INTRODUCTION
Stomach ulcers are common and afflict millions of individuals annually (http://www.mayoclinic.com)[1]. Like many gastrointestinal (GI) disorders, stomach ulcers cause significant pain, a hallmark feature of many ailments affecting visceral organs. Although stomach ulcers predicate a type of visceral hypersensitivity, surprisingly the mechanisms underlying the pain associated with gastric ulceration as with some other GI conditions [i.e., irritable bowel syndrome (IBS), functional dyspepsia, pancreatitis] remain poorly understood[2-4]. Several preclinical models of gastric or duodenal ulceration have been established that highly resemble human ulcers in terms of pathological features including the indomethacin-induced gastropathy and acetic acid models[5-9]. While these and other models have been used to aid the development of novel disease ameliorating therapies (i.e., proton pump inhibitors and histamine receptor antagonists) as well as elucidate the notable involvement of Helicobacter pylori infection in ulcer formation, a direct behavioral measure of the pain associated with ulceration in these models has not been reported[10]. Furthermore, despite the success of these drugs in healing ulcer lesions, sensory aberrations leading to such pain have not been clearly delineated and often remain a chief complaint for many patients[10,11]. This is especially true for those individuals who are actively treated for ulcers but who also require concomitant therapy with non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin, analgesics commonly used for other chronic conditions such as osteoarthritis (OA) and cardiovascular disease[12,13]. In conditions like OA, these agents are used to treat musculoskeletal pain, but paradoxically cause or exacerbate stomach pain on their own[13,14]. NSAIDs and salicylates are ulcerogenic and therefore, chronic use can exacerbate existing gastric injury or lead to new ulcer formation[15]. For these and other patients, it has been hypothesized that persistent or unresolved visceral pain, despite the etiology, may be due to aberrations in primary afferent function or hypersensitivity, peripheral sensitization, and/or psychological/genetic abnormalities[16-20].
With this in mind, we characterized the pain associated with gastric ulceration. By combining a clinically relevant stomach ulcer model with a predictive behavioral endpoint, we investigated some potential mechanisms producing visceral hypersensitivity. To this end, we used the indomethacin-induced gastropathy model to model the mucosal injury and concomitant pain associated with NSAID use. This model recapitulates the human condition in that indomethacin is orally administered to mice to produce mucosal damage, inflammation and referred visceral hyperalgesia[21-23]. Like in other GI disorders, ulcer pain is diffuse. Moreover, it can be referred to somatic structures and may present itself atypically given the dichotomization of sensory fibers that innervate visceral tissues[24-27]. Therefore, since ulcer pain is reportedly present upon palpation or mechanical stimulation of the abdomen both in dogs[28] and humans[29], we extrapolated this to mice and quantified the referred abdominal hypersensitivity by measuring the number of behavioral responses evoked by von Frey fiber stimulation[23,30]. We then investigated the pharmacological role of guanylate cyclase C (GC-C) and opioid receptors as well as TRPs, ASICS and sodium channels in this regard since all have been implicated in visceral hypersensitivity and/or functional bowel disorders to some degree[31-35].
MATERIALS AND METHODS
Animals
Male CD-1 mice (Harlan Laboratories, Indianapolis, IN, United States) weighing 20-25 g were housed on cob bedding (five/cage) in a climate-controlled room and maintained on a 12-h light/dark cycle with free access to food and water. Animals were acclimated to the Purdue Pharma L.P. animal facility for one week prior to testing.
Animal care and use statement
All studies were approved by the Purdue Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Ethical Guidelines of the International Association for the Study of Pain (http://www.iasp-pain.org) and are reported in accordance with the ARRIVE guidelines (http://www.nc3rs.org.uk). All efforts were made to minimize the number of animals used and to avoid any undue pain.
Ulcer pain model
As previously described, mice were fasted overnight then dosed orally with 30 mg/kg indomethacin to develop the ulcer model[23,30]. Control animals received vehicle (10 mL/kg, p.o.). Morphine was administered 2 h post-indomethacin while the other compounds were administered 3 h post-indomethacin or 1 h before testing. Stomachs from a separate set of vehicle- and indomethacin-dosed mice were dissected and photographed to ensure ulcer model development.
Behavior
Referred abdominal hypersensitivity from indomethacin-induced gastric ulceration was quantified by measuring the threshold to withdrawal from the application of a tactile stimulus to the abdominal area[23]. Briefly, the abdominal area was shaved and the mice were subsequently placed inside Plexiglas boxes situated on elevated wire screen mesh flooring conducive to von Frey probing. Following a habituation period, baseline tactile sensitivity was assessed. Tactile hypersensitivity was then measured 4 and 24 h following indomethacin administration using von Frey filaments applied to the upper abdomen (bending force 0.005-3.58 g starting with the 0.407 g fiber). The upper abdominal region was stimulated with an incremental series of eight monofilaments of increasing logarithmic stiffness. The 50% withdrawal threshold was determined using the up-down method of Dixon, modified by Dixon[36] and Chaplan et al[37]. First, an intermediate von Frey monofilament (number 3.58) was applied to the abdomen causing a slight bending. If a positive response was noted (abdominal/whole body withdrawal or licking), a smaller filament was then tested. Conversely, if no response occurred, the next larger filament was tested. If an animal did not respond to monofilament application, a cut-off value of 3.58 was assigned to that mouse[23,30]. A separate set of naïve mice were also dosed with the highest doses of the compounds of interest. von Frey thresholds were determined 4 h and 24 h post-indomethacin administration, noting that at least four hours is needed before a pain response can be reliably measured.
Rotarod
To delineate efficacy and assess changes in motor performance following compound administration, naïve mice were tested on an automated accelerating rotarod (Ugo Basile, Italy). For training and testing, the rotarod speed was increased from 4 to 40 rpm over a 300 second period. The maximum time spent on the apparatus was set to 300 s. Mice received two training sessions separated by a minimum of 2 h on the first day then 24 h later they were administered compound or vehicle. The latency to fall was assessed 1 , 2 and 24 h post-compound administration. It is important to note that only the highest dose tested in the efficacy assessment was evaluated in the rotarod assay.
Blinding and randomization
All testing was conducted in a blinded manner with experimenters involved in the study being unaware of the group assignment of any animal they were testing. Dosing solutions were coded using A-E. Animals were dosed in a blinded fashion after pre-treatment baseline assessment such that animals were assigned to treatment groups based on baseline response thresholds so that group means were approximately equal (i.e., randomization). Briefly, animals were ranked by response threshold from lowest to highest and treatments were assigned as follows (e.g., A, B, C, D, E, B, C, D, E, A, C, D, E, A, B, D, E, A, B, C, etc.). The animals were then dosed in sequence based on animal number, so that the distribution of treatment across a given set of animals was not predictable.
Statistical analysis
All data are expressed as the mean ± SEM. Data were analyzed using a two-way repeated measures analysis of variance (ANOVA) using the factors of treatment and time. The Bonferonni multiple comparisons test was subsequently performed for post-hoc comparison using GraphPad Prism software (version 5.04; GraphPad Sofware, Inc., United States). Statistical significance was set to P < 0.05. The statistical methods of this study were reviewed by Salvatore Colucci, a biomedical statistician employed by Purdue Pharma L.P.
Compounds
Indomethacin, AMG 9810, HC-030031, carbamazepine, amiloride HCl and morphine were obtained from Sigma-Aldrich (St. Louis, MO, United States). Linaclotide, and asimadoline HCl were obtained from Toronto Research Chemicals (Toronto, ON, Canada). AMG 9810, HC-030031, asimadoline and linaclotide were administered p.o. as suspensions in 0.5% methylcellulose. Amiloride HCl was formulated in 25% hydroxypropyl-β-cyclodextran and administered intraperitoneally while morphine was dissolved in saline and given subcutaneously. Also, with the exception of morphine, all compounds were administered one hour prior to behavioral testing (three hours post-indomethacin dosing). Morphine was administered two hours before testing (or two hours after indomethacin). Route, vehicle and pre-treatment time for all compounds were chosen based on preliminary dosing experiments, pharmacokinetic (PK) data and/or previously published work. All doses are expressed as the free base and were administered in a dose volume of 10 mL/kg.
RESULTS
Ulcer model
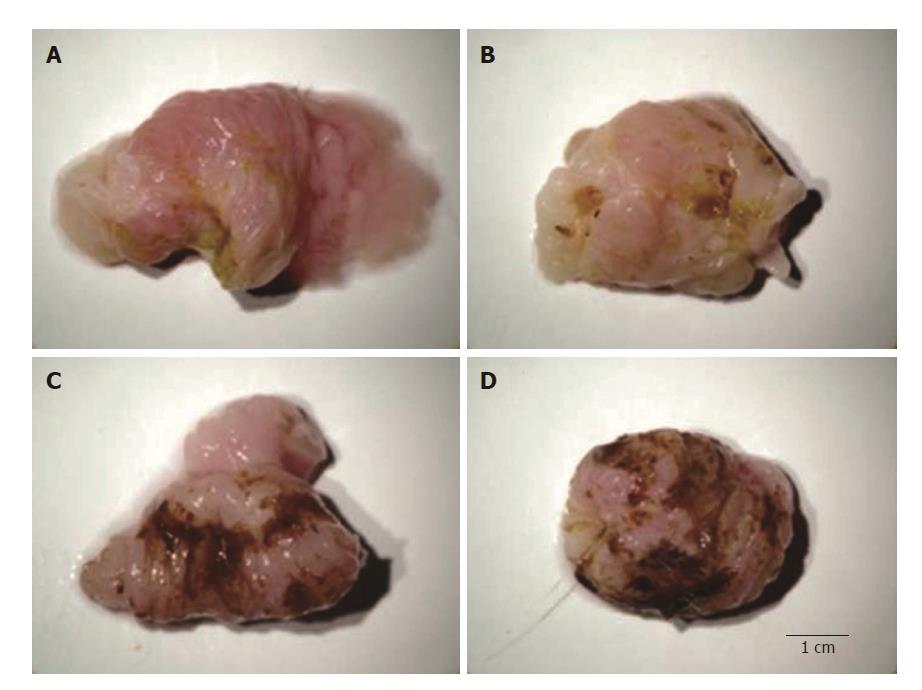
Model development: We removed stomachs from vehicle- and indomethacin-dosed mice (4-h post-indomethacin administration). Mice were humanely euthanized. Their stomachs were grossly dissected, rinsed with saline and then photographed to demonstrate model development as shown in Figure 1. It is important to note that we found that visceral sensitivity is present in indomethacin-dosed animals irrespective of the degree of mucosal injury present. Behaviorally, severe mucosal damage does not always produce the most robust pain response.
Figure 1 Representative digital photographs of stomachs dissected from mice orally dosed with vehicle (A) or 30 mg/kg indomethacin (B-D).
Stomachs were scored as follows: A: score 0 = no lesion (normal mucosa = vehicle); B: score 1 = light petechial; C: score 2 = diffuse bleeding or aphtha; and: score 3 = multiple aphtha.
Efficacy assessment
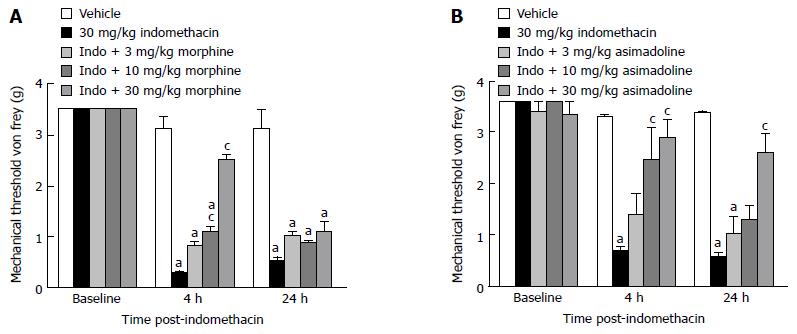
Opioid receptors: We examined the effect two distinct opioid receptor agonists had on visceral hypersensitivity. Firstly, we assessed the effect morphine (Figure 2A), the prototypical mu opioid receptor (MOR) agonist, had on the evoked visceral pain response. Results showed successful ulcer model development both 4 and 24-h post-indomethacin dosing (P < 0.05 vs vehicle), and that morphine attenuated the indomethacin-induced visceral hypersensitivity with a minimal effective dose (MED) equal to 10 mg/kg. Although there was a main effect of treatment [F (4, 54) = 36, P < 0.05 vs indomethacin] and a significant interaction with time [F (8, 79) = 21, P < 0.05 vs indomethacin], the effect morphine elicited on the pain behavior was only noted 2 h post-dosing (4 h post-indomethacin) for each of the higher doses tested, 10-30 mg/kg. Morphine was not efficacious in the assessment conducted 24-h post-indomethacin dosing. Nonetheless, the short-lived efficacy is consistent with the half-life of this drug. Subsequently, we next examined the effect asimadoline, a selective kappa opioid receptor (KOR) agonist, had in this pain model as well. Results showed consistent model development both 4 and 24-h post-indomethacin dosing (P < 0.05 vs vehicle). Asimadoline attenuated the indomethacin-induced visceral hypersensitivity with a MED= 10 mg/kg (Figure 2B). Like morphine, asimadoline was also efficacious in the model (10-30 mg/kg). There was a main effect of treatment [F (4, 63) = 25, P < 0.05 vs indomethacin] and a significant interaction with time [F (2, 78) = 52, P < 0.05]. However, unlike morphine, the kappa driven opioidergic effect was more prolonged such that the 30 mg/kg dose of asimadoline continued to attenuate the evoked pain response even at the 24-h assessment.
Figure 2 Effect of opioid receptor agonism on gastric ulcer pain.
A: Dose-response and time course for the effect of a mu opioid receptor agonist on referred gastric ulcer pain. Morphine significantly attenuated referred gastric ulcer pain when dosed 10-30 mg/kg; B: Dose-response and time course for the effect of a selective kappa opioid receptor agonist on referred gastric ulcer pain. Asimadoline significantly attenuated referred gastric ulcer pain when dosed 10-30 mg/kg. All data are expressed as the mean ± SEM, where n = 8-10 mice/group. aP < 0.05 vs vehicle, cP < 0.05 vs indomethacin.
TRP channels
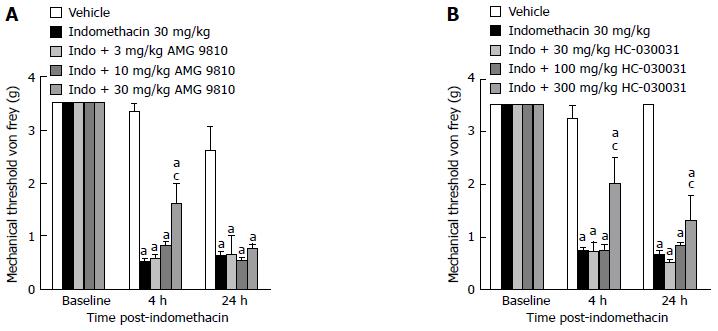
Next, we assessed the effect two distinct TRP channel receptor antagonists had on the visceral hypersensitivity. Firstly, we assessed the effect that AMG 9810, the most studied TRPV1 receptor compound, had on the evoked visceral pain response. In the ulcer pain model, indomethacin-dosed mice demonstrated reduced mechanical thresholds to von Frey fiber application compared to vehicle-treated animals as previously shown both 4 and 24-h post-dosing (Figure 3A, P < 0.05 vs vehicle). Systemic treatment with the TRPV1 antagonist AMG 9810 attenuated the referred ulcer pain by increasing the mechanical threshold at which a behavioral response was elicited. Although there was a main effect of treatment [F (4, 44) = 43.2, P < 0.05 vs indomethacin alone] and a significant interaction with time [F (8, 75)= 15, P < 0.05], only the 30 mg/kg dose significantly attenuated the visceral hypersensitivity present 4 h post-dosing as revealed by post hoc comparison. Efficacy was not observed at the 24-h assessment.
Figure 3 Effect of transient receptor potential channel blockade on gastric ulcer pain.
A: Dose-response and time course efficacy for selective TRPV1 channel blockade on referred gastric ulcer pain. AMG 9810 significantly attenuated referred gastric ulcer pain when dosed at 30 mg/kg; B: Dose-response and time course for the effect of selective TRPA1 channel blockade on referred gastric ulcer pain. HC-030031 significantly attenuated referred gastric ulcer pain when dosed at 300 mg/kg. All data are expressed as the mean SEM where n = 10 mice/group. aP < 0.05 vs vehicle, cP < 0.05 vs indomethacin.
We then examined the effect HC-030031, a selective transient receptor potential ankyrin 1 receptor (TRPA1) antagonist, had in this pain model as well. Indomethacin-dosed mice demonstrated reduced mechanical thresholds to von Frey fiber application compared to vehicle-treated animals as previously shown both 4 and 24-h post-dosing (Figure 3B, P < 0.05 vs vehicle). Similar to TRPV1 blockade, the systemic treatment with the TRPA1 antagonist HC-030031 also significantly attenuated the referred ulcer pain in indomethacin dosed mice (Figure 3B). Although there was a main effect of treatment [F (4, 46) = 38, P < 0.05 vs indomethacin alone]and a significant interaction with time [F (8, 71)= 15.4, P < 0.05], only the 300 mg/kg dose attenuated the visceral hypersensitivity as noted by post-hoc comparison. Unlike the TRPV1 antagonist AMG9810, the effect of 300 mg/kg HC-030031 persisted at the 24-h assessment.
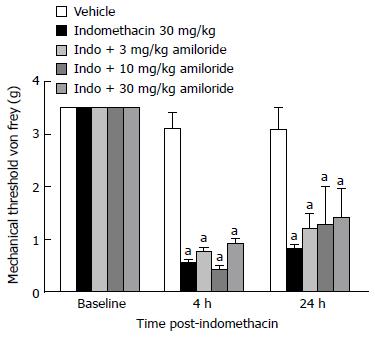
ASICs: We also investigated the sensory role of ASICs in ulcer pain. In the ulcer pain model, indomethacin-dosed mice demonstrated reduced mechanical thresholds to von Frey fiber application compared to vehicle-treated animals as previously shown both 4 and 24-h post-dosing (Figure 4; P < 0.05). Surprisingly, however, the non-selective ASIC blocker amiloride did not significantly reduce the referred ulcer pain at any doses tested when evaluated by post-hoc comparison. Referred abdominal hypersensitivity was present in all dose groups 4 and 24-h post-dosing.
Figure 4 Effect of acid-sensing ion channels channel blockade on gastric ulcer pain.
Dose-response and time course for the effect of non-selective ASIC channel blockade on referred gastric ulcer pain. Amiloride did not attenuate referred gastric ulcer pain at any of the doses tested. Data are expressed as the mean SEM where n = 10 mice/group. aP < 0.05 vs vehicle.
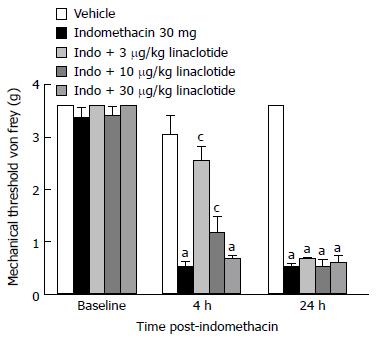
Guanylate cyclase-C: We determined if the newly developed GC-C agonist, linaclotide, was efficacious at attenuating ulcer pain. Indomethacin dosed mice demonstrated reduced mechanical thresholds compared to vehicle-dosed animals both 4 and 24-h post-dosing (Figure 5; P < 0.05). There was a main effect of indomethacin treatment [F (4, 62) = 486, P < 0.05] and a significant interaction with time, [F (2, 128) = 64; P < 0.05] 4 h post-dosing. 3-10 μg/kg linaclotide (MED = 3 μg/kg) significantly attenuated the indomethacin-induced mechanical hypersensitivity (P < 0.05). Surprisingly, the 3 μg/kg dose produced the most efficacious response while the 30 μg/kg dose was without effect. Visceral hypersensitivity was still notably present in mice that received 30 ug/kg linaclotide. That is, their tactile response was consistent with vehicle-dosed controls. The three doses of linaclotide tested were not efficacious in the ulcer pain model during the 24-h assessment.
Figure 5 Effect of guanylate cyclase C agonism on gastric ulcer pain.
A: Dose-response and time course for the effect of linaclotide on referred gastric ulcer pain. Linaclotide significantly attenuated referred gastric ulcer pain when dosed 3-10 μg/kg. Data are expressed as the mean SEM where n = 9-10 mice/group. aP < 0.05 vs vehicle, cP < 0.05 vs indomethacin.
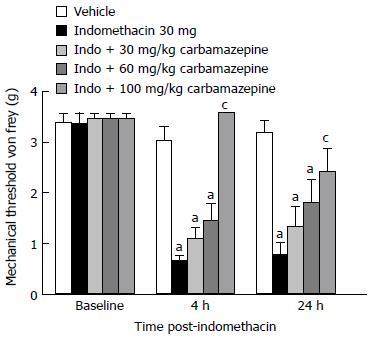
Sodium channels: Lastly, we determined if the non-selective sodium channel blocker, carbamazepine, was efficacious in the ulcer model. Indomethacin dosed mice demonstrated reduced mechanical thresholds compared to vehicle-treated animals both 4 and 24-h post-dosing (Figure 6, P < 0.05). There was a main effect of indomethacin treatment (F (4, 58)= 14, P < 0.05) and a significant interaction with time (F (2, 71) = 65, P < 0.05). The 100 mg/kg dose of carbamazepine significantly reversed the referred tactile hypersensitivity noted during the 4-h assessment (P < 0.05). Those animals that received the 100 mg/kg dose of carbamazepine also demonstrated significantly less visceral hypersensitivity during the 24-h assessment as well (P < 0.05 vs indomethacin).
Figure 6 Effect of sodium channel blockade on gastric ulcer pain.
Dose-response and time course for the effect of carbamazepine on referred gastric ulcer pain. Carbamazepine significantly attenuate referred gastric ulcer pain when dosed at 100 mg/kg. Data are expressed as the mean ± SEM with 9-10 mice/group. aP < 0.05 vs vehicle, cP < 0.05 vs indomethacin.
Side effect assessment
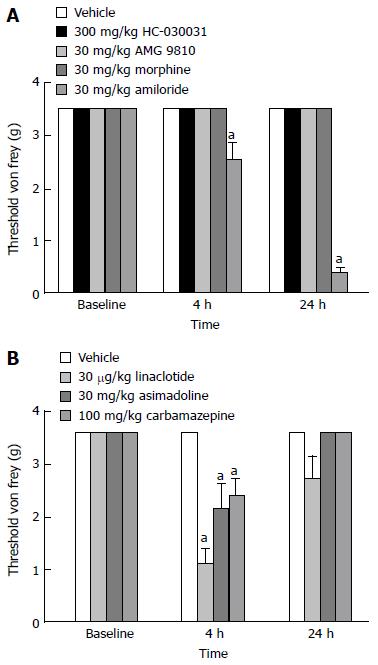
von Frey Threshold: To determine whether the compounds used in these experiments had any activity in the absence of visceral hypersensitivity, we assessed whether the highest doses tested in the efficacy model affected the von Frey thresholds of naïve animals. Post hoc analysis revealed that amiloride (Figure 7A; P < 0.05 vs vehicle), linaclotide, asimadoline and carbamazepine (Figure 7B; P < 0.05 vs vehicle) altered the von Frey thresholds of naïve mice when dosed at the highest dose examined in the ulcer model. Tactile sensitivity was enhanced 4-h after the dosing of linaclotide, asimadoline and carbamazepine, and 4 and 24-h after the administration of amiloride.
Figure 7 Effect of compound administration on von Frey threshold in naïve mice.
A: The non-selective ASIC blocker amiloride significantly decreased abdominal threshold in mice 4 and 24-h post-administration when dosed at 30 mg/kg. B: Linaclotide, asimadoline and carbamazepine significantly decreased abdominal threshold in mice 4-h post-administration. Data are expressed as the mean ± SEM, where n = 9-10 mice/group. aP < 0.05 vs vehicle.
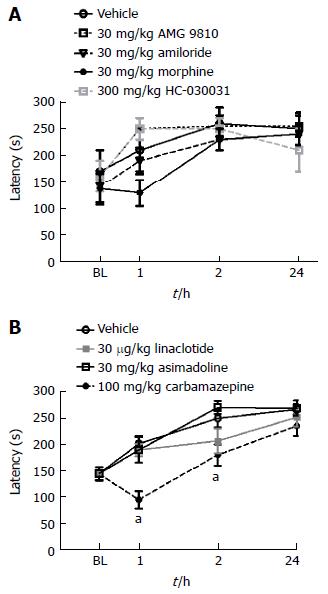
Rotarod performance: To place the efficacy of the compounds assessed into context with any inherent side effect liability, the highest dose of each compound examined in the ulcer model was subsequently evaluated in naïve mice using the rotarod assay. Morphine produced a slight but non-significant decrease in rotorad performance one hour after administration (Figure 8A), while carbamazepine produced significant deficits in rotarod performance both 1 and 2 h following dosing (Figure 8B; P < 0.05 vs vehicle). Conversely, AMG 9810, HC-030031, linaclotide, asimadoline and amilioride did not produce any deficits in motor performance as show.
Figure 8 Effect of compound administration on rotarod performance.
A: AMG 9810, amiloride, morphine and HC-030031 did not produce any significant deficits in rotarod performance 1-24 h post-administration; B: Linaclotide and asimadoline were without effect while carbamazepine significantly altered rotarod performance 1 and 2-h post-administration, aP < 0.05. All data are expressed as the mean ± SEM, where n = 10 mice/group.
DISCUSSION
Pain is a characteristic feature of many chronic disorders affecting the GI tract. In this study we measured the pain associated with NSAID-induced gastropathy[13,14] and then investigated relevant pharmacological mechanisms underlying its development and maintenance.
Firstly, our findings show that the NSAID-induced gastropathy model in mice consistently produces stomach ulceration and referred abdominal hypersensitivity that can be reliably measured. Importantly, the model is robust enough that proper pharmacological evaluation can be conducted. Since gastric ulcers are a source of visceral pain which can be referred to somatic dermatomes upon palpation of the abdomen[28,29], this study was able to measure the threshold at which mice responded to abdominal application of von Frey filaments. Although this response was used as an index of the pain, it was no surprise that the mice demonstrated such robust behavior given the macroscopic changes depicted in Figure 1.
In characterizing the mechanisms associated with this pain model, we first assessed the effects of two distinct opioid analgesics. We determined that the MOR agonist, morphine, attenuated the ulcer pain in a dose- and time-dependent manner. This was not unexpected given the robust presence of MORs on enteric neurons of the GI tract, as well as this compound’s well-published in vitro and in vivo pharmacological profile[38]. Interestingly and unknowing to us at the time of dosing was that the effect morphine had on ulcer pain may extend beyond its historical pain-ameliorating actions at the receptor level. That is, morphine may also provide gastric defense. An early study reported that morphine is protective to the stomach because it increases mucous production and decreases acid secretion[39]. However, contrary to this effect, it is also noteworthy to mention that morphine may be pro-ulcerogenic in certain circumstances as well. Esplugues and Whittle[40] had suggested that morphine can potentiate acid- and ethanol-induced gastric injury, although the experimental conditions employed herein did not support this result. Nonetheless, in this investigation, we demonstrated that morphine attenuated referred visceral pain at doses that did not alter von Frey thresholds or produce ataxia in naïve mice. However, it is important to note that the utility of MOR agonists for the treatment of visceral pain needs to be judiciously scrutinized given their effect on GI motility.
We next examined the efficacy of asimadoline, a potent KOR agonist. Asimadoline has 500-fold selectivity for kappa versus MORs and it is purported to produce its analgesic actions peripherally rather than centrally[41,42]. Previous work in animals has suggested that KOR agonists may represent a more viable approach to treating painful visceral conditions due to the nature of KOR expression and function[43,44]. In this regard, we hypothesized that asimadoline may be beneficial in attenuating visceral hypersensitivity. Interestingly, on a dose-per-dose basis, asimadoline was more efficacious than morphine in the model. Like morphine, it did not produce any deficits in rotarod performance at the highest dose tested; however, the 30 mg/kg dose did enhance tactile sensitivity in naïve animals. While Delgado-Aros et al[45] found that asimadoline produced hyperalgesia in patients dosed with high doses of the compound, there is a disconnect between human and rodent. In the human condition, these results may suggest that the KOR system becomes engaged during pathological conditions of persistent hypersensitivity[41,46] and that it does not significantly alter noxious sensation in healthy states[44]; however, these findings/assumptions are not consistent with ours since the mice tested were naïve and not subjected to ulcer induction. Although continued research is necessary to further our understanding on how KOR agonists modulate visceral pain, there is clinical evidence to support such an indication. Most notably, Cara Therapeutics have recently developed a peripherally restricted compound, CR665, which has demonstrated efficacy in human visceral pain[47-48].
TRP channels have received a significant amount of attention for their alleged role in pain including visceral hypersensitivity[35,49]. Since TRP channels transduce noxious stimuli at the peripheral terminals of nociceptors, these channels are well-poised anatomically to function as the first integrators of the pain response[50]. In support of this Akbar and colleagues[51] demonstrated a 3.5-fold increase in the density of TRPV1 immuno-reactive fibres in the colonic biopsies of patients with IBS compared to healthy controls. Transient receptor potential vanilloid-1 (TRPV1) is a ligand-gated nonselective polymodal cation channel that integrates a variety of pain stimuli[49]. Since noxious GI events are conveyed to the CNS by vagal and spinal afferents, and TRPV1 is expressed within both dorsal root and nodose ganglia innervating the GI tract[52,53], we examined whether selective blockade of the TRPV1 receptor would attenuate ulcer pain. Our results showing the ability of AMG 9810 to attenuate pain in the ulcer model suggests that the irritating effects of indomethacin on the gastric mucosa may engage the TRPV1 receptor either directly or indirectly such that these afferent neurons become hypersensitive to the hydrochloric acid that is normally innocuous to the stomach. Overall, the net effect may be analogous to proton activation of the channel. Alternately, it also becomes noteworthy to consider early reports supporting the protective effects of capsaicin-sensitive afferent neurons on the gastric mucosa[16,54]. While activation is believed to be protective, ablation of capsaicin-sensitive neurons impairs mucosal defense. With regard to our findings, one may have expected antagonism of TRPV1 to be detrimental. However, unlike nerve ablation which removes the entire nerve terminal and its functionality, an antagonist would leave the protective effects of these neurons intact.
Like TRPV1, TRPA1 is a non-selective cation channel that is a propagator of painful signaling that is activated by a variety of stimuli as well[55]. TRPA1 is mainly expressed in small diameter peptidergic nociceptors of the DRG, nodose and trigeminal ganglia along with TRPV1. TRPA1 has also been shown to be extensively expressed in enterochromaffin cells of colonic myenteric neurons as well as within non-neuronal tissue such as the small intestine and pancreas[55-57]. Therefore, together with its profile, co-expression pattern and connectivity to afferents that potentially impact the stomach, we hypothesized that TRPA1 would be a potential mediator of ulcer pain. We found that the TRPA1 antagonist, HC-030031, attenuated abdominal hypersensitivity in the ulcer model. This result is in agreement with a study by Kondo et al[57] who demonstrated the efficacy of this compound in the context of a noxious gastric distention model. However, these findings are in direct contrast to a recent study by Kojima et al[58] who examined the effect of the TRPA1 agonist, ASP7663, in a similar model. Surprisingly, they maintained that TRPA1 agonism, not antagonism, represents a rational approach to treating visceral pain and that the analgesic effect of ASP7663 on colorectal distension-induced abdominal pain is mediated by direct desensitization of the TRPA1 channel.
Another potential transducer of noxious signals in the GI tract are ASICs (ASIC 1-3), a family of proton-gated sodium channels expressed within primary afferent neurons. Since a positive correlation between pain and local acidity has been reported and protons trigger inward currents through sodium channels in visceral sensory neurons causing hyper-excitability[21,59,60], we were surprised that amiloride did not attenuate the referred ulcer pain at any of the doses tested. Another study by Jones et al[61] examining the effect of amiloride on afferent fiber sensitivity to circumferential stretch of the colon also failed to see efficacy. The authors suggested that different ASIC subunits may exert opposing effects on mechanosensation. Likewise, different subunits may be involved in mechanical hypersensitivity of the colon as shown in a study using ASIC3 knockout mice[62] and this too may be reflective of the enhanced tactile sensitivity observed following dosing of the non-selective blocker amiloride to naïve animals and its lack of efficacy in the ulcer model.
Voltage-gated sodium channels have also received a significant amount of attention in recent years for their diverse role in pain. Given this, we investigated the role of the non-selective sodium channel blocker, carbamazepine, in the ulcer model. Unlike a prior efficacy report[63], we found carbamazepine produced a positive signal when administered at a high dose, 100 mg/kg. However, it is difficult to conclude if this was an efficacious response or a mixed result driven by the sedative properties of this molecule. As expected, carbamazepine also affected other measures of neurological function, such as rotarod performance and innate tactile threshold, thereby complicating interpretation of the reflexive endpoint used. Nonetheless, sodium channels appear to be important in pain as they have been causally linked to human conditions like IBS[64]. Notably, a number of selective sodium channel blockers targeting Nav 1.7 and Nav 1.8 in the periphery have entered clinical development[65]. Given the expression pattern of these targets as well as their selectivity profiles, one would expect that the not so distant future will provide novel sodium channel blockers that may alleviate conditions associated with visceral hypersensitivity although the discovery and developmental path for these compounds thus far has been very challenging.
Lastly, we examined whether linaclotide (Linzess™), a first-in-class, orally administered synthetic peptide of the guanylin peptide family, would produce an efficacious response in the ulcer model[33]. Linaclotide, a GC-C agonist, and its active metabolite are purported to bind to GC-C to act locally within the GI lumen to elevate cGMP. Mechanistically, Silos-Santiago et al[66] suggest that the cGMP significantly decreases the firing rates of sensitized afferent neurons in response to mechanical stimuli to lessen the pain response. In agreement with this study, we found that linaclotide was efficacious in attenuating ulcer pain when dosed 3-10 μg/kg. Surprisingly, however, the maximum effect was observed when linaclotide was administered at the lowest dose, 3 μg/kg. Although we would not have predicted this result, this finding did align with findings published previously by Eutamene et al[67] using the colorectal distension model. Similarly, this study showed that higher doses of linaclotide, 30 μg/kg, did not affect colorectal hypersensitivity, while lower doses did attenuate it. Interestingly, IBS clinical trial results examining the efficacy of linaclotide in patients confirmed these preclinical findings as well such that the FDA approved package insert for this compound states that continued abdominal pain (7% vs 5% placebo) is a possible side effect of therapy (http://www.linzesshcp.com). Although the mechanism for this paradoxical effect is unclear, it could be related to the physiochemical-pharmacokinetic properties of the molecule. Linaclotide has no oral bioavailability when given at efficacious doses; it is purported to act locally within the GI tract. However, with increasing dosage, the PK of linaclotide is documented to be non-linear. So, it is conceivable that such reduced anti-hyperalgesic effects at higher doses may be attributable to a corresponding loss of pharmacological specificity[67].
Visceral hypersensitivity underlies the pain experienced by patients presenting with various GI-related disorders. Although there is an unmet need to model visceral pain pre-clinically, this can be very challenging given the unique sensory innervation of visceral organs as well as our incomplete understanding of disease etiology[68]. Nonetheless, given the characteristics of visceral pain, both favorable (i.e., not produced by noxious stimuli) and unfavorable (i.e., pain is referred, diffuse, poorly localized)[27], we have demonstrated that the ulcer model is uniquely sensitive for assessing the effects of compounds directed against several well-known pain mechanisms. Furthermore, while we think this model is well-suited for drug discovery namely because of its ease and its reliability in performing stringent pharmacological evaluation (i.e., dose-response data), it does have its limitations. For one, it is an acute model of visceral pain. While acute visceral pain is burdensome, the unmet need lies with the undulating hypersensitivity that persists from chronicity like in IBS. Also, most discovery groups perform their pain studies in rats, so consistency with regard to species selection does differ as this model was validated in mice. Lastly, functional GI disorders represent a heterogeneous group of disorders that include genetic and environmental contributors. Notably, these factors are difficult to recapitulate in animals. While these limitations are well-recognized, together with its clinical relevance, we believe that the NSAID-induced gastropathy model may help uncover additional targets contributing to persistent GI pain of ill-defined etiology and further advance future drug discovery efforts for this unmet need.