Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5722
Peer-review started: February 14, 2017
First decision: April 7, 2017
Revised: May 14, 2017
Accepted: June 9, 2017
Article in press: June 12, 2017
Published online: August 21, 2017
Processing time: 186 Days and 14.3 Hours
To investigate the protective effects of Foeniculum vulgare root bark (FVRB), a traditional Uyghur medicine, against carbon tetrachloride (CCl4)-induced hepatic fibrosis in mice.
Mice were randomly divided into eight groups (n = 20 each). Except for the normal control group, mice in the rest groups were intraperitoneally injected (i.p.) with 0.1% CCl4-olive oil mixture at 10 mL/kg twice a week to induce liver fibrosis. After 4 wk, mice were treated concurrently with the 70% ethanol extract of FVRB (88, 176, 352 and 704 mg/kg, respectively) daily by oral gavage for 4 wk to evaluate its protective effects. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglyceride (TG), hexadecenoic acid (HA), laminin (LN), glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) in liver tissues were measured. Hematoxylin-eosin (H and E) staining and Masson trichrome (MT) staining were performed to assess histopathological changes in the liver. The expression of transforming growth factor β1 (TGF-β1), matrix metalloprotein 9 (MMP-9) and metallopeptidase inhibitor 1 (TIMP-1) was detected by immunohistochemical analysis. Additionally, TGF-β1 and alpha-smooth muscle actin (α-SMA) protein expression was measured by Western blot.
A significant reduction in serum levels of AST, ALT, TG, HA and LN was observed in the FVRB-treated groups, suggesting that FVRB displayed hepatoprotective effects. Also, the depletion of GSH, SOD, and MDA accumulation in liver tissues was suppressed by FVRB. The expression of TGF-β1, MMP-9 and TIMP-1 determined by immunohistochemistry was markedly reduced in a dose-dependent manner by FVRB treatment. Furthermore, protective effects of FVRB against CCl4-induced liver injury were confirmed by histopathological studies. Protein expression of TGF-β1 and α-SMA detected by Western blot was decreased by FVRB treatment.
Our results indicate that FVRB may be a promising agent against hepatic fibrosis and its possible mechanisms are inhibiting lipid peroxidation and reducing collagen formation in liver tissue of liver fibrosis mice.
Core tip: Hepatic fibrosis is a wound-healing pathological process resulting from chronic hepatic injuries. In the present study, hepatoprotective effects of Foeniculum vulgare root bark (FVRB), a traditional Uyghur medicine, against carbon tetrachloride (CCl4)-induced hepatic fibrosis in mice were investigated. FVRB reduced serum levels of aspartate aminotransferase, alanine aminotransferase, triglyceride, hexadecenoic acid and laminin. Furthermore, FVRB inhibited CCl4-induced TGF-β1, MMP-9, TIMP-1 expression and histopathological changes. Our study indicated that the protective effects of FVRB are through inhibiting lipid peroxidation and collagen formation in liver tissue of liver fibrosis mice.
- Citation: Zhang C, Tian X, Zhang K, Li GY, Wang HY, Wang JH. Protective effects of Foeniculum vulgare root bark extract against carbon tetrachloride-induced hepatic fibrosis in mice. World J Gastroenterol 2017; 23(31): 5722-5731
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5722.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5722
Hepatic fibrosis is a wound-healing pathological process resulting from chronic hepatic injuries, which is characterized by the accumulation of extracellular matrix (ECM)[1]. It occurs during most continuous and chronic liver diseases, driven by inflammatory responses to tissue injury, which ultimately lead to liver cirrhosis. Previous studies indicated that activation of hepatic stellate cells (HSCs) plays an important role in the progress of hepatic fibrosis[2,3]. Activation of HSCs increases cell proliferation, producing large amounts of ECM components including hexadecenoic acid (HA) and laminin (LN)[4,5]. In addition, aberrant activity of transforming growth factor β1 (TGF-β1) or members of the platelet derived growth factor family are also the most prominent drivers to activate and transdifferent HSCs into myofibroblast[6,7]. Further, several chemokines that modulate the inflammatory reaction are involved in the progression of HSC activation and the fibrotic insult[8,9]. Many studies have demonstrated that the reversion of fibrosis can be achieved, particularly in the early course of the disease. Currently, treatment of liver damage mainly consists of inhibiting early activation and proliferation of HSCs and collagen fiber growth, and promoting HSC apoptosis and collagen degradation.
Many studies indicated that Foeniculum vulgare root bark (FVRB), a traditional Uyghur medicine, contains many chemical constituents, such as saccharides, glycosides, lactone compounds, phenols, tannins, flavonoids, alkaloids, volatile oil, grease, triterpenes and sterols[10,11]. In addition, FVRB has been traditionally used for the treatment of several pathophysiological conditions in China, exhibiting the activity of dispelling coldness, warming kidney and stomach, removing dampness, and alleviating swelling and pain[12-15]. For the first time, the present study was aimed to investigate the protective effects of FVRB against CCl4-induced liver injury in vivo and the possible mechanisms involved.
Male Kunming mice weighing 20 ± 2 g were supplied by the Experimental Animal Center of Urumqi (Urumqi, China). Mice were housed at room temperature under a 12 h light/dark cycle (lights on at 08:00 h) and were fed a standard diet ad libitum. All animal care and experimental procedures were approved by the Institute Ethnics Committee of Shihezi University.
FVRB was obtained from Uygur Pharmaceutical Company (Uygur, China). FVRB was extracted with 70% ethanol by using the method of heating reflux and steam drying. A voucher specimen (No. 20070820) has been deposited in School of Pharmacy, Shihezi University.
CCl4 was obtained from Tianjin Guangfu Science and Technology Development Co. (Tianjin, China). Yiganling Pian (batch number 150102044) containing 38.5 mg of Silybum marianum each piece was purchased from Shanxi Lijun Chinese Medicine Co. (Shanxi, China). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride, malondialdehyde (MDA), reduced glutathione hormone (GSH), and superoxide dismutase (SOD) assay kits were all purchased from Nanjing Jiancheng (Nanjing, China). Hyaluronic acid (HA) and laminin (LN) assay kits were obtained from Xitang Co. (Shanghai, China). Rabbit primary antibodies against TGF-β1, alpha-smooth muscle actin (α-SMA), matrix metalloprotein 9 (MMP-9), and metallopeptidase inhibitor 1 (TIMP-1), and horseradish peroxidase labeled secondary antibody were purchased from BOSTER (Wuhan, China).
Mice were randomly divided into eight groups (n = 20 each): A-H. Group A (normal control group) was allowed free access to water and food. In the other seven experimental groups (B-H), the mice were treated with CCl4 (10 mL/kg, i.p.) in olive oil (1:1000, v/v), twice a week for eight weeks. Group B served as a solvent control group, in which mice were given olive oil at 10 mL/kg at the fifth week. Group C was a model group, in which mice were given water at 10 mL/kg at the fifth week. Groups D, E, F, and G were orally administered with FVRB (88, 176, 352 and 704 mg/kg, respectively) once daily from the fifth week for four weeks. Group H was a positive control group, in which mice were treated with Yiganling Pian (200 mg/kg).
Mice were anesthetized with ethyl ether and blood samples were harvested. The blood was centrifuged at 3500 rpm for 10 min at 4 °C to obtain the supernatant serum, which was stored at 80 °C until further use for biochemical analysis. Liver, spleen and kidney were dissected out and washed immediately with ice cold saline to weigh.
Serum was collected as mentioned above. ALT, AST and TG were determined according to the manufacturer’s protocols using a Microplate Reader Thermo 3001. The absorbance of ALT and AST reactions was read at 505 nm and the absorbance of TG reaction was read at 546 nm. The enzyme activity is calculated as U/L. HA and LN levels were determined by enzyme-linked immunosorbent assay using the commercial kits. The absorbance of the reaction was read at 450 nm.
Liver tissues samples were homogenized in physiological saline to give a 10% (w/v) liver homogenate, which was then centrifuged at 2500 rpm for 15 min at 4 °C. The supernatant was used for the measurement of MDA, GSH and SOD activities with the commercial kits following the manufacture’s protocols. Data are expressed as U/mg of protein.
Liver specimens were fixed in 10% formalin and then embedded in paraffin. Four-micrometer-thick sections were obtained from paraffin blocks and stained with hematoxylin and eosin (H and E) and Masson’s trichrome (MT) before they were examined under a light microscope. The images were randomly taken from ten fields under a light microscope (200 × magnification).
TGF-β1, MMP-9 and TIMP-1 expression levels in the liver were measured by immunohistochemistry. The liver tissues were sectioned and incubated with rabbit anti-TGF-β1 antibody (1:100), rabbit anti-MMP-9 antibody (1:100), and rabbit anti-TIMP-1 antibody (1:100). Then the slides were processed using an immunohistochemical staining kit. After that, the slides were counterstained with hematoxylin and mounted with a glycerin gel. In the negative control groups, the primary antibodies were replaced with PBS. The sections were observed under a microscope (Nikon 80i).
Total protein was extracted from the liver tissue and the protein concentration was determination by BCA method. The protein was separated by SDS-polyacrylamide gel electrophoresis, followed by transfer to PVDF membrane. The membranes were blocked with 5% nonfat milk in TBST buffer for 1 h. Then target proteins were incubated overnight at 4 °C with TGF-β1 and α-SMA primary antibodies (1:1000). After washing four times with TBST, the membranes were incubated with HRP-conjugated secondary antibody (1:10000) for 1 h at room temperature. Then the membranes were immersed in an enhanced chemiluminescence detection solution. Protein was analyzed by the gray value of the band, which is expressed as the ratio of the target protein and the β-actin protein.
All quantitative data are expressed as mean ± SE. Data were analyzed with SPSS 13.0 software. Statistical significance between groups was determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple range post hoc test. P < 0.05 was considered statistically significant.
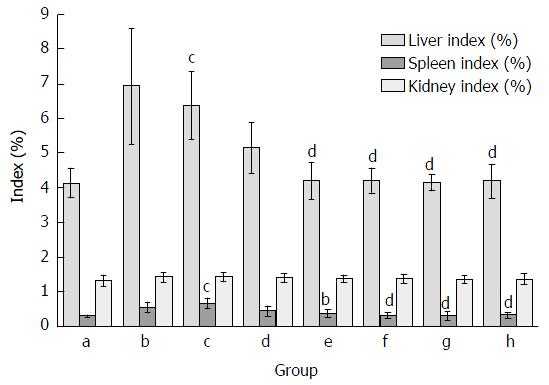
As demonstrated in Figure 1, organ indexes including liver, spleen and kidney coefficients were measured in mice. Similar to previous studies[16], liver and spleen indexes were significantly increased in mice treated with CCl4. Compared with the model group, the increase of liver index and spleen index in CCl4-treated group was reduced by FVRB and Yiganling Pian treatment. However, there were no significant differences in the kidney coefficient between the groups.
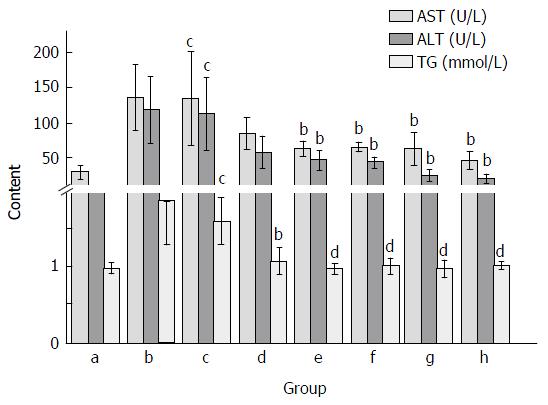
As shown in Figure 2, CCl4 treatment markedly elevated serum AST, ALT and TG activities as compared with the normal control group. The AST and ALT activities after CCl4 treatment were about 5 and 4 times higher than that of the normal group, respectively. However, Yiganling Pian treatment markedly inhibited the increase of serum AST, ALT and TG after long-term CCl4 injection in mice (P < 0.05; Figure 2). Similarly, the administration of FVRB at different dosages significantly decreased AST, and ALT and TG activities (P < 0.05 or P < 0.01; Figure 2).
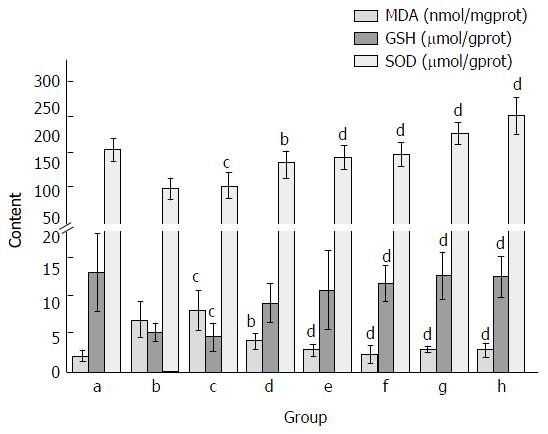
Lipid peroxidation was evaluated by measuring MDA content in liver tissue. In the CCl4 treatment group, the content of MDA was elevated as compared with the normal control group. The administration of FVRB significantly decreased MDA content in a dose-dependent manner. Also, as compared with the normal control group, CCl4 treatment markedly decreased the GSH level and SOD activity in liver tissue. However, treatment with the extract of FVRB (352 and 704 mg/kg) markedly recovered the CCl4-induced GSH depletion (P < 0.01; Figure 3). In addition, FVRB treatment (88, 176, 352 and 704 mg/kg) significantly restored the depletion of SOD activity in a dose-dependent manner (Figure 3). Similarly, Yiganling Pian treatment (200 mg/kg) increased the GSH content and SOD activity as compared with the CCl4 group (P < 0.01; Figure 3). Further, there was no significant difference in the levels of MDA, GSH and SOD between the CCl4 group and the solvent control group.
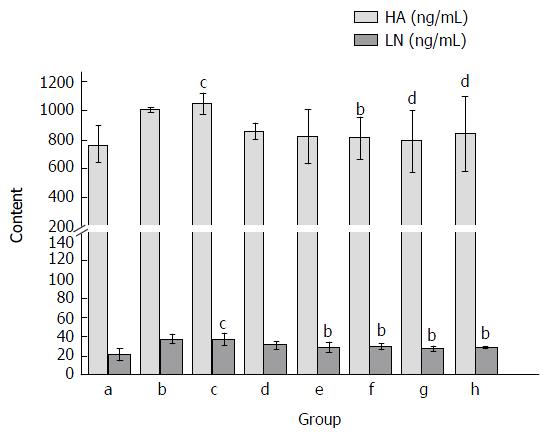
After CCl4 administration, the levels of serum HA and LN were significantly increased as compared with the normal control group (P < 0.05; Figure 4). Treatment with Yiganling Pian (200 mg/kg) significantly decreased the levels of serum HA and LN (P < 0.05; Figure 4). Meanwhile, FVRB treatment markedly decreased the elevation of serum HA and LN in a dose-dependent manner after long-term CCl4 injection in mice (P < 0.05; Figure 4).
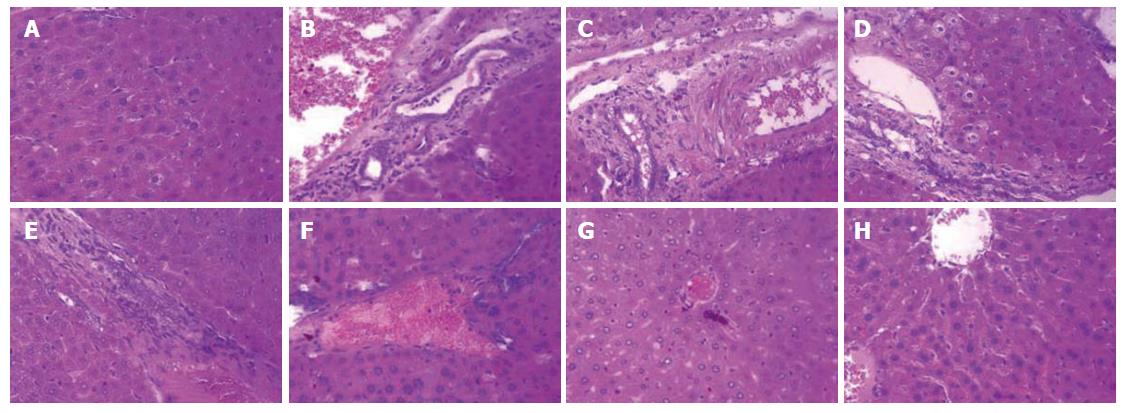
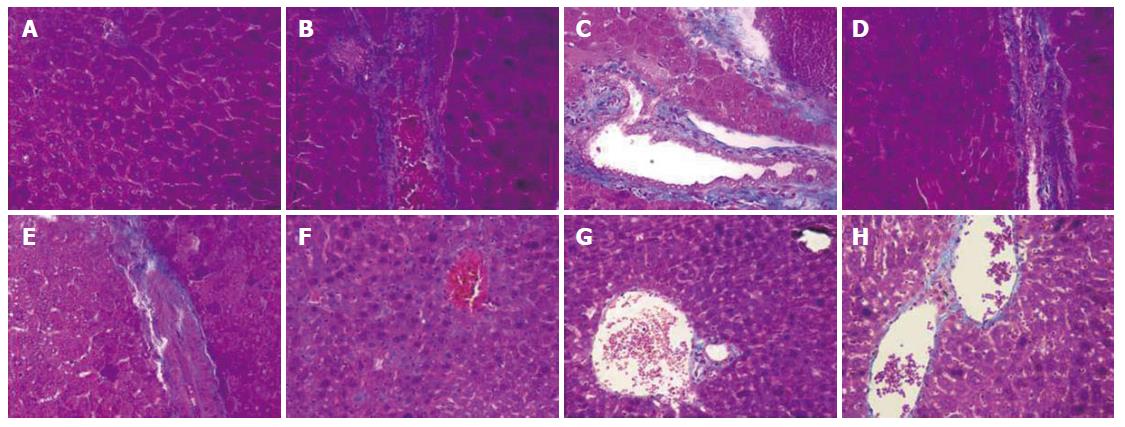
As shown in Figure 5A, the liver sections of the normal control group exhibited the normal cellular structure with well-preserved cytoplasm, prominent nucleolus and central vein. In contrast, the liver sections of the CCl4-treated group and solvent control group exhibited significant pathological changes, such as fibrosis, ballooning degeneration, steatosis, disseminated macrovesicular and microvesicular changes (Figure 5B and C). There was focal necrosis as well as piecemeal necrosis and fibrosis of portal areas. However, the FVRB and Yiganling Pian treatment groups showed a few to milder degree of leukocytes infiltration and necrosis (Figure 5E-H). In addition, as compared to the normal control group, the livers of mice treated with CCl4 and the solvent exhibited extensive accumulation of connective tissue, leading to the formation of continuous fibrotic septa, nodules of regeneration, and noticeable alterations in the central vein (Figure 6A-C). However, treatment with FVRB and Yiganling Pian significantly attenuated CCl4-induced alterations (Figure 6E-H). The severe hepatic fibrosis was reduced by treatment with FVRB, which was in good correlation with the results of hepatic antioxidant enzyme activities and serum aminotransferase activities.
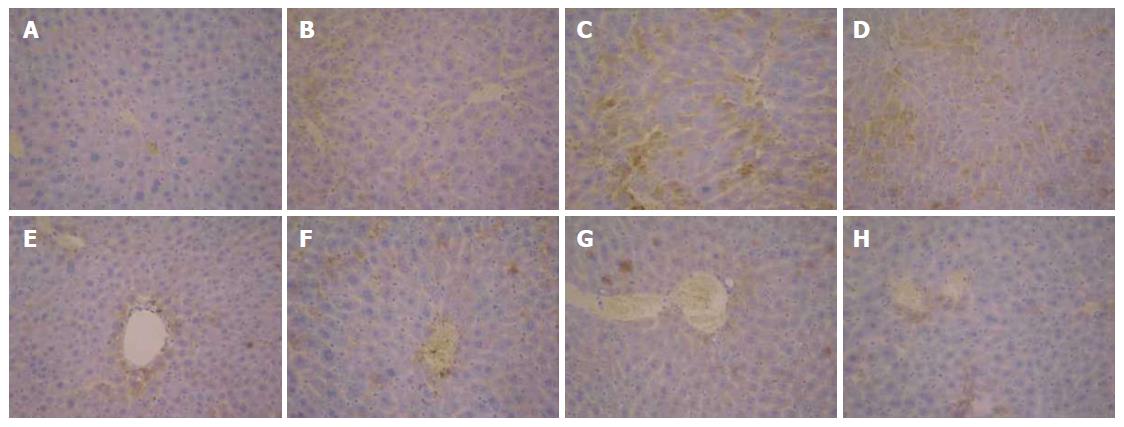
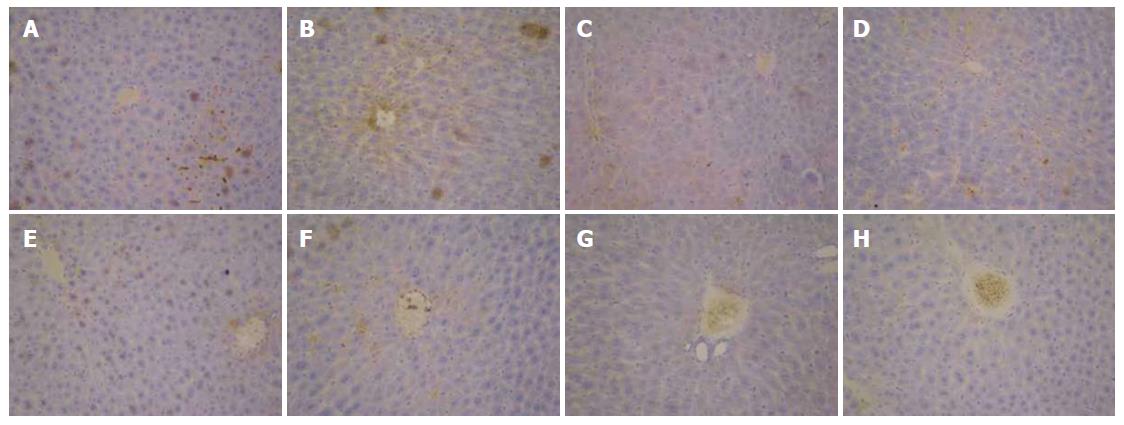
As shown in Figure 7A, the expression of TGF-β1 in the normal control group was only observed in the portal area and central vein, with a relatively shallow, narrow range. In Figure 7B and C, the expression of TGF-β1 in the model group was mainly distributed in the portal area and central vein, with brown granules showing a wide distribution. However, the positive expression of TGF-β1 was significantly decreased in the FVRB treatment group (Figure 7D-G). The positive control group also showed a good reduction in the expression of TGF-β1 (Figure 7H).
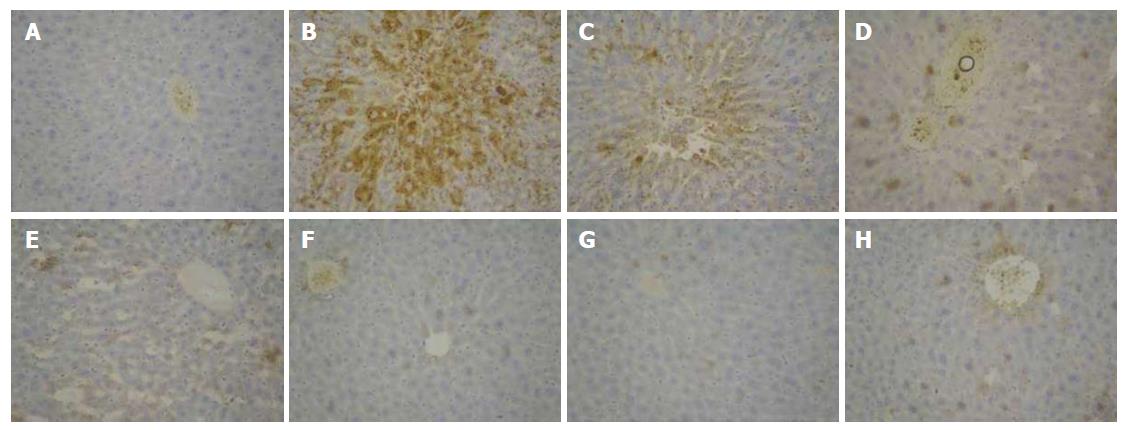
Immunohistochemical expression of MMP-9 protein is shown in Figure 8. Positive MMP-9 staining appeased as brown granules in the cytoplasm and membrane. The overall color of the normal group was light brown, while the model group was significantly different. Compared with the model group, the positive expression in the treatment group was decreased, especially in the dose group of 704 mg/kg.
The results of TIMP-1 protein expression are shown in Figure 9. In the normal control group, there was positive expression of TIMP-1 in peripheral blood vessels and bile duct wall of the portal area (Figure 9A). In the solvent group and the model group, the positive staining of TIMP-1 was distributed in the fiber spacing and the central vein, and the brown yellow was obviously visible (Figure 9B and C). Compared with the model group, the positive expression of TIMP-1 in the FVRB treatment groups was markedly decreased (Figure 9 D-G). Further, there was a small amount of positive TIMP-1 staining in the Yiganling Pian positive control group (Figure 9H).
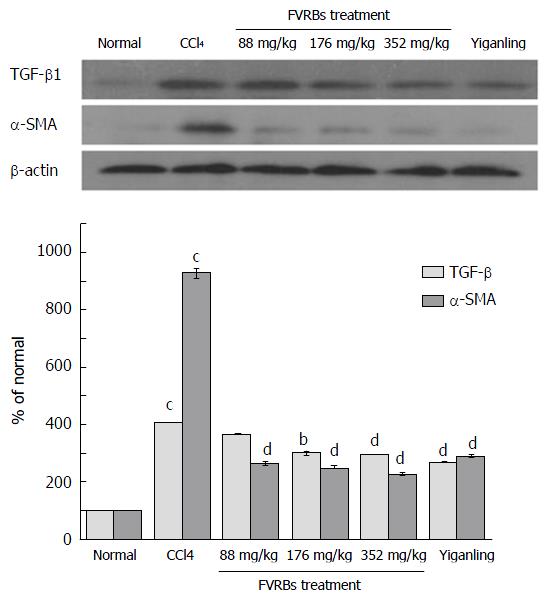
As illustrated in Figure 10, compared to the normal control group, the expression of TGF-β1 and α-SMA in the CCl4 treatment group was significantly increased (P < 0.01). However, FVRB treatment markedly decreased TGF-β1 and α-SMA expression as compared with the CCl4 treatment group (P < 0.05 or P < 0.01). Meanwhile, treatment with Yiganling Pian also significantly attenuated TGF-β1 and α-SMA expression (P < 0.01).
Liver fibrosis is usually regarded as an outcome of chronic liver injury in the process of long-term wound healing[17-19]. In the present study, CCl4-induced liver injury, the most commonly used model for hepatic fibrosis[20,21], was induced. We detected the levels of ALT, AST, TG, HA and LN to assess liver function and the degree of liver fibrosis. There is evidence that natural substances may have a protective role against CCl4-induced liver injury[22,23]. Considerable efforts have been made in the study of natural products with hepatoprotective activities[24,25]. Our study showed that CCl4 caused a significant increase in serum levels of ALT, AST, TG, HA and LN in mice. However, FVRB treatment significantly altered these trends. Its hepatoprotective effect was further confirmed by histopathological observation that FVRB attenuated CCl4-induced necro-inflammatory and fibrogenic effects.
There is growing evidence that oxidative stress contributes to the development of liver fibrosis by activating various signaling pathways involved in fibrogenesis[26,27]. The tissue concentration of MDA, a product of lipid peroxidation during liver fibrogenesis, was assayed. Also, the SOD and GSH activities were measured. In the present study, in the CCl4-treated mice, the MDA level in liver tissue was elevated and the activities of SOD and GSH were decreased. Reversal of these trends by FVRB treatment suggests that FVRB prevented the progression of liver fibrosis by inhibiting oxidative stress in the liver.
The histopathological studies are a direct means for assessing the protective effect of FVRB. HE and Masson staining results showed that FVRB could reduce liver necrosis, significantly inhibit collagen fiber hyperplasia, improve liver tissue structure and reduce fiber tissue. These results further confirmed that FVRB dose-dependently decreased hepatic histopathological changes.
The activation of HSCs is the central event in the pathogenesis of liver fibrosis[28-30]. In recent years, many studies found that TGF-β1 is the strongest factor inducing fibrosis, and it is also an important factor to promote the activation of HSCs[28,31]. Our result confirmed that administration of FVRB reduced the expression of TGF-β1 protein. Therefore, the anti-fibrotic effect of FVRB may be mediated by its inhibitory effect on TGF-β1. In addition, α-SMA expression also increases the generation and proliferation of chemotactic factors that are capable of recruiting inflammatory cells[32,33]. From the results we know that expression of α-SMA was enhanced by CCl4 treatment, while the administration of FVRB prevented the development of fibrosis perhaps through the inhibition of α-SMA.
The major pathological change of liver fibrosis is the excessive accumulation of collagen and other extracellular matrixes[34]. Under normal circumstances, the synthesis and decomposition of collagen is balanced. Once the synthesis is over than decomposition, it will cause the accumulation of collagen in the liver, leading to the formation of liver fibrosis[35]. Matrix metalloproteinases (MMPs) can promote the degradation of extracellular matrix, which is consistent with the previous finding that the tissue inhibitors of metalloproteinase (TIMPs) can reduce liver fibrosis severity[36,37]. Among the MMP family members, MMP-9 plays an essential role in fibrosis formation. Many studies have shown that MMP-9 is elevated in patients with liver fibrosis. TIMP-1 can inhibit MMP-9[38], preventing the degradation of ECM and thereby promoting liver fibrosis. In the present study, the expression of MMP-9 and TIMP-1 proteins showed significant differences between CCl4 and FVRB treatment groups, which suggests that MMP-9 and TIMP-1 are related to the protective effects of FVRB against the formation of liver fibrosis.
In conclusion, FVRB dose-dependently ameliorated hepatic oxidative stress and suppressed inflammation in CCl4-injured liver fibrosis, and its mechanisms against liver fibrosis may be related with inhibiting lipid peroxidation formation in liver tissue of liver fibrosis mice and reducing the collagen formation by suppressing protein expression of TGF-β1, α-SMA, MMP-9 and TIMP-1. Thus, FVRB may have potential therapeutic utilities for protecting against liver fibrosis. Further experimental studies are necessary to determine the effective constituents of FVRB.
Hepatic fibrosis is a wound-healing pathological process resulting from chronic hepatic injuries, which is characterized by the accumulation of extracellular matrix. It occurs during most continuous and chronic liver diseases, driven by inflammatory responses to injury tissues, which ultimately lead to liver cirrhosis.
Currently, treatment of liver damage mainly consists of inhibiting early activation and proliferation of hepatic stellate cells (HSCs) and collagen fiber growth, and promoting HSC apoptosis and collagen degradation. Foeniculum vulgare root bark (FVRB), a traditional Uyghur medicine, has been used for the treatment of several pathophysiological conditions in China, exhibiting the activity of dispelling coldness, warming kidney and stomach, removing dampness, and alleviating swelling and pain.
For the first time, the present study was aimed to investigate the protective effects of FVRB against CCl4-induced liver injury in vivo. And its mechanisms against liver fibrosis may be related with inhibiting lipid peroxidation in liver tissue of liver fibrosis mice and inhibiting the collagen formation by suppressing protein expression of transforming growth factor-β1, alpha-smooth muscle actin, matrix metalloprotein 9 and metallopeptidase inhibitor 1.
FVRB dose-dependently ameliorated hepatic oxidative stress and suppressed inflammation in CCl4-injured liver injury. Thus, FVRB may have potential therapeutic utilities for protecting against liver fibrosis.
Liver fibrosis is usually regarded as an outcome of chronic liver injury in the process of long-term wound healing.
The authors’ aim was to investigate the protective effects of FVRB extract against carbon tetrachloride-induced hepatic fibrosis in mice. The methods are appropriate, and the results are moderate.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bacskay I, Lee HC S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Li D
| 1. | Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Tai CJ, Choong CY, Lin YC, Shi YC, Tai CJ. The anti-hepatic fibrosis activity of ergosterol depended on upregulation of PPARgamma in HSC-T6 cells. Food Funct. 2016;7:1915-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Zhao J, Tang N, Wu K, Dai W, Ye C, Shi J, Zhang J, Ning B, Zeng X, Lin Y. MiR-21 simultaneously regulates ERK1 signaling in HSC activation and hepatocyte EMT in hepatic fibrosis. PLoS One. 2014;9:e108005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Mòdol T, Brice N, Ruiz de Galarreta M, García Garzón A, Iraburu MJ, Martínez-Irujo JJ, López-Zabalza MJ. Fibronectin peptides as potential regulators of hepatic fibrosis through apoptosis of hepatic stellate cells. J Cell Physiol. 2015;230:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Thomas RG, Moon MJ, Kim JH, Lee JH, Jeong YY. Effectiveness of Losartan-Loaded Hyaluronic Acid (HA) Micelles for the Reduction of Advanced Hepatic Fibrosis in C3H/HeN Mice Model. PLoS One. 2015;10:e0145512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Peng J, Li X, Feng Q, Chen L, Xu L, Hu Y. Anti-fibrotic effect of Cordyceps sinensis polysaccharide: Inhibiting HSC activation, TGF-β1/Smad signalling, MMPs and TIMPs. Exp Biol Med (Maywood). 2013;238:668-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | He Y, Huang C, Sun X, Long XR, Lv XW, Li J. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal. 2012;24:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Wasmuth HE, Weiskirchen R. [Pathogenesis of liver fibrosis: modulation of stellate cells by chemokines]. Z Gastroenterol. 2010;48:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Parejo I, Jauregui O, Sánchez-Rabaneda F, Viladomat F, Bastida J, Codina C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography-negative electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2004;52:3679-3687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:842674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 12. | Tripathi P, Tripathi R, Patel RK, Pancholi SS. Investigation of antimutagenic potential of Foeniculum vulgare essential oil on cyclophosphamide induced genotoxicity and oxidative stress in mice. Drug Chem Toxicol. 2013;36:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Berrington D, Lall N. Anticancer Activity of Certain Herbs and Spices on the Cervical Epithelial Carcinoma (HeLa) Cell Line. Evid Based Complement Alternat Med. 2012;2012:564927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Bogucka-Kocka A, Smolarz HD, Kocki J. Apoptotic activities of ethanol extracts from some Apiaceae on human leukaemia cell lines. Fitoterapia. 2008;79:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Singh B, Kale RK. Chemomodulatory action of Foeniculum vulgare (Fennel) on skin and forestomach papillomagenesis, enzymes associated with xenobiotic metabolism and antioxidant status in murine model system. Food Chem Toxicol. 2008;46:3842-3850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Tsai CF, Hsu YW, Chen WK, Chang WH, Yen CC, Ho YC, Lu FJ. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2009;47:2031-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260-7276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 289] [Cited by in RCA: 292] [Article Influence: 26.5] [Reference Citation Analysis (4)] |
| 18. | Getachew Y, Cusimano FA, Gopal P, Reisman SA, Shay JW. The Synthetic Triterpenoid RTA 405 (CDDO-EA) Halts Progression of Liver Fibrosis and Reduces Hepatocellular Carcinoma Size Resulting in Increased Survival in an Experimental Model of Chronic Liver Injury. Toxicol Sci. 2016;149:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Melino M, Gadd VL, Alexander KA, Beattie L, Lineburg KE, Martinez M, Teal B, Le Texier L, Irvine KM, Miller GC. Spatiotemporal Characterization of the Cellular and Molecular Contributors to Liver Fibrosis in a Murine Hepatotoxic-Injury Model. Am J Pathol. 2016;186:524-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Huang SS, Chen DZ, Wu H, Chen RC, Du SJ, Dong JJ, Liang G, Xu LM, Wang XD, Yang YP. Cannabinoid receptors are involved in the protective effect of a novel curcumin derivative C66 against CCl4-induced liver fibrosis. Eur J Pharmacol. 2016;779:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Hafez MM, Hamed SS, El-Khadragy MF, Hassan ZK, Al Rejaie SS, Sayed-Ahmed MM, Al-Harbi NO, Al-Hosaini KA, Al-Harbi MM, Alhoshani AR. Effect of ginseng extract on the TGF-β1 signaling pathway in CCl4-induced liver fibrosis in rats. BMC Complement Altern Med. 2017;17:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Sun Y, Jia L, Huang Z, Wang J, Lu J, Li J. Hepatoprotective effect against CCl4-induced acute liver damage in mice and High-performance liquid chromatography mass spectrometric method for analysis of the constituents of extract of Rubus crataegifolius. Nat Prod Res. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Pareek A, Godavarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Vladimir-Knežević S, Cvijanović O, Blažeković B, Kindl M, Štefan MB, Domitrović R. Hepatoprotective effects of Micromeria croatica ethanolic extract against CCl4-induced liver injury in mice. BMC Complement Altern Med. 2015;15:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wahid A, Hamed AN, Eltahir HM, Abouzied MM. Hepatoprotective activity of ethanolic extract of Salix subserrata against CCl4-induced chronic hepatotoxicity in rats. BMC Complement Altern Med. 2016;16:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Heeba GH, Mahmoud ME. Therapeutic potential of morin against liver fibrosis in rats: modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ Toxicol Pharmacol. 2014;37:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Domitrović R, Jakovac H, Marchesi VV, Blažeković B. Resolution of liver fibrosis by isoquinoline alkaloid berberine in CCl4-intoxicated mice is mediated by suppression of oxidative stress and upregulation of MMP-2 expression. J Med Food. 2013;16:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | He YH, Li Z, Ni MM, Zhang XY, Li MF, Meng XM, Huang C, Li J. Cryptolepine derivative-6h inhibits liver fibrosis in TGF-β1-induced HSC-T6 cells by targeting the Shh pathway. Can J Physiol Pharmacol. 2016;94:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Wang Q, Dai X, Yang W, Wang H, Zhao H, Yang F, Yang Y, Li J, Lv X. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int Immunopharmacol. 2015;25:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Wu T, Chen JM, Xiao TG, Shu XB, Xu HC, Yang LL, Xing LJ, Zheng PY, Ji G. Qinggan Huoxue Recipe suppresses epithelial-to-mesenchymal transition in alcoholic liver fibrosis through TGF-β1/Smad signaling pathway. World J Gastroenterol. 2016;22:4695-4706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Tang LX, He RH, Yang G, Tan JJ, Zhou L, Meng XM, Huang XR, Lan HY. Asiatic acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS One. 2012;7:e31350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Zhou DJ, Mu D, Jiang MD, Zheng SM, Zhang Y, He S, Weng M, Zeng WZ. Hepatoprotective effect of juglone on dimethylnitrosamine-induced liver fibrosis and its effect on hepatic antioxidant defence and the expression levels of α-SMA and collagen III. Mol Med Rep. 2015;12:4095-4102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Zakaria S, Youssef M, Moussa M, Akl M, El-Ahwany E, El-Raziky M, Mostafa O, Helmy AH, El-Hindawi A. Value of α-smooth muscle actin and glial fibrillary acidic protein in predicting early hepatic fibrosis in chronic hepatitis C virus infection. Arch Med Sci. 2010;6:356-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Wu K, Huang R, Wu H, Liu Y, Yang C, Cao S, Hou X, Chen B, DaI J, Wu C. Collagen-binding vascular endothelial growth factor attenuates CCl4-induced liver fibrosis in mice. Mol Med Rep. 2016;14:4680-4686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Fuchs BC, Wang H, Yang Y, Wei L, Polasek M, Schühle DT, Lauwers GY, Parkar A, Sinskey AJ, Tanabe KK. Molecular MRI of collagen to diagnose and stage liver fibrosis. J Hepatol. 2013;59:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Cong M, Liu T, Wang P, Fan X, Yang A, Bai Y, Peng Z, Wu P, Tong X, Chen J. Antifibrotic effects of a recombinant adeno-associated virus carrying small interfering RNA targeting TIMP-1 in rat liver fibrosis. Am J Pathol. 2013;182:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Peng WH, Tien YC, Huang CY, Huang TH, Liao JC, Kuo CL, Lin YC. Fraxinus rhynchophylla ethanol extract attenuates carbon tetrachloride-induced liver fibrosis in rats via down-regulating the expressions of uPA, MMP-2, MMP-9 and TIMP-1. J Ethnopharmacol. 2010;127:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Latronico T, Mascia C, Pati I, Zuccala P, Mengoni F, Marocco R, Tieghi T, Belvisi V, Lichtner M, Vullo V. Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors. Int J Mol Sci. 2016;17:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |