Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5887
Peer-review started: December 28, 2016
First decision: April 21, 2017
Revised: May 8, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: August 28, 2017
Processing time: 247 Days and 9.2 Hours
To investigate and compare the effects of tocotrienol and omeprazole on gastric growth factors in rats exposed to water-immersion restraint stress (WIRS).
Twenty-eight male Wistar rats were randomly assigned to four groups of seven rats. The two control groups were administered vitamin-free palm oil (vehicle) and the two treatment groups were given omeprazole (20 mg/kg) or tocotrienol (60 mg/kg) by oral gavage. After 28 d of treatment, rats from one control group and both treated groups were subjected to WIRS one time for 3.5 h. Gastric lesions were measured and gastric tissues were obtained to measure vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and transforming growth factor-alpha (TGF-α) mRNA expression.
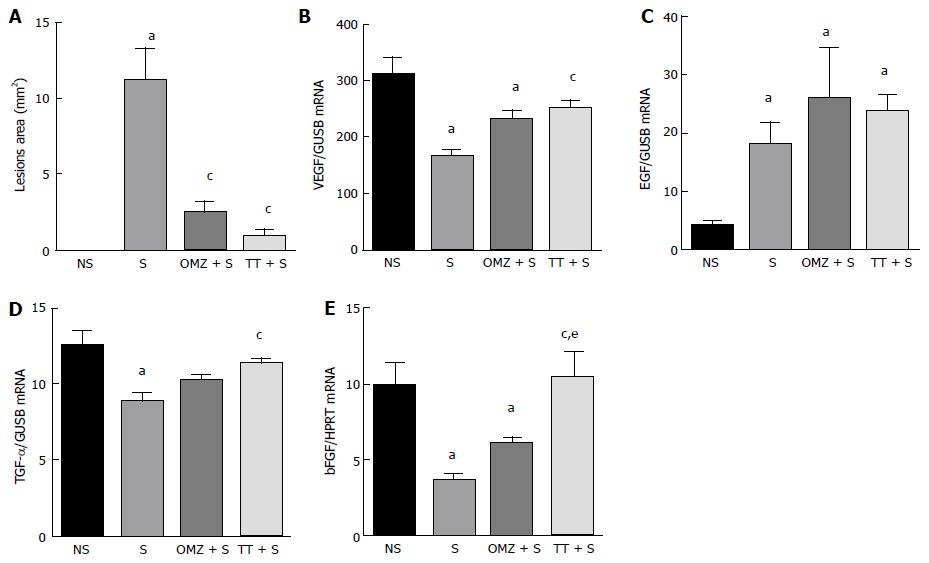
Rats exposed to WIRS for 3.5 h demonstrated the presence of considerable ulcers in the form of gastric erosion. The lesion index in the stressed control (S) group was increased (P < 0.001) compared to the tocotrienol treated and omeprazole treated groups. Stress led to a decrease in gastric VEGF (P < 0.001), bFGF (P < 0.001) and TGF-α (P < 0.001) mRNA levels and caused an increase in EGF mRNA (P < 0.001) that was statistically significant compared to the non-stressed control group. Although both treatment agents exerted similar ulcer reducing ability, only treatment with tocotrienol led to increased expression of VEGF (P = 0.008), bFGF (P = 0.001) and TGF-α (P = 0.002) mRNA.
Tocotrienol provides gastroprotective effects in WIRS-induced ulcers. Compared to omeprazole, tocotrienol exerts a similar protective effect, albeit through multiple mechanisms of protection, particularly through up-regulation of growth factors that assist in repair of gastric tissue injuries.
Core tip: During the process of ulcer healing, growth factors such as epidermal growth factor, transforming growth factor-alpha, basic fibroblast growth factor and vascular endothelial growth factor acts by activating the migration of cells from the edge of the ulcer and cell proliferation together with the formation of granulation tissue and angiogenesis. Rats exposed to stress develop gastric mucosal ulcers and changes in expression of these growth factors surrounding the ulcers had been reported. Tocotrienol effects on gastric mucosal growth factors were compared to those by omeprazole in this study. The findings suggest that in contrast with omeprazole, tocotrienol has a protective effect on the gastric mucosa through its effect on these growth factors.
- Citation: Nur Azlina MF, Qodriyah HMS, Chua KH, Kamisah Y. Comparison between tocotrienol and omeprazole on gastric growth factors in stress-exposed rats. World J Gastroenterol 2017; 23(32): 5887-5894
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5887
Stress ulcers often occur in critically ill patients as a result of major stressful events, such as trauma, shock, surgery, sepsis and burns. The responses to stress are both psychological and physiological. Physiological responses include neurohormonal and immunological activation, which includes release of corticotropin-releasing factor[1], while the psychological responses include anxiety, depression, feeling of helplessness, fear, etc.
The pathological basis for the development of stress ulcers is multifactorial and includes changes in gastric acid secretion[2], oxidative stress[3-5], impaired gastric blood flow[6], reduced gastric prostaglandin synthesis[7,8], inflammation[9,10], and inhibition of mucosal growth and proliferation. Growth factors, by contrast, play pivotal roles in prevention and repair of stress-induced gastric ulcers[11]. This is particularly true during recovery of the mucosa after stress-induced injuries. Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor-alpha (TGF-α) and epidermal growth factor (EGF) are crucial for reconstruction of damaged mucosal structures.
Among the growth factors involved, VEGF and bFGF are important factors because of their effects on angiogenesis. These growth factors are produced by endothelial cells, fibroblasts, macrophages and smooth muscle cells, and are involved in the regulation of physiological and pathological angiogenesis[12]. Angiogenesis and growth factors such as bFGF and VEGF play important roles in the repair of gastric ulcers caused by disturbances in the balance between factors that damage and factors that protect the stomach[13].
In animal studies, angiogenesis has been shown to play a role in the process of supplying oxygen and nutrients to ulcers of affected areas[14]. Malara et al[15] demonstrated a role for VEGF and angiogenesis in the repair of gastric ulcers caused by stress induced in rats. Research has also shown that a significant increase in the expression of VEGF protein followed by the formation of new blood vessels occurred as early as 1 d after formation of the ulcers[14]. Exogenous bFGF was found to assist in repair of gastric ulcers and other stress, and angiogenesis was also found to reduce gastric acid secretion[13]. Ernst et al[13] reported that there was a reduction in stress-induced endogenous bFGF and that this resulted in reduced gastric microcirculation, which plays an important role in the repair of ulcers. It is unknown if transcription of these growth factors are activated in response to stress-induced gastric injury.
Tocotrienol has been shown to prevent gastric ulcer development in rats exposed to noxious stimuli including ethanol, non-steroidal anti-inflammatory drugs (NSAIDs) and stress[8,16]. Tocotrienol, in comparison to tocopherol, was reported to be a more potent antioxidant[17]. Other than its antioxidant capabilities, tocotrienol has also be shown to have anti-inflammatory effects[10], which may play an effective role in reducing damage to the gastric mucosa due to stress.
The gastric ulcer formation is complex in nature and involves multiple pathways that play a role in the prevention and repair[18]. The ulcer model that was used in this study was water-immersion restraint stress (WIRS). This experimental model was chosen because of its reproducibility, reliability and validity[15,19]. This model had been used to mimic clinical acute gastric ulcers formation in critically ill patients and is widely accepted for research involving the mechanism of stress-induced gastric ulcers[10,12]. The present study evaluated the limited information on the gastroprotective activity of palm-derived tocotrienol with relation to anti-ulcer properties and gastric growth factors. The purpose of the study is to contribute a better understanding of the pathophysiology of stress-induced gastric ulcers. In this study, tocotrienol was compared to omeprazole, one of the widely used drugs for peptic-ulcer disease in clinical settings.
Male Wistar rats (n = 28) were divided into four equally sized groups. Two control groups were fed a normal rat diet (non-stressed: NS and stressed: S), while the treatment groups received the same diet but with oral supplement of tocotrienol or omeprazole at 60 mg/kg or 20 mg/kg body weight, respectively, for 28 d. The tocotrienol dose was chosen based on our previous studies that demonstrated a protective effect on stress-induced gastric lesions[10,20]. The tocotrienol and omeprazole were diluted in vitamin-free palm oil, acting as vehicle, and administered by oral gavage using an 18G gavage needle. Both S and NS control groups were administered vitamin-free palm oil.
At the end of the treatment period, rats from the S control group and both treated groups were exposed to WIRS, by placing them in individual plastic restrainers measuring approximately 17 cm × 5 cm and immersing them in water neck deep one time for 3.5 h, as previously described by Aziz Ibrahim et al[16]. Following the restraining procedure, rats were sacrificed by exsanguination under anesthesia. Stomachs were then dissected along the greater curvature. The dissected stomachs were taken for evaluation of gastric ulcers and mRNA expression of gastric EGF, bFGF, VEGF, and TGF-α. Gastric tissues were homogenized using an Omni Bead Ruptor machine at 25 °C with the speed of 8 m/s for 20 s. Homogenates were centrifuged at 1500 × g for 5 min at 4 °C. Supernatants were then used for mRNA analysis.
All rats were kept on a regular night/day cycle, with natural light for a period of 12 h (0700 to 1900 h). Throughout the feeding period, all rats were habituated to handling to reduce stress-related disturbances. Rats were housed in large cages with wide wire-mesh floors to prevent coprophagy. Food and water were given ad libitum throughout the experiment. Ethical approval was obtained from Universiti Kebangsaan Malaysia Animal Ethics Committee (UKMAEC). Humane methods of euthanasia were practiced (i.e., exsanguination under anesthesia) by following guidelines of and with approval from UKMAEC. The anesthetic agent used was a combination of ketamine and xylazine (1:1 ratio).
The macroscopic assessment of stress-induced gastric lesions in the gastric mucosa was performed by two independent examiners who were blinded to the treatment. The assessment of lesions was done according to a semi-quantitative scale. Lesion size (mm) was determined by measuring each lesion area. Five petechial lesions were equal to 1 mm lesion. The total lesion area in each group of rats were averaged and expressed as the lesion index; this method was modified as previously described by Aziz Ibrahim et al[16].
mRNA levels of EGF, bFGF, VEGF and TGF-α from gastric tissues were assayed according to the manufacturer’s instructions using the standard QuantiGene Plex 2.0 assay kit (Genospectra, Fremont, CA, United States). Briefly, tissue lysates were transferred to a capture well in the presence of the gene-specific probe set and then hybridized at 53 °C overnight. Wells were washed twice with bDNA wash buffer and then incubated at 46 °C sequentially with an amplifier and an alkaline phosphatase-labeled probe, with a wash step in between incubations. After the final wash step, addition of streptavidin phycoerythrin generated a signal that was proportional to the amount of target RNA present in the sample. The luminescence signal was detected using a Luminex instrument. The protocol was followed as previously described by Zhang et al[21].
Statistical analyses were performed using PRISM software version 6.00 (Graphpad, San Diego, CA, United States). The results are expressed as the mean ± SE of the mean. Statistical significance (P < 0.05) was determined by ANOVA and Tukey’s post-hoc test.
Exposure to WIRS for 3.5 h caused the formation of ulcers in the form of gastric mucosal erosion and ulcers which were confined to the corpus of the stomach. The gastric lesion index (area in mm2) in the stressed-exposed (S) group was increased (11.92 ± 2.0 mm2; P = 0.001) compared to the tocotrienol-treated group (0.94 ± 0.30 mm2) and the omeprazole-treated group (2.44 ± 0.7 mm2), as shown in Figure 1A. Rats not exposed to stress did not develop any gastric lesions.
Figure 1B shows that VEGF mRNA expression in stressed control rats was decreased by 45% compared to NS rats (P < 0.0001). Pre-treatment with tocotrienol caused a statistically significant increase in VEGF expression compared to the stressed control group (P = 0.0075). However, pre-treatment with omeprazole did not enhance VEGF expression compared to the S control group (P = 0.0593).
Stress exposure caused an increase in EGF gene expression (P = 0.0001). Expression was increased in all groups exposed to stress and no differences were observed between the three stressed groups (S, tocotrienol-treated and omeprazole-treated) (Figure 1C), suggesting that tocotrienol and omeprazole had no effect on EGF mRNA levels in stress-exposed rats.
Exposure to immobilization stress caused a decrease in expression of TGF-α in gastric tissue (Figure 1D). Stress caused a significant reduction in the gastric expression as shown. Pre-treatment with tocotrienol caused an increase in TGF-α gene expression compared to the S control group (P = 0.0024). Similar levels of TGF-α were observed in the NS control group. This result suggests that tocotrienol had a protective effect by preventing the stress-induced decrease of TGF. By contrast, TGF-α gene expression in the omeprazole-treated rats was decreased to levels similar to those observed in the S control group (P > 0.999).
Stress caused a 63% decrease in bFGF gene expression (P = 0.0022), as compared to NS rats. bFGF expression in the tocotrienol-treated group was increased compared to stressed control rats (P = 0.0013) and there was no statistically significant change in bFGF levels when compared with the NS control rats. This suggests that pre-treatment with tocotrienol protects stress-induced rats by preventing a decrease in the level of bFGF to a level that was similar to that observed in the NS controls. bFGF expression in the tocotrienol-treated group was increased by 43% compared to the omeprazole-treated group (P = 0.0492). Omeprazole treatment had no protective effect when the bFGF levels were similar to those observed in S rats, as shown in Figure 1E.
Various genes are regulated at different rates and times during and after gastric mucosal injury. Most data regarding gene expression after mucosal injury models are taken from experimental animals. Together with the formation of granulation tissue, blood vessel and angiogenesis, growth factors act to enable the migration of cells from the edge of the ulcer and induce cell proliferation during the process of ulcer healing[7,22]. For example, EGF mRNA is detected immediately after ulcer induction, peaks during day 3, and continues to decrease 10 d after the induction of ulcers, whereas TGF-α mRNA expression increases 6 d after injury[23].
VEGF is a growth factor that helps tissue healing by stimulating angiogenesis, and is also important for the formation of connective tissue[24,25]. Antonisamy et al[26] showed that injury to the gastric mucosa through administration of indomethacin caused a striking decrease in VEGF content in the gastric mucosa. Findings from this study also showed that exposure to WIRS caused a significant decrease in VEGF gene expression in gastric tissue compared to rats that were not exposed to stress. In another study, pre-treatment with a single dose of oral VEGF protected the stomach against damage due to acute ethanol administration[27]. Furthermore, gastric ulcer healing was prolonged and angiogenesis was decreased in response to a reduction in expression of VEGF[28]. Finally, up-regulation of VEGF has been shown to play an important role in the healing of acute gastric injury[29].
Our findings show that tocotrienol led to increased VEGF expression in stress-induced rats. Studies that evaluate the effect of tocotrienols on growth factor expression in gastric ulcers are limited. δ-tocotrienol has been shown to decrease the expression of VEGF in tumor cells, thereby reducing angiogenesis in these cells[30-32]. The tocotrienol administered in this study contained less than 4% δ-tocotrienol and consisted mostly of other isomers, the most being isomeric γ-tocotrienol (approximately 50%). The effect obtained from this study leads us to assume that the other isomers in the tocotrienol mixture used in this study might assist in the healing process of ulcers or provide gastric mucosal protection against injury by promoting the process of granulation tissue formation and angiogenesis through VEGF expression.
Unlike tocotrienol, omeprazole administration did not decrease VEGF expression in stress-induced rats. This is inconsistent with results from a study by Kobayashi et al[30] that showed that administration of lansoprazole (another proton pump inhibitor) led to increased expression of the VEGF gene in rats with gastric ulcers induced with acetic acid. These results suggest that proton pump inhibitors have an additional impact on protection of gastric ulcer formation (i.e., regulation of growth factor expression) other than prevention of gastric acid secretion[30]. However, this effect was not observed in our study using omeprazole, suggesting that not all proton pump inhibitors have the same effect on growth factors. Abdul-Aziz et al[27] reported similar results, i.e., omeprazole reduced gastric ulcers but did not enhance VEGF expression levels.
In addition to VEGF, the growth of the mucosa is under the influence of various other growth factors, such as EGF and polyamines, which play important roles in tissue maintenance and repair. EGF is secreted into the intestinal lumen and into the bloodstream after being produced in saliva and pancreatic gland, and excreted via the urine as urogastrone[33]. It is well established that EGF is necessary for the maintenance of mucosal integrity. Furthermore, accumulation of EGF in the region of gastric mucosal injury promotes the local lesion healing process[1].
Milani et al[24] showed that expression of growth factors detected in gastric mucosal cells, constantly fluctuates even under normal conditions. In the absence of induction of lesions, vitamin E does not significantly affect the function of growth factors where a decline in immunoreactivity of the EGF receptor (EGF-R) has been reported[34]. The results of this study also showed that rats that were not exposed to stress had low levels of EGF gene expression. Exposure to NSAIDs causes gastric ulcers to form and leads to decreased levels of EGF in gastric mucosa[26]. Increased expression of both EGF and EGF-R in the ulcer area also contributes to the repair process[35]. This reaction may occur in response to the sharp decline of this growth factor during gastric injury.
Exposure to stress led to formation of gastric lesions and caused an increase in gene expression of EGF that was statistically significant compared to the non-stressed control group. Increased expression of EGF has been reported to accelerate healing of gastroduodenal ulcers by increasing gastric mucin production and reducing gastric acid secretion[26]. This indicates that expression of EGF levels increase in response to injury of the gastric mucosa in order to restore the tissue back to its original state.
In this study, however, pre-treatment with tocotrienol or omeprazole did not change gastric tissue EGF expression; expression of EGF remained elevated in stress-exposed rats compared to rats that were not exposed to stress. This may have occurred due to the presence of gastric tissue injury in the treatment group that could have led to increased expression of EGF, which accelerates the recovery of gastric tissue caused by stress. Studies by Qodriyah et al[34] showed that EGF levels were increased compared to the control group under normal conditions after 8 wk of palm vitamin E (PVE) treatment. The results of their study suggest that, under normal circumstances, vitamin E also enhances expression of EGF. While in a state of gastric injury due to NSAIDs, expression of EGF remained elevated after pre-treatment with PVE[34], consistent with the results of this study.
EGF released from the salivary glands and TGF-α from the gastric mucosa act to maintain mucosal integrity and recovery during gastric mucosal injuries. Both these growth factors produce the same biological activities during recovery. For example, TGF-α and bFGF levels change when an injury occurs in the gastric mucosa. In this study, exposure to stress caused a significant decrease in bFGF gene expression. bFGFs have been shown to play a role in both angiogenesis and recovery of gastric ulcers in rats[36,37]. bFGF activation occurs in response to injury of the gastric mucosa, as demonstrated by the increased expression observed near ulcers[38]. Administration of bFGF (100 ng) locally into ulcers or systemically caused significant recovery in acetic acid-induced gastric ulcers[37]. bFGFs are also known to stimulate synthesis of prostaglandins locally[30,39], leading to increased formation of blood vessels[14,40] as well as proliferation of endothelial cells[41], sustaining and assisting the recovery of gastric tissues in the event of injury.
Pre-treatment with tocotrienol in this study led to increased expression of bFGF in rats that were exposed to stress. Vitamin E at 150 mg/kg has been shown to improve bFGF expression in mice that developed gastric mucosal injury due to NSAID exposure[34,42]. Rashid et al[42] showed that tocotrienol increased bFGF levels, thus reducing the formation of scar tissue. However, our study found that omeprazole had no effect on bFGF gene expression, which is in contrast to the tocotrienol-treated group. Studies that examine bFGF expression in response to omeprazole treatment are limited. Tsuji et al[43] found that administration of lansoprazole, also a proton pump inhibitor, helped repair gastric ulcers by increasing bFGF levels at the edge of the ulcer border. Pantoprazole helped promote angiogenesis in gastric lesions induced by NSAIDs through increased expression of bFGF[44]. This effect, however, was not observed with pre-treatment of omeprazole in this study.
Growth factors such as EGF, TGF-α, bFGF and VEGF activate migration and proliferation of cells at the edge of the ulcer and promote the formation of granulation tissue and angiogenesis during the process of ulcer repair[22]. EGF is required to maintain the integrity of gastric mucosa and promotes healing of injured tissue[26]. TGF-α accelerates replacement of the epithelium and regulates regeneration of epithelial cells in gastric tissues[33]. VEGF assists in repair of ulcers by stimulating angiogenesis and remodeling of connective tissues[24], while bFGF has been known to stimulate synthesis of local prostaglandins, which ultimately leads to increased formation of blood vessels[14,40] and endothelial cell proliferation[41]. This assists in maintenance and recovery of gastric tissue in the event of injury. The results of this study suggest that in contrast with omeprazole, tocotrienol has a protective effect on the gastric mucosa through regulation of these growth factors.
Here, we show that tocotrienol provides a gastroprotective effect in WIRS-induced ulcers and exerts similar effectiveness when compared to omeprazole. However, it displays a more diverse mechanism of protection, particularly through increased expression of bFGF, TGF-α and VEGF in a stress-induced gastric ulcer rat model in comparison to omeprazole. Thus, the effect of tocotrienol might be of therapeutic interest for the prevention and repair of gastric mucosal injuries due to other mechanisms.
We would like to acknowledge ExcelVite® Sdn. Bhd. for the supply of tocotrienol for this research. The authors also wish to thank En. Muhamad Arizi Aziz, Puan Juliana, Cik Hafizah, Puan Farhana and Puan Nurul Akmal for their technical assistance.
Stress is well known to induce gastric ulcers. Although the proposed pathogenesis is multifactorial, a common entity observed in peptic ulcer diseases is oxidative stress which overwhelms the endogenous antioxidant system. Thus, prevention and treatment using an antioxidant like tocotrienol is a logical therapeutic approach. This study focuses on the therapeutic ability of tocotrienol on reducing stress-induced gastric ulcers and its effects on gastric growth factors which plays an important role in the prevention and repair of ulcers.
Few studies had investigated the effect of tocotrienol from palm source on gastric growth factors.
Tocotrienol provides gastroprotective effect in water-immersion restraint stress-induced ulcers. Although tocotrienol provides similar effectiveness as compared to omeprazole, it has a more diverse mechanism of protection, particularly through up-regulation of basic fibroblast growth factor, transforming growth factor-alpha and vascular endothelial growth factor in a stress-induced gastric ulcer model.
Tocotrienol as therapeutic agent for the prevention and enhancing the repair of gastric mucosa against injures.
Stress ulcers can occur as a result of major stressful events, such as trauma, shock, surgery, sepsis and burns. Tocotrienol prevents gastric ulcer development in rats exposed to noxious stimuli including ethanol, non-steroidal anti-inflammatory drugs and stress.
The research is well conducted and the paper is well written. The series of experiments conducted were able to answer the objective of the study and the statistical tests used were scientifically sound.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Malaysia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Yuen KH S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Xu XR
| 1. | Guo S, Gao Q, Jiao Q, Hao W, Gao X, Cao JM. Gastric mucosal damage in water immersion stress: mechanism and prevention with GHRP-6. World J Gastroenterol. 2012;18:3145-3155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Dalia M, Abd El Motteleb, Mai MH. Gastroprotective effect of simvastatin against experimentally induced gastric ulcers in rats: Role of ATP-sensitive K+ channels. J Am Sci. 2011;7:760-768. |
| 3. | Brzozowski T, Konturek PC, Sliwowski Z, Drozdowicz D, Burnat G, Pajdo R, Pawlik M, Bielanski W, Kato I, Kuwahara A. Gastroprotective action of orexin-A against stress-induced gastric damage is mediated by endogenous prostaglandins, sensory afferent neuropeptides and nitric oxide. Regul Pept. 2008;148:6-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Nur Azlina MF, Rubaizah K, Muliana M, Nafeeza MI. Modulation of restraint stress induced gastric oxidative changes in rats by tocotrienol and tocopherol. Int J Pharmacol. 2009;5:58-64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Mohd Fahami NA, Ibrahim IA, Kamisah Y, Mohd Ismail N. Palm vitamin E reduces catecholamines, xanthine oxidase activity and gastric lesions in rats exposed to water-immersion restraint stress. BMC Gastroenterol. 2012;12:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Wang YB, Liu J, Yang ZX. Effects of intestinal mucosal blood flow and motility on intestinal mucosa. World J Gastroenterol. 2011;17:657-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Konturek PC, Brzozowski T, Duda A, Kwiecien S, Löber S, Dembinski A, Hahn EG, Konturek SJ. Epidermal growth factor and prostaglandin E(2) accelerate mucosal recovery from stress-induced gastric lesions via inhibition of apoptosis. J Physiol Paris. 2001;95:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Nur Azlina MF, Kamisah Y, Chua KH, Qodriyah HM. Tocotrienol Attenuates Stress-Induced Gastric Lesions via Activation of Prostaglandin and Upregulation of COX-1 mRNA. Evid Based Complement Alternat Med. 2013;2013:804796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G414-G423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Nur Azlina MF, Kamisah Y, Chua KH, Ibrahim IA, Qodriyah HM. Preventive Effects of Tocotrienol on Stress-Induced Gastric Mucosal Lesions and Its Relation to Oxidative and Inflammatory Biomarkers. PLoS One. 2015;10:e0139348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Konturek PC, Brzozowski T, Konturek SJ, Taut A, Sliwowski Z, Stachura J, Hahn EG. Activation of genes for growth factors and cyclooxygenases in rat gastric mucosa during recovery from stress damage. Eur J Pharmacol. 1998;342:55-65. [PubMed] |
| 12. | Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 252] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Ernst M, Inglese M, Waring P, Campbell IK, Bao S, Clay FJ, Alexander WS, Wicks IP, Tarlinton DM, Novak U. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189-203. [PubMed] |
| 14. | Schmassmann A, Peskar BM, Stettler C, Netzer P, Stroff T, Flogerzi B, Halter F. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br J Pharmacol. 1998;123:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Malara B, Jośko J, Tyrpień M, Malara P, Steplewska K. Dynamics of changes in vascular endothelial growth factor (VEGF) expression and angiogenesis in stress-induced gastric ulceration in rats. J Physiol Pharmacol. 2005;56:259-271. [PubMed] |
| 16. | Aziz Ibrahim IA, Kamisah Y, Nafeeza MI, Nur Azlina MF. The effects of palm vitamin E on stress hormone levels and gastric lesions in stress-induced rats. Arch Med Sci. 2012;8:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994;234:354-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | El-Moselhy MA, Abdel-Hamid NM, Abdel-Raheim SR. Gastroprotective effect of nicorandil in indomethacin and alcohol-induced acute ulcers. Appl Biochem Biotechnol. 2009;152:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Konturek SJ, Brzozowski T, Konturek PC, Zwirska-Korczala K, Reiter RJ. Day/night differences in stress-induced gastric lesions in rats with an intact pineal gland or after pinealectomy. J Pineal Res. 2008;44:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kamisah Y, Ibrahim AAI, Nafeeza MI, Nur Azlina MF. Palm tocotrienol rich fraction supplementation suppressed stress-induced gastric oxidative stress in rats. J Appl Phar Sci. 2011;1:118-122. |
| 21. | Zhang A, Pastor L, Nguyen Q, Luo Y, Yang W, Flagella M, Chavli R, Bui S, Nguyen CT, Zheng Z. Small interfering RNA and gene expression analysis using a multiplex branched DNA assay without RNA purification. J Biomol Screen. 2005;10:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Konturek PC, Konturek SJ, Brzozowski T, Ernst H. Epidermal growth factor and transforming growth factor-alpha: role in protection and healing of gastric mucosal lesions. Eur J Gastroenterol Hepatol. 1995;7:933-937. [PubMed] |
| 23. | Pohle T, Shahin M, Domschke W, Konturek JW. Effect of basic fibroblast growth factor on gastric ulcer healing and its own mRNA expression. Aliment Pharmacol Ther. 1999;13:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Milani S, Calabrò A. Role of growth factors and their receptors in gastric ulcer healing. Microsc Res Tech. 2001;53:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Szabo S, Vincze A. Growth factors in ulcer healing: lessons from recent studies. J Physiol Paris. 2000;94:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Antonisamy P, Kannan P, Aravinthan A, Duraipandiyan V, Arasu MV, Ignacimuthu S, Al-Dhabi NA, Kim JH. Gastroprotective activity of violacein isolated from Chromobacterium violaceum on indomethacin-induced gastric lesions in rats: investigation of potential mechanisms of action. ScientificWorldJournal. 2014;2014:616432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Abdul-Aziz MA. Anti-ulcerogenic activity of peel an extracts of Musa acuminata against ethanol-induced ulcer in vivo. Bachelor’s thesis, Universiti Selangor, Bestari Jaya Campus, Kuala Selangor, Selangor, Malaysia. 2011. . |
| 28. | Harsch IA, Brzozowski T, Bazela K, Konturek SJ, Kukharsky V, Pawlik T, Pawlowski E, Hahn EG, Konturek PC. Impaired gastric ulcer healing in diabetic rats: role of heat shock protein, growth factors, prostaglandins and proinflammatory cytokines. Eur J Pharmacol. 2003;481:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Jones MK, Kawanaka H, Baatar D, Szabó IL, Tsugawa K, Pai R, Koh GY, Kim I, Sarfeh IJ, Tarnawski AS. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001;121:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Kobayashi S, Nakajima N, Ito Y, Moriyama M. Effects of lansoprazole on the expression of VEGF and cellular proliferation in a rat model of acetic acid-induced gastric ulcer. J Gastroenterol. 2010;45:846-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Weng-Yew W, Selvaduray KR, Ming CH, Nesaretnam K. Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis. Nutr Cancer. 2009;61:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Selvaduray KR, Radhakrishnan AK, Kutty MK, Nesaretnam K. Palm tocotrienols decrease levels of pro-angiogenic markers in human umbilical vein endothelial cells (HUVEC) and murine mammary cancer cells. Genes Nutr. 2012;7:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Tétreault MP, Chailler P, Beaulieu JF, Rivard N, Ménard D. Epidermal growth factor receptor-dependent PI3K-activation promotes restitution of wounded human gastric epithelial monolayers. J Cell Physiol. 2008;214:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Qodriyah HMS, Kamsiah J, Kamisah Y, Gapor MT, Nafeeza MI. Kesan palm vitamin E ke atas gatser tikus aruhan dadah anti-inflamasi bukan steroid. Thesis Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia, 2006. . |
| 35. | Al Mofleh IA. Spices as Alternative Agents for Gastric Ulcer Prevention and Treatment, Peptic Ulcer Disease, Dr. Jianyuan Chai (Ed.), 2011ISBN: 978-953-307-976-9, InTech,. Available from: http://www.intechopen.com/books/peptic-ulcer-disease/spices-as-alternative-agents-for-gastric-ulcerprevention-and-treatment. |
| 36. | Hull MA, Brough JL, Powe DG, Carter GI, Jenkins D, Hawkey CJ. Expression of basic fibroblast growth factor in intact and ulcerated human gastric mucosa. Gut. 1998;43:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Brzozowski T, Konturek PC, Konturek SJ, Kwiecień S, Drozdowicz D, Bielanski W, Pajdo R, Ptak A, Nikiforuk A, Pawlik WW. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept. 2004;120:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50 Suppl 1:S24-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 39. | Kawaguchi H, Pilbeam CC, Gronowicz G, Abreu C, Fletcher BS, Herschman HR, Raisz LG, Hurley MM. Transcriptional induction of prostaglandin G/H synthase-2 by basic fibroblast growth factor. J Clin Invest. 1995;96:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Wang CL, Shi DZ, Yin HJ. [Effect of panax quinquefolius saponin on angiogenesis and expressions of VEGF and bFGF in myocardium of rats with acute myocardial infarction]. Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27:331-334. [PubMed] |
| 41. | Hull MA, Hewett PW, Brough JL, Hawkey CJ. Isolation and culture of human gastric endothelial cells. Gastroenterology. 1996;111:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Rashid SA, Halim AS, Muhammad NA. The effect of vitamin E on basic fibroblast growth factor level in human fibroblast cell culture. Med J Malaysia. 2008;63 Suppl A:69-70. [PubMed] |
| 43. | Tsuji S, Kawano S, Higashi T, Mukuda T, Imaizumi T, Tatsumi T, Miura N, Miyajima K, Fukuda M, Noguchi M. Gastric ulcer healing and basic fibroblast growth factor: effects of lansoprazole and famotidine. J Clin Gastroenterol. 1995;20 Suppl 2:S1-S4. [PubMed] |
| 44. | Lee JS, Lee GH, Hyun KY. Effects of GUF (Glycyrrhiza uralensis Fischer) extract in water-immersion restrain stress (wirs)-induced gastric injury in rats. Adv Sci Technol Lett. 2015;88:247-250. [DOI] [Full Text] |