Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5809
Peer-review started: March 23, 2017
First decision: June 5, 2017
Revised: June 27, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: August 21, 2017
Processing time: 151 Days and 12 Hours
To investigate blood cultures of deceased donors and report the confirmed transmission of bacterial infection from donors to liver recipients.
We retrospectively studied the results of blood cultures among our donation after cardiac death (DCD) donors and calculated the donor-derived bacterial infection rates among liver recipients. Study participants underwent liver transplantation between January 1, 2010 and February 1, 2017. The study involved a total of 67 recipients of liver grafts from 67 DCD donors. We extracted the data of donors’ and patients’ characteristics, culture results and clinical outcomes, especially the post-transplant complications in liver recipients, from electronic medical records. We analyzed the characteristics of the donors and the corresponding liver recipients with emphasis put on donor-derived infections.
Head trauma was the most common origin of death among our 67 DCD donors (46.3%). Blood taken prior to the procurement operation was cultured for 53 of the donors, with 17 episodes of bloodstream infections developing from 13 donors. The predominant organism isolated from the blood of donors was Gram-positive bacteria (70.6%). Only three (4.5%) of 67 liver recipients developed confirmed donor-derived bacterial infections, with two isolates of multidrug-resistant Klebsiella pneumoniae and one isolate of multidrug-resistant Enterobacter aerogenes. The liver recipients with donor-derived infections showed relation to higher crude mortality and graft loss rates (33.3% each) within 3 mo post transplantation, as compared to those without donor-derived infections (9.4% and 4.7%, respectively). All three liver recipients received appropriate antimicrobial therapy.
Liver recipients have high occurrence of donor-derived infections. The liver recipients with donor-derived multidrug-resistant Enterobacteriaceae infections can have good outcome if appropriate antimicrobial therapy is given.
Core tip: This study aimed to investigate blood cultures of donation after cardiac death (DCD) liver donors and report the confirmed transmission of bacterial infection from donors to liver recipients. The predominant organism isolated from the blood of donors was Gram-positive bacteria (70.6%). Only three (4.5%) of 67 liver recipients developed confirmed donor-derived bacterial infections, with two isolates of multidrug-resistant Klebsiella pneumoniae and one isolate of multidrug-resistant Enterobacter aerogenes. Our findings support that liver grafts from DCD donors with bloodstream infections owing to multidrug-resistant Enterobacteriaceae can be used if the donors and recipients receive appropriate antimicrobial therapy.
- Citation: Ye QF, Zhou W, Wan QQ. Donor-derived infections among Chinese donation after cardiac death liver recipients. World J Gastroenterol 2017; 23(31): 5809-5816
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5809.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5809
Liver transplantation is currently considered the therapy of choice for patients with end-stage liver disease. Organs from infected donors after cardiac death (DCD), such as those with bacteremia, are now utilized in response to the disparity between the available graft pool and the organ need for liver transplantation, which carries risk of transmission of infectious diseases and death[1-5]. The risk of unanticipated disease transmission to liver recipients via donor grafts has been gaining research attention. Donor-derived transmission of infections remains a rare complication of liver transplantation and the studies have supported its overall safety and favorable outcomes[6-13]; however, the outcome of this type of infection itself among liver recipients is controversial and some authors suggest that it is associated with significant mortality[14,15].
As the volume of liver recipients increases, the number of infections transmitted through DCD donors can also be expected to rise. Unfortunately, the data of DCD donor-derived infections following liver transplantation are currently lacking in China. We, therefore, aimed to investigate the blood cultures of DCD donors and report the cases of confirmed (proven/probable) transmission of bacterial and fungal infections from donors to liver recipients. The current study provided, to our best knowledge, the first finding of liver recipients experiencing confirmed donor-derived bacterial infections in China and this report represents the largest series of liver recipients with donor-derived infections due to multidrug-resistant (MDR) Enterobacteriaceae in the world thus far.
This retrospective analysis of a single-center population was conducted with the purpose of recording all liver recipients with donor-derived bacterial infections. All involved human participants had been initially recruited to the study between January 1, 2010 and February 1, 2017. We then searched the medical record systems of the Third Xiangya Hospital (Changsha, China) to identify all DCD donors and liver recipients who received grafts from DCD donors. The recorded information allowed for identification of individual participants during or after data collection.
The final study population consisted of 67 liver recipients of grafts from 67 DCD donors who had been admitted to the intensive care unit (ICU) of the Department of Transplant Surgery, the Third Xiangya Hospital of Central South University before organ procurement and transplantation. Multi-organ transplant recipients were excluded from the study. Data recorded included donor age, sex, category, length of ICU stay, number of donors with/without available results of blood cultures, bacteria and fungi isolated from donors, antibiotic administration, and cause of death. The procedures used for donor screening, donor treatment for bacterial and fungal infections, and obtainment of the liver graft were also recorded. Liver graft recipient data representing variables associated with donor-derived infections were collected from the medical records as well, and included age, sex, underlying liver diseases, site of infection, time of infection onset, organisms, antimicrobial use, immunosuppressive therapy, and crude mortality/graft loss. The data of post-liver transplant complications were collected for all liver recipients. For all liver graft recipients, the maintenance immunosuppression was tacrolimus/cyclosporin complemented with prednisone tapered to 5-10 mg/d. All liver graft recipients were followed for at least 3 mo post transplantation or until death.
In our hospital, any DCD donor with a history or suspicion of prior bloodstream infection from whom a liver graft will be harvested is subject to a detailed and appropriate investigation to ensure that infection is not present in the liver. To rule out the presence of active infection, our hospital also takes a complete history from the donor’s family and performs a thorough review of the medical records, including vital signs and findings of physical, radiographic and any available microbiologic tests. Most of the donors in this study also underwent routine culturing of blood, urine and sputum. Blood cultures were obtained to rule out occult donor infection, especially among donors at “increased” risk for bacteremia or fungemia[16]. Targeted antibiotics were administered to the infected donor for at least 24 h, with some degree of clinical response (improved white blood cell count and hemodynamics, and defervescence) and, if possible, the infected donor’s treatment included documentation of the infection resolution prior to donation.
All cases of organ donation were performed according to the protocols for China categories I, II and III donors[17]. After informed consent was obtained from the donor’s family, life supports were removed. After the legal 5-min standoff time, the donor immediately underwent a “super-rapid” procurement technique in the operating room. In brief, a rapid abdominal incision was made, followed by rapid cannulation of the abdominal aorta and superior mesenteric or portal vein. The liver was then perfused with University of Wisconsin solution and prompt hepatectomy was performed. The intra-abdominal organs were removed en bloc, submerged in University of Wisconsin solution at 4 °C, and placed in cold storage. The bile duct was flushed with physiological saline solution in situ and preserved ex situ with University of Wisconsin solution.
Second- or third-generation cephalosporins, semisynthetic penicillins/beta-lactamase inhibitors, or carbapenems, were prescribed according to the pre-transplant results of cultures and administered 1 h before the liver transplantation, with an additional dose given at 72 h post transplantation to the liver recipients without donor-derived infections. Recipients of liver graft from a bacteremic donor were treated with a 7-d to 14-d course of targeted antibiotics. Antifungal therapy was administered within 2 wk to a recipient of liver graft from a donor with fungemia if the liver recipient had no evidence of infection. Cases of established fungal infection were administered a 4-wk to 6-wk course, with at least 6 wk of treatment, when vascular involvement was present.
The deceased-organ donations were classified as follows: China category I (C-I), organ donation after brain death; China category II (C-II), organ donation after circulatory death; and China category III (C-III), organ donation after brain death followed by circulatory death[17].
Transmission of organisms was considered proven by clear evidence of the same infection in the donor and at least one of the recipients. Transmission of the organisms was considered probable by strong evidence suggesting but not proving infectious transmission, such as the infection being documented in more than one recipient but not diagnosed in the donor[18]. Both proven and probable transmissions were considered as confirmed transmission of infections[18]. Appropriate antimicrobial use in donors was considered when the infected donor had received targeted antimicrobial treatment for at least 24-48 h, optimally with some degree of clinical response[19]. Appropriate antimicrobial use in recipients was considered if the isolated organisms showed in vitro susceptibility to empirical antibiotics, which were administered within 48 h of sampling for culture[20].
We utilized the standardized definition of MDR Enterobacteriaceae, as previously defined by international consensus in 2012; specifically, MDR was considered with evidence of non-susceptibility to at least one agent in three or more appointed antimicrobial categories[21].
There were 67 liver recipients of grafts from 67 DCD donors during the study period. The characteristics of the donors are shown in Table 1. The DCD donors showed a male-dominated sex ratio (nearly 1:4) and were largely represented by young adults. The pre-retrieval ICU length of stay ranged from 1 d up to 41 d. Head trauma was the most common cause of death, followed by benign central nervous system tumor. The majority of donors were classified as China category III, followed by China category II.
| Characteristic | Value |
| Age in years, median (IQR) | 29.0 (19.0-44.0) |
| Sex | |
| Male | 55 (82.1) |
| Female | 12 (17.9) |
| Origin of death | |
| HT | 31 (46.3) |
| Benign CNS tumor | 18 (26.9) |
| CVA | 15 (22.4) |
| Anoxia | 2 (3.0) |
| Meningitis | 1 (1.5) |
| China classification of donation | |
| I | 5 (7.5) |
| II | 8 (11.9) |
| III | 54 (80.6) |
| ICU stay in days, median (IQR) | 5 (3.0-10.0) |
| Donors with positive culture | 13 |
| Blood culture, n/n | |
| Donors with/without available results | 53/14 |
| Donors with appropriate antimicrobial use/all donors with positive blood culture results | 10/13 |
For 53 of the donors (79.1%), the blood samples taken prior to the procurement operation were cultured and produced evidence of 17 bloodstream infections from 13 of the donors. Appropriate antimicrobial use, according to the positive blood culture results, was administered in 10 of those 13 donors. The results of blood cultures from the donors are shown in Table 2. The majority of pathogens isolated from the donor’s blood were coagulase-negative Staphylococci. Among the three donors who elicited infectious transmission to three liver recipients, one donor did not have an available result of blood culture and the other two donors had negative results of blood culture after being admitted to the ICU; none of these three donors underwent blood culture within 10 d prior to organ procurement.
| Organism | n (%) |
| Gram-positive bacteria | 12 (70.6) |
| Staphylococcus aureus | 3 (17.6) |
| Coagulase-negative staphylococci | 9 (52.9) |
| S. epidermidis | 4 (23.5) |
| S. hemolyticus | 1 (5.9) |
| S. capitis | 1 (5.9) |
| S. hominis | 2 (11.8) |
| S. simulans | 1 (5.9) |
| Gram-negative bacteria | 3 (17.6) |
| Klebsiella pneumoniae | 2 (11.8) |
| Acinetobacter baumannii | 1 (5.9) |
| Fungi | 2 (11.8) |
| Candida albicans | 1 (5.9) |
| Candida parapsilosis | 1 (5.9) |
Table 3 shows the characteristics of these three donors (D1-D3) and their corresponding liver recipients (R1-R3, representing 4.5% of the total recipient population), highlighting the relationship between donors and recipients with infection transmission. Several cultures (blood, urine, and abdominal drainage fluid) were routinely taken from the recipients following the liver transplantation. The confirmed donor-derived MDR bacterial infections included two isolates of Klebsiella pneumoniae and one isolate of Enterobacter aerogenes. Both of the Klebsiella pneumoniae isolates were extended-spectrum beta lactamase-producing rods. All three of the isolates (all being Enterobacteriaceae family members) were carbapene-susceptible.
| Donor | Diagnosis | Blood culture result | Recipient (sex/age) | Underlying liver disease(s) | Culture result (specimen)/time to infection onset | Inappropriate antimicrobial/immunosuppressive therapy | Outcome |
| D1 | Head trauma | Negative1 | R12 (Female/48 yr) | Polycystic liver disease | K. pneumoniae (Blood and abdominal drainage fluid)/1 d | No/Pred | Patient death and graft loss |
| D2 | Head trauma | Negative1 | R23 (Male/38 yr) | Hepatocellular carcinoma and cirrhosis due to hepatitis B virus infection | K. pneumoniae (Blood and abdominal drainage fluid)/1 d | No/FK506 + Pred | Patient and graft survival |
| D3 | Head trauma | Not available | R33 (Male/69 yr) | Heredofamilial amyloidosis | E. aerogenes (Blood)/3 d | No/FK506 + Pred | Patient and graft survival |
Additional isolates from four recipients of kidney grafts from these three donors (D1-D3) provided strong evidence of transmission of infectious bacteria through their similar resistance profiles. Thus, no recipient case of infection transmission could be classified as a proven donor-derived infection, and all three cases (R1-R3) were classified as probable donor-derived infections. No liver recipients developed donor-derived fungal infections. No recipients had a presumed or confirmed invasive bacterial or fungal infection before transplantation. The underlying liver diseases of these three recipients were polycystic liver disease, hepatocellular carcinoma and cirrhosis due to hepatitis B virus infection, and heredofamilial amyloidosis. All three donor-derived infections occurred within 3 d post transplantation, and were administered appropriate anti-microbial therapy prior to or immediately following diagnosis of the infection. All three recipients recovered from the donor-derived bacterial infections, but one died of septic shock with graft loss owing to other organisms. Liver recipients without donor-derived infections had lower rates of crude mortality and graft loss [6/64 (9.4%) and 3/64 (4.7%), respectively] within 3 mo post transplantation, as compared with those with donor-derived infections (33.3% each).
All complications experienced by the full liver recipient population (n = 67) are presented in Table 4. The most common complication was post-transplant infection; the 20 total infections occurred in the three liver recipients with donor-derived infections (4 infection episodes) and the 64 liver recipients without donor-derived infections (16 infection episodes). The next most common complication was vascular (9 vascular episodes), all cases of which occurred in the liver recipients without donor-derived infections. Tumor recurrence developed in six of the liver recipients without donor-derived infections and none of the recipients with donor-derived infections.
| Complication | Episodes of complications in three liver recipients with donor-derived infections | Episodes of complications in 64 liver recipients without donor-derived infections |
| Infection | 4 | 16 |
| Pneumonia | 1 | 7 |
| Peritonitis | 0 | 3 |
| Bloodstream infection | 3 | 5 |
| Surgical site infection | 0 | 1 |
| Vascular complications | 0 | 9 |
| Portal vein thrombosis | 0 | 1 |
| Cerebral embolism | 0 | 1 |
| Hepatic artery thrombosis | 0 | 1 |
| Abdominal bleeding | 0 | 5 |
| Gastrointestinal bleeding | 0 | 1 |
| Biliary complications | 3 | 2 |
| Bile leakage | 0 | 0 |
| Biliary strictures | 1 | 2 |
| Stone formation | 1 | 0 |
| Bilomas | 1 | 0 |
| Recurrent tumor | 0 | 6 |
| Acute rejection | 0 | 4 |
| Graft-versus-host disease | 0 | 2 |
| Graft dysfunction | 0 | 3 |
| Adhesive ileus | 0 | 1 |
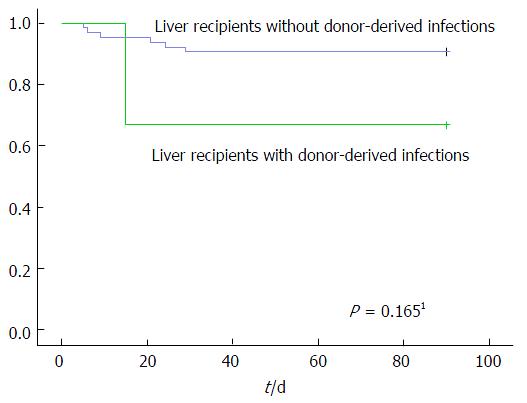
Figure 1 shows the Kaplan-Meier curves for 3-mo survival after liver transplantation. There was no difference in survival between the liver recipients with and without donor-derived infection (log-rank test, P = 0.165).
Although there are numerous reports of fatal donor-derived infections affecting around 3% of solid organ transplants, in the current era of organ shortage marginal organ donors are increasingly utilized[22,23]. More recent data demonstrate that most bacterial isolates appear to be irrelevant to subsequent recipient outcome and that the grafts from bacteremic donors may be safely used if bacteremia in the donor has been ideally treated and antibiotic therapy is continued in the organ recipient[2,6,7,24,25].
Our present study found 19.4% (13/67) of donors having available results of blood cultures developed bloodstream infections, which is in line with literature reports suggesting that about 5%-11.3% of all deceased donors have unrecognized bacteremia at the time of donation[2,6,8,25]. We also found that three (4.5%) of 67 donors transmitted bacterial infections to three liver recipients, which agrees with the 2013 report[26] from the Organ Procurement and Transplantation Network Ad Hoc Disease Transmission Advisory Committee that reported 15 (12.8%) out of 117 donors with infections developed proven/probable transmission of bacterial (except for tuberculosis) and fungal infections.
Doucette et al[3] proposed that the observed higher risk of transmission in liver recipients, as compared to other solid organ recipients, may be mainly attributed to bacterial retention in and poor antibiotic penetration of the liver graft. Some recipient-related factors were also proposed, such as a high Model for End-Stage Liver Disease (commonly known as MELD) score, leukopenia, and immunosuppression.
Coagulase-negative Staphylococci (such as Staphylococcus epidermidis) and Staphylococcus aureus represent the more commonly isolated microorganisms from donor blood cultures[7,8,25]. In our donor blood cultures, the majority of isolates were coagulase-negative Staphylococci and S. aureus (together 70.6%; 12 of 17 pathogens). Coagulase-negative Staphylococci also represent the most common microorganisms isolated from preservation fluid[2,15,27]. None of the cases of donor-derived infections in liver recipients of our study were due to Gram-positive bacteria, agreeing with previous studies that have suggested lower risk of transmission of Gram-positive bacteria (vs Gram-negative bacteria) from a donor to a recipient[6,28-30]. This could be due to several reasons, such as low pathogenicity of coagulase-negative Staphylococci, potential contamination in donor culture (i.e., not a true pathogen), or catheter line-associated infection in donor (i.e., non-systemic infections).
All three donor-derived infections in our current study were caused by MDR Gram-negative bacteria, agreeing with a previous report suggesting that Gram-negative bacteria account for about 80% of transmissions to recipients[9]. Although rare, donor-derived infections caused by bacteria, in particular MDR bacteria, can have devastating consequences for organ transplant recipients[9-11,29,31]. The data reported to the United States’ Organ Procurement and Transplantation Network from 2005 to 2011 showed the donor-derived infection-attributable recipient mortality rate to be 29.2% (19/65)[6]. These previous studies are consistent with our present study, wherein one (33.3%) of three liver recipients with MDR Klebsiella pneumoniae infection died from subsequent septic shock due to other organisms and the rate of graft loss was 33.3%. In contrast with our finding, however, others have reported a favorable outcome among liver recipients with donor-derived infections due to MDR Gram-negative bacteria or methicillin-resistant S. aureus (commonly known as MRSA)[3,5,9-11,23,32-34]. Further data are needed to assess the effects of donor-derived bacterial or fungal infections and appropriate antimicrobial use on allograft function and recipient survival over a long-term follow-up period.
The current study has provided, to the best of our knowledge, the first data of liver recipients with confirmed donor-derived bacterial infections treated in a single transplant center in China; in addition, the report represents the largest of liver recipients with donor-derived infections due to MDR Enterobacteriaceae in the world thus far. Since both the morbidity and mortality rates of donor-derived infections are high in China, the findings from the current study support future efforts towards a better understanding of potential risks for disease transmission and highlight the necessity for a standardized critical incident reporting system in the Chinese transplant system to improve short- and long-term allograft and recipient survival.
The principal limitation of our study was the single-center, retrospective study design, which did not allow for the investigation of donor-derived MDR bacterial infections by genetic or molecular analysis. Improvements in new screening technologies, such as the use of whole genome sequencing, have recently proven a powerful advance in the investigation of donor-derived MDR bacterial infections[35]. Since we used drug-sensitive test screening for donor-derived infections rather than a gene-level technique, it is possible that our testing algorithm over-estimated the transmission events.
Nonetheless, our findings underscore the importance of blood culture being performed as close to the donation time as possible and the importance of testing preservation fluid culture; since the reported contamination rates of the latter range widely (from 9.5% to 98.4%)[2,15,27], this sample was not routinely obtained at our institution. Our findings also indicate that donors with infections should receive antibiotics directed at the identified bacteria or fungi 24-48 h prior to procurement, optimally yielding evidence of clinical improvement.
In conclusion, DCD donors in our institute had a high rate of bacterial infection, with Gram-positive bacteria being the predominant isolate, whereas the donor-derived infections developed by liver recipients were Gram-negative predominant. Our findings strongly support organ donation as the common source of bacterial infection in the liver recipients. Although the number of these cases in our study was too small to draw conclusions, we support the use of liver grafts from the DCD donor pool, including those with infections owing to MDR Enterobacteriaceae if the donors and recipients receive appropriate antimicrobial therapy.
Nowadays, the use of bacteremic donors to fulfill the disparity between the limited donor pool and the increasing need for organs is an expanding practice, which may result in an increased risk of transmission of infectious diseases and death. However, the data of donation after cardiac death (DCD) donor-derived infections following liver transplantation are currently lacking in China. As limited data are available in Chinese liver recipients of grafts from DCD donors, this study aimed to investigate the blood cultures of DCD donors and report the cases of confirmed (proven/probable) transmission of bacterial and fungal infections from donors to liver recipients.
The use of infectious donors to fulfill the disparity between the limited donor pool and the increasing need for organs has become an important issue in the field of transplantation. Furthermore, the use of donors with infections caused by multidrug-resistant (MDR) bacteria is controversial and the recipients with MDR infections have a high mortality. This study shows excellent outcome for liver recipients with donor-derived MDR infections.
This is the first study to analyze liver recipients experiencing confirmed donor-derived bacterial infections in China and the largest report of liver recipients with donor-derived infections due to MDR Enterobacteriaceae in the world thus far.
Liver grafts from the pool of DCD donors with bloodstream infections owing to MDR Enterobacteriaceae can be used if the donors have been treated and have documentation of resolution of infection prior to donation, and if the corresponding liver recipients are treated with a 7- to 14-d course of antibiotics targeted to the organism isolated from a bacteremic donor.
Confirmed transmission includes a proven or probable transmission. A proven transmission was indicated by clear evidence of the same infectious disease in the donor and at least one of the recipients. A probable transmission was indicated by strong evidence suggesting, but not proving, a disease transmission, such as the disease being documented in more than one recipient but not being diagnosed in the donor. MDR bacteria were defined as those non-susceptible to at least one agent in three or more appointed antimicrobial categories.
Although sample size was small, this paper provides important information. This report shows excellent outcomes for recipients with donor-derived infections.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boin IFSF, Hori T, Inoue K S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Xu XR
| 1. | Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:961-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Cerutti E, Stratta C, Romagnoli R, Serra R, Lepore M, Fop F, Mascia L, Lupo F, Franchello A, Panio A. Bacterial- and fungal-positive cultures in organ donors: clinical impact in liver transplantation. Liver Transpl. 2006;12:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Doucette KE, Al-Saif M, Kneteman N, Chui L, Tyrrell GJ, Kumar D, Humar A. Donor-derived bacteremia in liver transplant recipients despite antibiotic prophylaxis. Am J Transplant. 2013;13:1080-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Camargo JF. Donor-derived infections in solid organ transplant recipients: Challenging the 30-day paradigm. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Miceli MH, Gonulalan M, Perri MB, Samuel L, Al Fares MA, Brown K, Bruno DA, Zervos M, Ramesh M, Alangaden G. Transmission of infection to liver transplant recipients from donors with infective endocarditis: lessons learned. Transpl Infect Dis. 2015;17:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ison MG, Grossi P; AST Infectious Diseases Community of Practice. Donor-derived infections in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Freeman RB, Giatras I, Falagas ME, Supran S, O'Connor K, Bradley J, Snydman DR, Delmonico FL. Outcome of transplantation of organs procured from bacteremic donors. Transplantation. 1999;68:1107-1111. [PubMed] |
| 8. | Lumbreras C, Sanz F, González A, Pérez G, Ramos MJ, Aguado JM, Lizasoain M, Andrés A, Moreno E, Gómez MA. Clinical significance of donor-unrecognized bacteremia in the outcome of solid-organ transplant recipients. Clin Infect Dis. 2001;33:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Mularoni A, Bertani A, Vizzini G, Gona F, Campanella M, Spada M, Gruttadauria S, Vitulo P, Conaldi P, Luca A. Outcome of Transplantation Using Organs From Donors Infected or Colonized With Carbapenem-Resistant Gram-Negative Bacteria. Am J Transplant. 2015;15:2674-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Mills JP, Wilck MB, Weikert BC, Porrett PM, Timko D, Alby K, Bonomo RA, Blumberg EA. Successful treatment of a disseminated infection with extensively drug-resistant Klebsiella pneumoniae in a liver transplant recipient with a fosfomycin-based multidrug regimen. Transpl Infect Dis. 2016;18:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Ariza-Heredia EJ, Patel R, Blumberg EA, Walker RC, Lewis R, Evans J, Sankar A, Willliams MD, Rogers J, Milano C. Outcomes of transplantation using organs from a donor infected with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. Transpl Infect Dis. 2012;14:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Zibari GB, Lipka J, Zizzi H, Abreo KD, Jacobbi L, McDonald JC. The use of contaminated donor organs in transplantation. Clin Transplant. 2000;14:397-400. [PubMed] |
| 13. | Cohen J, Michowiz R, Ashkenazi T, Pitlik S, Singer P. Successful organ transplantation from donors with Acinetobacter baumannii septic shock. Transplantation. 2006;81:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | van der Vliet JA, Tidow G, Kootstra G, van Saene HF, Krom RA, Sloof MJ, Weening JJ, Tegzess AM, Meijer S, van Boven WP. Transplantation of contaminated organs. Br J Surg. 1980;67:596-598. [PubMed] |
| 15. | Janny S, Bert F, Dondero F, Durand F, Guerrini P, Merckx P, Nicolas-Chanoine MH, Belghiti J, Mantz J, Paugam-Burtz C. Microbiological findings of culture-positive preservation fluid in liver transplantation. Transpl Infect Dis. 2011;13:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Fischer SA, Lu K; AST Infectious Diseases Community of Practice. Screening of donor and recipient in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:9-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Huang J, Wang H, Fan ST, Zhao B, Zhang Z, Hao L, Huo F, Liu Y. The national program for deceased organ donation in China. Transplantation. 2013;96:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Garzoni C, Ison MG. Uniform definitions for donor-derived infectious disease transmissions in solid organ transplantation. Transplantation. 2011;92:1297-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Fishman JA, Greenwald MA, Grossi PA. Transmission of infection with human allografts: essential considerations in donor screening. Clin Infect Dis. 2012;55:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Gao F, Ye Q, Wan Q, Liu S, Zhou J. Distribution and resistance of pathogens in liver transplant recipients with Acinetobacter baumannii infection. Ther Clin Risk Manag. 2015;11:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8770] [Article Influence: 626.4] [Reference Citation Analysis (0)] |
| 22. | Kirchner VA, Pruett TL. Receiving the Unwanted Gift: Infection Transmission through Organ Transplantation. Surg Infect (Larchmt). 2016;17:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Altman DR, Sebra R, Hand J, Attie O, Deikus G, Carpini KW, Patel G, Rana M, Arvelakis A, Grewal P. Transmission of methicillin-resistant Staphylococcus aureus via deceased donor liver transplantation confirmed by whole genome sequencing. Am J Transplant. 2014;14:2640-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Goldberg E, Bishara J, Lev S, Singer P, Cohen J. Organ transplantation from a donor colonized with a multidrug-resistant organism: a case report. Transpl Infect Dis. 2012;14:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | González-Segura C, Pascual M, García Huete L, Cañizares R, Torras J, Corral L, Santos P, Ramos R, Pujol M. Donors with positive blood culture: could they transmit infections to the recipients? Transplant Proc. 2005;37:3664-3666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Green M, Covington S, Taranto S, Wolfe C, Bell W, Biggins SW, Conti D, DeStefano GD, Dominguez E, Ennis D. Donor-derived transmission events in 2013: a report of the Organ Procurement Transplant Network Ad Hoc Disease Transmission Advisory Committee. Transplantation. 2015;99:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Ruiz P, Gastaca M, Gonzalez J, Hernandez MJ, Ventoso A, Valdivieso A, Montejo M, Ortiz de Urbina J. Incidence and clinical relevance of bacterial contamination in preservation solution for liver transplantation. Transplant Proc. 2009;41:2169-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Sifri CD, Ison MG. Highly resistant bacteria and donor-derived infections: treading in uncharted territory. Transpl Infect Dis. 2012;14:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Watkins AC, Vedula GV, Horan J, Dellicarpini K, Pak SW, Daly T, Samstein B, Kato T, Emond JC, Guarrera JV. The deceased organ donor with an "open abdomen": proceed with caution. Transpl Infect Dis. 2012;14:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Bull DA, Stahl RD, McMahan DL, Jones KW, Hawkins JA, Renlund DG, Taylor DO, Karwande SV. The high risk heart donor: potential pitfalls. J Heart Lung Transplant. 1995;14:424-428. [PubMed] |
| 31. | Martins N, Martins IS, de Freitas WV, de Matos JA, Magalhães AC, Girão VB, Dias RC, de Souza TC, Pellegrino FL, Costa LD. Severe infection in a lung transplant recipient caused by donor-transmitted carbapenem-resistant Acinetobacter baumannii. Transpl Infect Dis. 2012;14:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Wendt JM, Kaul D, Limbago BM, Ramesh M, Cohle S, Denison AM, Driebe EM, Rasheed JK, Zaki SR, Blau DM. Transmission of methicillin-resistant Staphylococcus aureus infection through solid organ transplantation: confirmation via whole genome sequencing. Am J Transplant. 2014;14:2633-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Johnston L, Chui L, Chang N, Macdonald S, McKenzie M, Kennedy W, Haldane D, Bethune R, Taylor G, Hanakowski M. Cross-Canada spread of methicillin-resistant Staphylococcus aureus via transplant organs. Clin Infect Dis. 1999;29:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Giani T, Conte V, Mandalà S, D'Andrea MM, Luzzaro F, Conaldi PG, Grossi P, Rossolini GM. Cross-infection of solid organ transplant recipients by a multidrug-resistant Klebsiella pneumoniae isolate producing the OXA-48 carbapenemase, likely derived from a multiorgan donor. J Clin Microbiol. 2014;52:2702-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Fishman JA, Grossi PA. Donor-derived infection--the challenge for transplant safety. Nat Rev Nephrol. 2014;10:663-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |