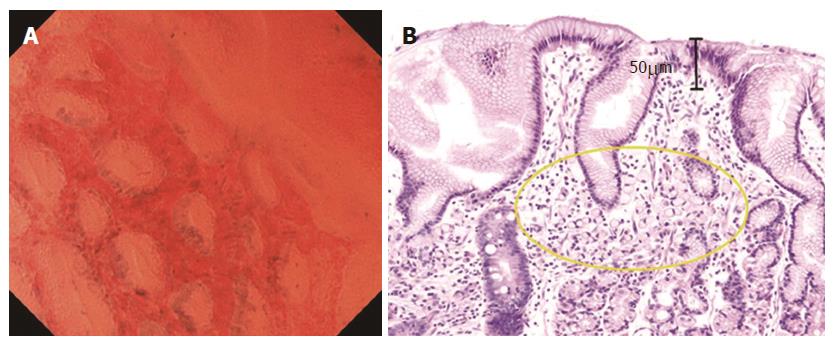
Normal mucosa (without intestinal metaplasia). In the fundic gland mucosa, the foveolar epithelium was arranged in a circle, and the glandular lumens were round (Figure 1A).
In the pyloric gland mucosa, the foveolar epithelium was arranged in ridges, and the glandular lumens were slit-like (Figure 2A). The arrangement of the epithelial cells was regular, closely resembling that seen in H&E-stained horizontal tissue sections (Figures 1B and 2B). The nuclei were faintly stained, showing no atypia.
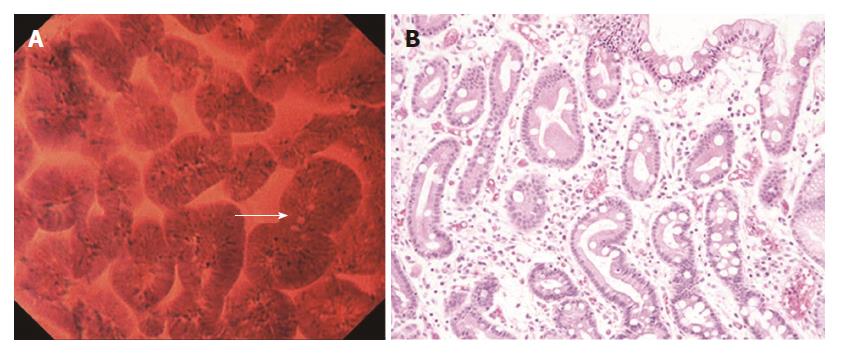
In the intestinal metaplastic mucosa, the epithelium exhibited a villous architecture (varying in size). The gland lumens were widened due to epithelial atrophy (Figure 3A). Some epithelial cells had vacuoles, suggestive of goblet cells. These findings were very similar to those in H&E-stained horizontal sections (Figure 3B). Nuclei were stained more intensely than in the normal mucosa, but demonstrated no atypia.
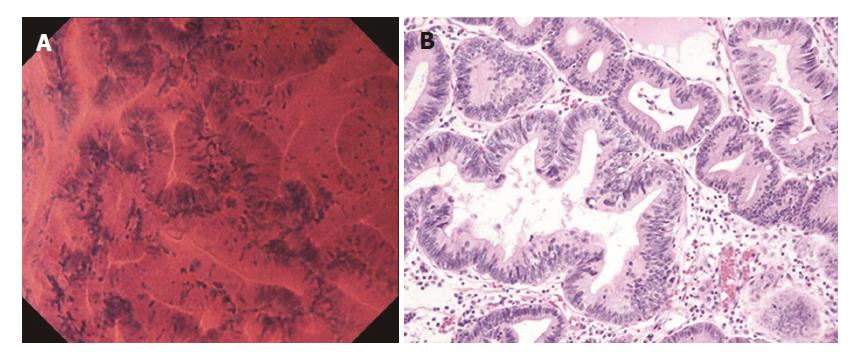
In well differentiated adenocarcinoma, the glands were branched irregularly and the width of the lumen varied. The epithelium was arranged in a disorderly fashion (Figure 4A). The nuclei were deeply stained and pseudostratified, and showed an increased nuclear density and nuclear atypia. In poorly differentiated adenocarcinoma, the tubular architecture was completely lost, and the nuclei were hyper-chromatic and anisokaryotic (Figure 5A). These findings closely resembled those in H&E-stained horizontal sections (Figures 4B and 5B).
The endocytoscopic images obtained were analyzed separately by an endoscopist A (Ryoji M) and a pathologist B (Satou A), who were blinded to clinical information. The diagnosing physicians interpreted three photographs chosen by the examining physician, and classified the image quality as evaluable (nuclei and glandular structures evaluable) or unevaluable (neither nuclei nor gland structures evaluable), and determined whether the tissue was cancerous or non-cancerous if the image was of evaluable quality. The results were then compared with the actual pathology.
Primary endpoint: Diagnostic accuracy: The endocytoscopic diagnosis performed independently by the endoscopist and the pathologist were compared with the pathological diagnosis, and sensitivity, specificity, and accuracy were evaluated.
Secondary endpoint: Diagnostic concordance rate between endoscopist and pathologist. To evaluate the diagnostic concordance rate between the endoscopist and the pathologist in the diagnosis of gastric cancer using endocytoscopy, inter-observer agreement was calculated.
The statistical software package SPSS 19.0 was used for data analysis. The endoscopic diagnoses by the endoscopist and the pathologist were compared using the χ2 test, and a P-value of less than 0.05 was considered as significant. For evaluable images, the unweighted κ-statistic was calculated to evaluate inter-observer agreement.
The endocytoscopy images of the cancerous areas were assessed as evaluable in 25 (83.3%) and 27 (90%) of the 30 patients by endoscopist A and pathologist B, respectively, and those of the non-cancerous areas as evaluable in 28 (93.3%) and 23 (76.7%) patients, by the endoscopist and the pathologist, respectively. The images that were assessed as unevaluable were excluded from the analysis. Endocytoscopic images of lesions in the lesser curvature of the gastric body are prone to artifacts due to cardiac pulsation, and it was difficult to place the endocytoscope in the desired position to visualize lesions in the fornix or greater curvature of the gastric body, thus complicating observation in these areas.
Table 2 shows the interpretation of endocytoscopic images by the endoscopist and the pathologist. Calculated from these findings, the sensitivity, specificity, and diagnostic accuracy of endocytoscopy in the diagnosis of gastric cancer using evaluable images were 88.0%, 92.9%, and 90.6% as performed by endoscopist A, and 88.9%, 91.3%, and 90.0% as performed by pathologist B, respectively (Table 3). Using the χ2 test, no significant difference was found between the two observers, and the κ-value was 0.745.
| Endocytoscopic diagnosis | ||||
| Endoscopist A | Pathologist B | |||
| Histological diagnosis | Cancer | Non-cancer | Cancer | Non-cancer |
| Cancer | 22 | 3 | 24 | 3 |
| Non-cancer | 2 | 26 | 2 | 21 |
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | |
| Endoscopist A | 88.00% | 92.90% | 91.70% | 89.70% | 90.60% |
| (76.6-93.3) | (82.7-97.6) | (79.8-97.2) | (79.8-94.2) | (79.8-95.6) | |
| Pathologist B | 88.90% | 91.30% | 92.30% | 87.50% | 90.00% |
| (78.4-93.8) | (79.0-97.1) | (81.4-97.4) | (75.7-93.0) | (78.7-95.3) | |
| P-value | 0.92 | 0.84 | 0.93 | 0.81 | 0.92 |
No complications were observed in this study, and none of the patients required temporary discontinuation of endocytoscopy.
At present, magnifying endoscopy facilitates observation of the mucosal surface structure as indicated by its surface patterns and the vascular structure as revealed by microvessels, thus mainly allowing evaluation of structural atypia. On the other hand, the resolution that endocytoscopy offers, allows visualization of individual cell nuclei and enables in vivo tissue observation, yielding results comparable to those obtained from histopathological images. Using an integrated endocytoscopy system, it is now possible to perform continuous observation of tissues with conventional to ultra-high magnification, creating the possibility of performing real-time virtual biopsies without obtaining physical lesion biopsies.
Endocytoscopy requires vital staining in order to evaluate the cellular and nuclear morphology, and methylene blue is generally used for this purpose. However, the diversity and poor staining of the gastric mucosa due to the presence of mucus[14] make observation and evaluation difficult. Therefore, to date, few studies have reported in vivo experiments in humans with gastric cancer[10-12].
Kodashima et al[15] attempted to optimize the staining conditions (staining time and dye concentration) for endocytoscopy in porcine experiments using different dyes, and reported that the ability of crystal violet to stain esophageal, gastric, and colonic tissues was inferior, and the optimal staining conditions were as follows: staining of esophageal tissue with 1% methylene blue solution for 60 s; staining of gastric and colonic tissues with 0.25% toluidine blue solution for 60 s. A double-staining method using crystal violet and methylene blue has recently been developed. In this method, methylene blue stains cellular nuclei purple, and crystal violet stains cells pink, thereby enabling the visualization of images resembling those of H&E staining. Kudo et al[6] and Minami et al[16] have demonstrated usefulness of the methylene blue and crystal violet double-staining method in the evaluation of neoplastic lesions in the colon and the esophagus, respectively. This same double-staining method has been used in the present study. Further studies are needed to compare this double-staining method with others such as the crystal violet and toluidine blue double-staining method.
The use of endocytoscopy in this study enabled us to evaluate the morphology of glandular lumens, arrangement of gastric foveolar cells, degree of nuclear stainability, and presence or absence of nuclear pseudostratification.
To determine the diagnostic performance of endocytoscopy to differentiate benign from malignant lesions, structural atypia were evaluated based on the morphology of glandular lumens and arrangement of foveolar cells; nuclear atypia were assessed based on the degree of nuclear chromasia and presence or absence of nuclear pseudostratification. Lesions with or without structural or nuclear atypia were diagnosed as cancerous or non-cancerous, respectively. Endocytoscopic images obtained from the normal gastric mucosa, gastric mucosa with intestinal metaplasia, and gastric cancers, were similar to those obtained from H&E-stained horizontal tissue sections. Thus, using endocytoscopic images, we were able to differentiate cancerous from non-cancerous lesions with high sensitivity, specificity, and accuracy. In addition, the findings that goblet cells were observed in the intestinal metaplastic mucosa were similar to those reported by Chiu et al[17].
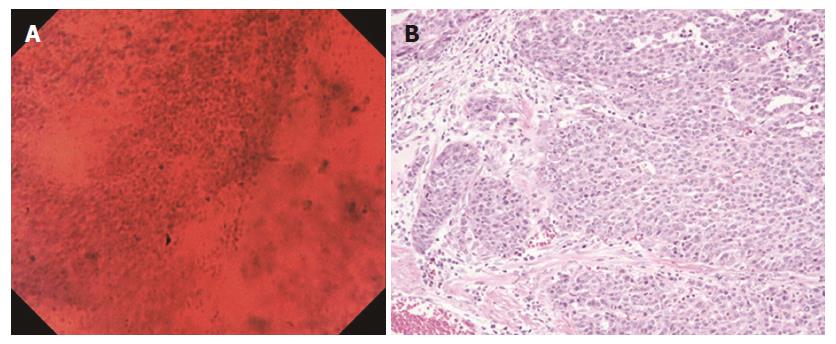
Although endocytoscopy enables in vivo observation of the mucosal tissue at the microscopic level, it only provides information about cells and nuclei of the superficial layer of the mucosa. In this study, both the pathologist and the endoscopist classified a signet ring cell carcinoma as a false-negative by endocytoscopy (Figures 6A and B). Although Fasoli et al[18]. reported that signet ring cells can be observed in resected gastric cancer specimens with endocytoscopy[18], the visualization by endocytoscopy is limited to the superficial mucosal layer (up to 50 μm in depth). In some poorly differentiated adenocarcinomas, carcinoma cells may spread in the lamina propria without any distortion to gland structure (Figure 6B). In these cases, the diagnosis of carcinoma with endocytoscopy alone is difficult.
This study has several limitations. First, only the best images that were chosen by the examining physician were evaluated. That is, the diagnostic capability of endocytoscopy was not evaluated in real-time. Second, the stomach moves actively due to peristalsis, aortic pulsation, and respiration, making focusing and fiber manipulation during endocytoscopic observation difficult for beginners. Third, the endoscopist requires knowledge of pathology when evaluating endocytoscopic findings. Fourth, this study did not include patients with adenoma. Finally, the sample size was small, implying that additional studies with a larger number of patients is indicated to confirm our findings.
In conclusion, we were able to obtain high quality endocytoscopic images, which were adequate for the differentiation of benign lesions from malignant ones. As this was a retrospective pilot study involving a small number of patients, a large-scale prospective study is indicated.
Endocytoscopy, which allows for ultra-high-magnifying observation of the gastrointestinal mucosa, has recently been developed, enabling the visualization of the gastrointestinal mucosa at resolutions approaching those of histology. Although the usefulness of virtual biopsy using endocytoscopy in the diagnosis of esophageal cancer, colorectal cancer, lung cancer, and ulcerative colitis has been reported, few studies are available on the diagnosis of gastric cancer.
The gold standard for the diagnosis of malignant gastrointestinal tumors is histopathological examination, and definitive diagnosis requires endoscopic biopsy. As endocytoscopy provides real-time microscopic images in vivo, its application to histological diagnosis by virtual biopsy (without the need to take biopsies) is promising.
Endocytoscopy allows recognition of nuclei and cells, thereby producing in vivo images similar to those produced by histological study of biopsies. This study suggested the usefulness of virtual biopsy for gastric cancer.
Using endocytoscopy, it is expected that we will be able to diagnose gastric cancer with virtual biopsies, eliminating the need for physical biopsies. The need for tissue biopsies, and associated limitations like bleeding and fibrosis at biopsy site may be pre-empted for gastric cancer diagnosis, improving patient outcomes.
Endocytoscopy involves an ultra-high magnification endoscope that allows observation of nuclei and cells in vivo. Virtual biopsy denotes a procedure to obtain a tissue image of high resolution with nuclear and cellular visualization, without performing and analyzing a physical tissue biopsy.
This manuscript addresses the usefulness of endocytoscopy for distinguishing gastric cancer. Although this pilot study involved a small number of patients, the content of this manuscript is interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sugimoto S, Watari J S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279-284. [PubMed] |
| 3. | Otsuka Y, Niwa Y, Ohmiya N, Ando N, Ohashi A, Hirooka Y, Goto H. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, Shimoyama Y, Nagasaka T, Maeda O, Ando T. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 2006;38:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Banno K, Niwa Y, Miyahara R, Nakamura M, Nagaya T, Nagasaka T, Watanabe O, Ando T, Kawashima H, Ohmiya N. Confocal endomicroscopy for phenotypic diagnosis of gastric cancer. J Gastroenterol Hepatol. 2010;25:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Shah PL, Kemp SV, Newton RC, Elson DS, Nicholson AG, Yang GZ. Clinical Correlation between Real-Time Endocytoscopy, Confocal Endomicroscopy, and Histopathology in the Central Airways. Respiration. 2017;93:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Nakazato Y, Naganuma M, Sugimoto S, Bessho R, Arai M, Kiyohara H, Ono K, Nanki K, Mutaguchi M, Mizuno S. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy. 2017;49:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Kaise M, Kimura R, Nomura K, Kuribayashi Y, Kikuchi D, Iizuka T, Ohkura Y. Accuracy and concordance of endocytoscopic atypia for the diagnosis of gastric cancer. Endoscopy. 2014;46:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kaise M, Ohkura Y, Iizuka T, Kimura R, Nomura K, Kuribayashi Y, Yamada A, Yamashita S, Furuhata T, Kikuchi D. Endocytoscopy is a promising modality with high diagnostic accuracy for gastric cancer. Endoscopy. 2015;47:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Kumagai Y, Takubo K, Kawada K, Higashi M, Ishiguro T, Sobajima J, Fukuchi M, Ishibashi KI, Mochiki E, Aida J. A newly developed continuous zoom-focus endocytoscope. Endoscopy. 2017;49:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [PubMed] |
| 14. | Inoue H, Kazawa T, Sato Y, Satodate H, Sasajima K, Kudo SE, Shiokawa A. In vivo observation of living cancer cells in the esophagus, stomach, and colon using catheter-type contact endoscope, “Endo-Cytoscopy system”. Gastrointest Endosc Clin N Am. 2004;14:589-594, x-xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kodashima S, Fujishiro M, Takubo K, Kammori M, Nomura S, Kakushima N, Muraki Y, Tateishi A, Kaminishi M, Omata M. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Minami H, Inoue H, Yokoyama A, Ikeda H, Satodate H, Hamatani S, Haji A, Kudo S. Recent advancement of observing living cells in the esophagus using CM double staining: endocytoscopic atypia classification. Dis Esophagus. 2012;25:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Chiu PW, Ng EK, To KF, Teoh AY, Lam CC, Chan FK, Sung JJ, Lau JY. Recognition of goblet cells upon endocytoscopy indicates the presence of gastric intestinal metaplasia. Dig Endosc. 2014;26:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Fasoli A, Pugliese V, Furnari M, Gatteschi B, Truini M, Meroni E. Signet ring cell carcinoma of the stomach: correlation between endocytoscopy and histology. Endoscopy. 2009;41 Suppl 2:E65-E66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |