Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5692
Peer-review started: January 9, 2017
First decision: March 16, 2017
Revised: May 9, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: August 21, 2017
Processing time: 224 Days and 22.1 Hours
To evaluate the role of tissue factor (TF) and protease activated receptor (PAR)-2 in liver fibrosis.
Using CCl4 administration for eight weeks, we induced hepatic fibrosis in wild-type C57BL/6 mice and in mice with deletion of the cytoplasmic signalling domain of TF (TF§CT/§CT), deletion of PAR-2 (PAR-2-/-) and combined deletion of TF signalling domain and PAR-2 (TF§CT/§CT/PAR-2-/-). Hepatic fibrosis area was assessed by quantitative imaging of picrosirius red staining. Hepatic collagen content was assessed by hydroxyproline levels. Hepatic stellate cells (αSMA positive) and hepatic macrophages (CD68 positive) were identified by immunohistochemistry. Hepatic gene expression was determined by PCR and liver TGFβ1 content by ELISA.
CCl4 treated mice with deletion of the PAR-2 gene (PAR-2-/-) and the cytoplasmic domain of TF (TF§CT/§CT) developed significantly less hepatic fibrosis, characterised by reduced liver fibrosis area and hydroxyproline content, compared to control wildtype mice treated with CCl4. The observed reduction in histological fibrosis was accompanied by a significant decrease in the hepatic content of TGFβ, the prototypic fibrogenic cytokine, as well as fewer activated hepatic stellate cells and hepatic macrophages. Deletion of the TF cytoplasmic signalling domain reduced hepatic fibrosis to levels similar to those observed in mice lacking PAR-2 signalling but combined deletion provided no added protection against fibrosis indicating a lack of mutual modulating effects that have been observed in other contexts such as angiogenic responses.
Tissue factor cytoplasmic domain is involved in TF-PAR-2 signalling initiating hepatic fibrosis and is a potential therapeutic target, as its deletion would not impact coagulation.
Core tip: No effective anti-fibrotic therapies are available for patients with cirrhosis. PAR-2, a receptor that activates coagulation and inflammation, promotes hepatic fibrosis; whether tissue factor (TF), the primary initiator of the coagulation cascade, affects hepatic fibrosis is unknown. We found that deletion of the TF cytoplasmic domain reduces fibrosis through an effect on hepatic stellate cell activation, possibly mediated through reduced hepatic macrophage activation. Currently available direct thrombin inhibitors may be useful in preventing hepatic fibrosis while therapeutic targeting of the cytoplasmic domain of TF may be useful in patients with advanced liver disease, as its deletion does not alter coagulation.
- Citation: Knight V, Lourensz D, Tchongue J, Correia J, Tipping P, Sievert W. Cytoplasmic domain of tissue factor promotes liver fibrosis in mice. World J Gastroenterol 2017; 23(31): 5692-5699
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5692.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5692
Chronic hepatocyte injury leads to a wound healing response that can result in excessive inflammation, collagen deposition and advanced hepatic fibrosis. The coagulation system is involved in the acute response to injury with formation of a fibrin clot, but also plays an important role in the inflammatory signalling that perpetuates wound healing and tissue remodelling. The links between inflammation and coagulation occur through tissue factor (TF) and through protease activated receptors (PAR) although the relative contributions of each to inflammatory and fibrotic outcomes are unclear. TF is a transmembrane glycoprotein receptor with a pivotal role in hemostasis initiated by binding FVIIa, its principal ligand. Clot formation following TF ligation is well known, however downstream events also include expression of pro-inflammatory cytokines such as interleukin (IL)-6. Evidence for this relationship is seen in healthy human subjects who demonstrate IL6 and IL8 induction following exposure to recombinant FVIIa and in mice expressing low TF levels (< 1% of wildtype) which show reduced inflammatory cytokine expression and mortality following lipopolysaccharide (LPS) exposure[1,2].
The TF molecule has three domains; binding of the extracellular domain to Factor VII/VIIa initiates coagulation, the transmembrane domain has anchoring properties necessary for normal coagulation and the cytoplasmic domain, which is not required for coagulant activity, plays a role in intracellular signalling. For example, binding of FVIIa to macrophage TF leads to phospholipase C-dependent intracellular calcium fluxes and reactive oxygen species production that requires an intact TF cytoplasmic domain[3]. In the liver, hepatocytes are the primary source of Factor VII/VIIa. Constitutive hepatocyte expression of TF mRNA is low but can be upregulated in circulating monocytes by LPS[4], monocyte chemotactic protein-1 and platelet derived growth factor as well as by direct contact with platelets[4,5].
The TF cytoplasmic domain also plays a regulatory role in PAR-2 signalling. Mice with deletion of the cytoplasmic domain (TF§CT/§CT) demonstrate enhanced PAR-2 dependent angiogenesis suggesting that the TF cytoplasmic domain acts as a negative regulator of PAR-2 signalling[6]. Context may be important, however, as others have shown that the TF cytoplasmic domain is necessary for PAR-2 regulated inflammation[7]. A role for TF-PAR-2 signalling in the development of obesity and insulin resistance has recently been demonstrated. TF-VIIa-PAR-2 signalling in adipocytes regulates weight gain and in macrophages promotes inflammation and insulin resistance[8]. Such pathways may play a role in the development of non-alcoholic fatty liver disease (NAFLD) as direct thrombin inhibition has been shown to reduce hepatic inflammation in murine NAFLD[9].
The aim of our study was to evaluate the role of the TF cytoplasmic domain in the hepatic inflammatory response and liver fibrogenesis following chronic injury. We have previously shown that PAR-2 deficiency ameliorates experimental liver fibrosis[10]. Given the observed interactions between TF cytoplasmic domain and PAR-2 in angiogenesis and inflammation, we explored a possible relationship between the TF cytoplasmic domain and the PAR-2 receptor in the development of liver fibrosis in mice with deletion of the TF cytoplasmic domain, deletion of PAR-2 and deletion of both the TF cytoplasmic domain and PAR-2.
Mice on a 25%Swiss/25%129S/50% MF-1 background with deletion of 18 of the 20 amino acids of the TF cytoplasmic domain (TF§CT/ §CT) were generated by Cre-lox recombination as previously described and backcrossed 9 generations onto a C57BL6 background[11]. TF§CT/§CT mice have normal development, coagulation and fertility. PAR-2 knockout mice were generated as previously described[10]. Mice with deletion of both the TF cytoplasmic domain and PAR 2 (TF§CT/§CT/PAR-2-/-) were generated; these mice have normal development, coagulation and fertility. Mice were allowed food and water ad libitum and were housed at a constant temperature in 12:12 hour light-dark cycle. Experimental protocols were approved by the Monash University Animal Ethics Committee and mice received humane care as specified under the Australian code of practice for the care and use of animals for scientific purposes.
Liver fibrosis was induced in male mice by twice-weekly intraperitoneal injections of 1 μL/g body weight CCl4 mixed with olive oil (1:10). Starting between 8-10 wk of age, four groups of mice were studied. TF§CT/§CT (n = 9), TF§CT/§CT/PAR-2-/- (n = 6) and wild type (WT) C57BL/6 (n = 10) all received CCl4 for 8 wk. A control group of WT C57BL/6 mice (n = 8) received olive oil alone for 8 wk as a vehicle control.
Liver fibrosis area: Four micron thick sections of paraffin embedded liver tissue were deparaffinised and stained with picrosirius red (Sirius red F3BA 0.1% wt/vol in saturated picric acid) for 90 min, washed in acetic acid and water (5:1000), dehydrated in ethanol and mounted in neutral DPX. Fifteen consecutive non-overlapping fields were acquired for each mouse liver, the image digitised and fibrosis area analysed by Scion Image for Windows (vAlpha 4.0.3.2, Scion Corporation, Frederick, MD, United States).
Hepatic collagen content: Hydroxyproline is an amino acid that stabilises collagen deposited in the liver and is exclusively associated with collagenous connective tissue and therefore is a good surrogate for quantification of collagen deposition[12]. Hepatic hydroxyproline content was quantified using liver tissue frozen in liquid nitrogen as previously described with minor modification[13]. Briefly, liver samples were weighed and hydrolysed in 2.5 mL of 6 N HCl at 110 °C for 18 h in Teflon coated tubes. The hydrolysate was centrifuged at 3000 rpm for 10 min; the pH of the resulting supernatant was adjusted to 7.4 and absorbance measured at 558 nm. Total hydroxyproline content was measured against a standard curve prepared with trans-4-hydroxy-L-proline (Sigma-Aldrich, St Louis, MO, United States) preparations in the range of 0.156 to 5.0 μg/mL and expressed per milligram of wet tissue weight.
Hepatic stellate cell and macrophage immunohistochemistry: To identify activated hepatic stellate cells (myofibroblasts) and hepatic macrophages, paraformaldehyde fixed 4 micron thick liver tissue sections were stained with primary antibody for αSMA (monoclonal mouse anti-mouse α-smooth muscle actin, Sigma, St Louis, MO, United States) and CD68 (rat anti mouse CD68, FA11 a gift of Dr G Koch, Cambridge United Kingdom, 1:100). The following secondary antibodies were used: αSMA biotinylated rabbit anti-mouse IgG2a antibody (Invitrogen, Camarillo, CA, United States, 1:300) and CD68 polyclonal rabbit anti rat IgG (DAKO #E0468 1:150). In brief sections were dewaxed, rehydrated and then blocked with 0.6% hydrogen peroxide and cellular apoptosis susceptibility protein blocking solution (Invitrogen, Camarillo, CA, United States). Primary antibody incubations for 30 min at room temperature (αSMA) and overnight at 4 °C (CD68) were followed by application of secondary antibody. Staining was amplified using avidin-biotin complex kit (Vector Laboratories, Burlingame, CA, United States) and detected with 3,3’ diaminobenzidine (Dako, Carpentaria, CA, United States). Slides were counterstained with Harris hematoxylin. For quantification of immunoreactivity, 15 consecutive non-overlapping fields at 250 × magnification (αSMA, CD68) were scored using a graticule eyepiece in a blinded fashion. Negative controls consisted of a mouse IgG1 isotype control antibody (Dako, Glostrup, Denmark) and water substituting for the primary antibody.
Mouse RNA was extracted using the Qiagen RNeasy mini kit according to the manufacturer’s instructions (Qiagen Pty Ltd, Hilden, Germany). Briefly, 0.02 to 0.03 g of liver was placed in RNAse free tube. 600 μL of RLT was added to each sample which was then vortexed for 15 min. The resultant lysate was pipetted into a QIA shredder spin column and centrifuged initially at full speed for 2 min, then the column was removed and the lysate was centrifuged for a further 3 min. The resultant supernatant was removed and added to equal volume of 50% ethanol, transferred to an RNeasy spin column, centrifuged for 15 s and the flow through discarded. Seven hundred microliter Buffer RW1 was added and the column was centrifuged for 15 s and followed by the addition of two aliquots of 500 μL of RPE, centrifuging in between for 15 s and 2 min respectively. The RNeasy spin column was placed in a new collection tube, centrifuged for 1 min and then 30 μL of RNase-free water was added directly to the spin column membrane, centrifuged and then a further 30 μL of RNase-free water was added. Four microliter of RNa was added to 76 μL of RNase free water. The RNA concentration was measured with a Nanodrop ND-100 spectrophotometer (Thermo Scientific, Waltham, MA, United States). RNA was used to generate cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States) as per manufacturer’s instructions. Briefly, 2 × RT master mix was prepared from supplied components as follows: 2.0 μL 10 × RT buffer, 0.8 μL 25 × dNTP mix (100 mmol/L), 10 × RT Random Primers, 1.0 μL MultiscribeTM Reverse Transcriptase, 1.0 μL RNase Inhibitor and 3.2 μL of Nuclease free water. Nineteen microliter of 2 × RT master mix was combined with 10 μL of RNA containing 1 μg RNA. The tubes were centrifuged. The PCR program involved Step 1: 25 °Cfor 10 min, Step 2: 37 °C for 120 min, Step 3: 85 °C for 5 s, and Step 4: 4 °C for 10 min and then samples were collected and stored at -80 °C.
Real time PCR analysis was performed (Power Sybr Green, Roche, Manheim, Germany) using a Rotor Gene 3000 light cycler (Qiagen Pty Ltd, Sydney, Australia) and the specific target mRNA of interest quantified as a ratio relative to 18S RNA content of the sample. 200 ng of cDNA was used per reaction. The primer sequences used in analysis are found in Table 1.
| Target gene | Primer sequence (5’-3’) |
| 18s | Forward: 5’-GTAACCCGTTGAACCCCATTC-3’ |
| Reverse: 5’-GCCTCACTAAACCATCCAATC G-3’ | |
| TGFβ | Forward: 5’-TGCCCTCTACAACCAACACA-3’ |
| Reverse: 5’-GTTGGACAACTGCTCCACCT-3 | |
| MMP-2 | Forward: 5’-ACCCAGATGTGGCCAACTAC-3’ |
| Reverse: 5’-TCATTTTAAGGCCCGAGCAA-3’ | |
| TIMP-1 | Forward: 5’-ACGAGACCACCTTATACCAGCCG-3’ |
| Reverse: 5’-GCGGTTCTGGGACTTGTGGGC-3’ | |
| PAR-1 | Forward: 5’-CTCCTCAAGGAGCAGACCCAC-3’ |
| Reverse: 5’-AGACCGTGGAAACGATCAAC-3’ | |
| PAR-2 | Mm00433160_m1, using TaqMan Gene Expression Master Mix (Applied Biosystems) |
| 18S | Hs03003631_g1 using TaqMan Gene Expression Master Mix (Applied Biosystems) |
Extracts were prepared from snap frozen liver by homogenisation in lysis buffer (Tris-HCl 50 mmol/L, NaCl 150 mmol/L, EDTA 1 mmol/L, 1% Triton X-100, 0.5% Tween-20, 0.1% SDS) containing a protease inhibitor cocktail (#11836170, Roche Diagnostics, Mannheim, Germany) followed by centrifugation at 14000 × g for 15 min at 4 °C. Supernatants were collected and activated with acetic acid/urea prior to analysis. TGFβ1 content of liver protein extracts were measured using a mouse TGFβ1 ELISA kit (R and D Systems Inc, Minneapolis, MN, United States). Plates were read using the Bio-Rad microplate reader at 450 nm and TGFβ1 concentrations were calculated from the standard curve by the plate reader software.
Data are expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA with Newman-Keuls post-test for multiple comparisons or Student’s t-test for comparisons between two groups as appropriate, using GraphPad Prism 5.03 for Windows (GraphPad Software, Inc, La Jolla, United States). A P value < 0.05 was considered significant.
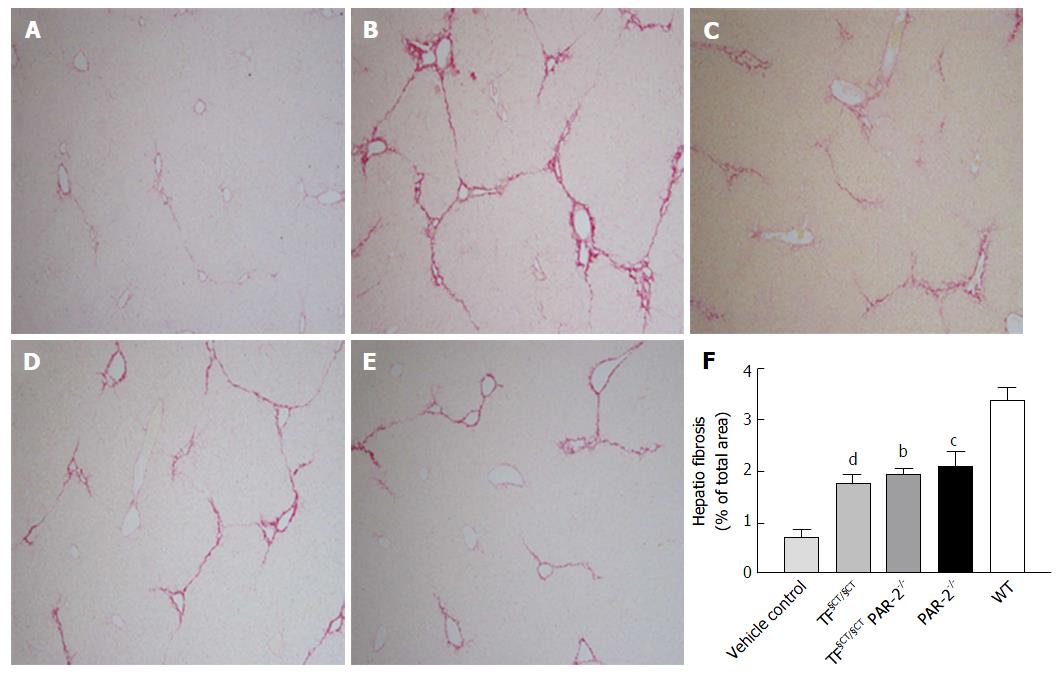
Quantitative analysis of histological fibrosis by computer-assisted morphometry in CCl4-treated WT mice showed a marked increase in liver fibrosis area (LFA) at 8 wk (3.39% ± 0.26%) compared to vehicle controls (LFA 0.7% ± 014%) (P < 0.0001). CCl4 administration induced similar levels of fibrosis in TF§CT/§CT and TF§CT/§CT/PAR-2-/- mice (1.76% ± 0.17% and 1.94% ± 0.11% respectively), which were similar to that seen with PAR-2-/- mice (LFA 2.09% ± 0.28%). Compared to WT mice, at 8 wk LFA was significantly lower in the PAR-2-/- (P < 0.001), TF§CT/ §CT (P < 0.0001) and TF§CT/ §CT/PAR-2-/- mice (P < 0.01) (Figure 1A-F).
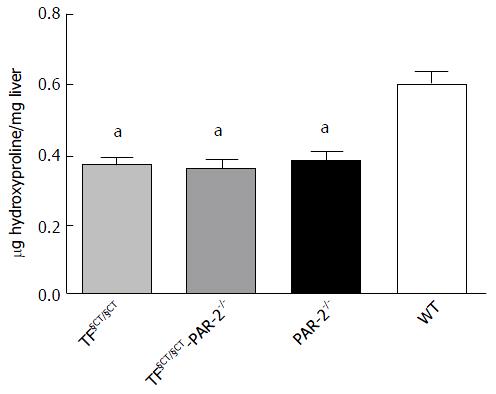
Histological assessment of fibrosis area correlated with the amount of liver hydroxyproline, a surrogate marker for collagen content. TF§CT/§CT and TF§CT/§CT/PAR-2-/- mice showed significantly less hepatic hydroxyproline content (0.37 ± 0.017 μg/mg and 0.37 ± 0.02 μg/mg) compared to WT mice (0.61 ± 0.03 μg/mg) at 8 wk (P < 0.05). Hydroxyproline content in TF§CT/ §CT and TF§CT §CT/PAR-2-/- mice were similar to that seen in the PAR-2-/- mice (0.39 ± 0.02 μg/mg) (Figure 2).
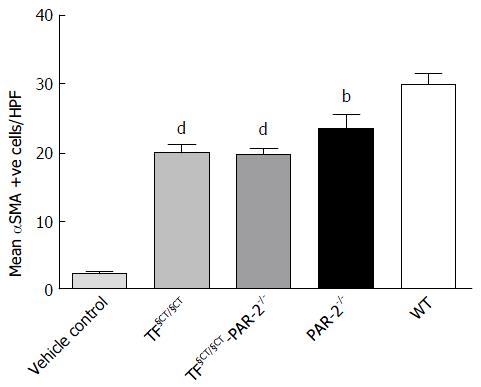
We identified αSMA positive cells as a marker of HSC activation and myofibroblast differentiation. There were significantly fewer αSMA positive cells seen histologically in the TF§CT/§CT and TF§CT/§CT/PAR-2-/- groups compared to WT (19.99, 19.96 and 30.09 mean αSMA positive cells/hpf respectively, P < 0.0001) (Figure 3). The reduction observed in αSMA positive cells in the TF§CT/§CT and TF§CT/§CT/PAR-2-/- groups was similar to that observed in PAR-2-/- alone.
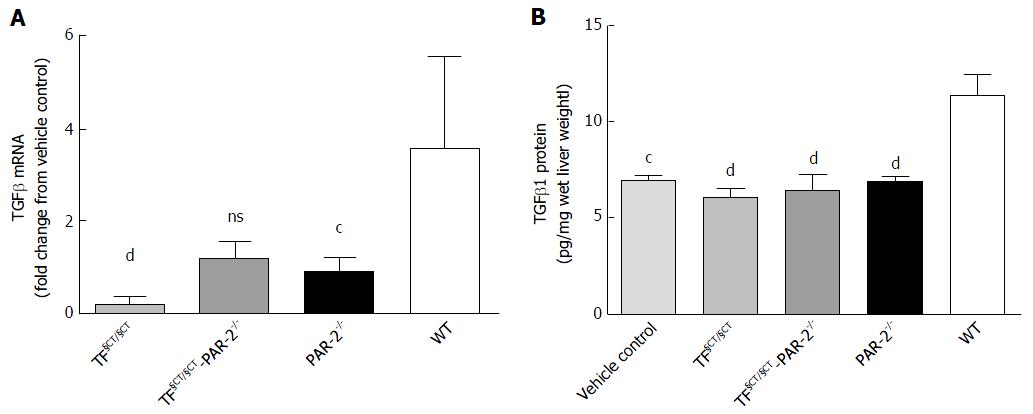
CCl4 induced hepatic fibrosis was associated with upregulation of TGFβ mRNA (3.57 fold greater than control) and protein (11.41 ± 0.98 pg/mg liver vs control 7.01 ± 0.01 pg/mg in WT mice) at 8 wk. In TF§CT/§CT mice, TGFβ mRNA expression was significantly reduced (0.22 ± 0.05 fold greater than control, P < 0.0001). In TF§CT/§CT/PAR-2-/- mice there was a trend towards TGFβ mRNA reduction (1.21 ± 0.14 fold greater than control, P = NS) (Figure 4A). In both the TF§CT/§CT and TF§CT/§CT/PAR-2-/- mice, TGFβ protein was similar to controls (6.14 ± 0.33, 6.45 ± 0.84 and 7.01 ± 0.18 pg/mg wet liver weight respectively) and significantly lower than the TGFβ protein level in wildtype mice (P < 0.0001) (Figure 4B).
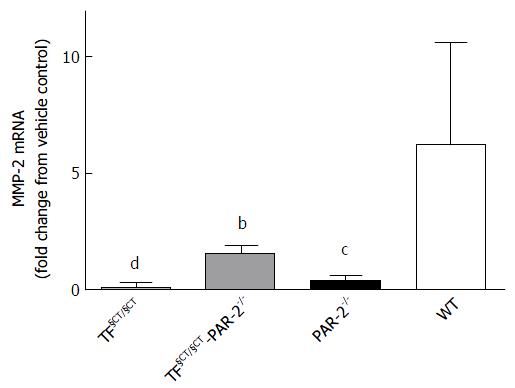
Matrix metalloproteinases (MMP) regulate matrix composition and turnover and in turn are regulated by specific tissue inhibitors, TIMPs. The balance of the expression of different MMPs changes throughout the development of liver injury, but in general there is upregulation of the basement membrane-like MMPs, such as MMP2 and 9 and down regulation of interstitial type MMPs such as MMP1 (MMP13 in mice). In WT mice treated with CCl4 for 8 wk, MMP2 mRNA increased (6.29 ± 1.45 fold greater than control), consistent with active ECM remodelling during development of hepatic fibrosis (Figure 5). MMP-2 mRNA expression was significantly lower in TF§CT/§CT and TF§CT/§CT/PAR-2-/- mice compared to WT mice (P < 0.0001 and P < 0.01 respectively), suggesting ECM remodelling is reduced in association with the lower levels of fibrosis seen at 8 wk in these mice.
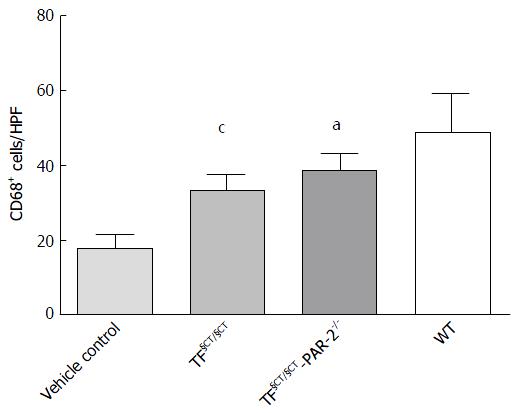
We used CD68 (macrosialin) to identify activated macrophages. There were significantly fewer CD68+ hepatic macrophages in both the TF§CT/§CT and TF§CT §CT/PAR-2-/- mice compared to WT mice (33.24 ± 1.47, 38.70 ± 1.78, 48.75 ± 2.87 positive cells/hpf; P < 0.001, P < 0.05 respectively) (Figure 6). We observed similar findings in PAR-2-/- mice suggesting a role for PAR-2 in macrophage recruitment and activation[9]. The current findings suggest that the TF cytoplasmic domain, as well as PAR-2, may be involved in this process.
There is growing evidence in animal models and in humans that injury-related signalling through the pivotal coagulation receptors, TF and PAR, can drive hepatic fibrogenesis. A recent study of acute injury in mice given a single injection of CCl4 demonstrated elevated hepatocyte TF levels and tissue injury that were blocked by specific TF antisense oligonucleotide therapy[14]. In man, TF levels have been found to be over 100 fold higher in patients with cirrhosis compared to non-cirrhotic patients[15]. PAR-1 polymorphisms were shown to influence the hepatic fibrosis rate in Brazilian and European patients with chronic hepatitis C infection[16]. Resistance to activated protein C occurs in patients heterozygous for the factor V Leiden mutation leading to increased thrombin activity, which is a mitogen for hepatic stellate cells. Factor V Leiden heterozygosity has been associated with rapid fibrosis progression in hepatitis C patients[17]. Given our previous study showing that PAR-2 deletion decreased hepatic fibrosis in mice and data showing that the TF cytoplasmic domain induces proinflammatory effects in macrophages we examined the relative contributions of TF and PAR-2 in the development of toxin-induced liver fibrosis.
We found that mice with deletion of the TF cytoplasmic domain had lower hepatic collagen content and less histological fibrosis than wild type mice following 8 wk of CCL4 exposure. This was accompanied by fewer αSMA positive cells histologically suggesting reduced hepatic stellate cell activation. There was significantly lower gene and protein expression of the key profibrogenic cytokine TGFβ and a reduction in MMP2 expression in TF§CT/§CT mice. These data suggest that activation of the cytoplasmic domain of TF promotes hepatic fibrosis by inducing a profibrogenic phenotype in hepatic stellate cells. In angiogenesis, the cytoplasmic domain of TF acts as a negative regulator of PAR-2. If that were true in this model of liver fibrosis, then loss of the cytoplasmic domain and thus loss of negative regulation of PAR-2, should lead to increased fibrosis through increased PAR-2 activation. However this was not observed in our study. In fact, the protection from experimentally induced liver fibrosis that the TF§CT/§CT mice were afforded was similar to that which we previously observed in mice with PAR 2 deficiency[10]. This observation is consistent with the observation in other studies that TF may be necessary for PAR-2 mediated inflammation[7].
We found that mice with dual receptor deletion (TF§CT/§CT/PAR-2-/-) in this experimental model also exhibited reduced fibrosis, reduced stellate cell activation and reduced expression of TGFβ. If the mechanisms underlying fibrogenesis through the TF cytoplasmic domain and PAR-2 were independent, an additive effect and thus greater reduction in fibrosis would potentially have been observed in these mice. However, the extent of fibrosis reduction seen in the TF§CT/§CT/PAR-2-/- mice was similar to that seen in TF§CT/ §CT mice and PAR-2-/- mice, suggesting that the TF cytoplasmic domain and PAR-2 may signal through a common downstream pathway to promote fibrosis.
Our studies in TF§CT/§CT mice demonstrated significantly fewer CD68+ activated macrophages supporting the view that TF cytoplasmic domain signalling is important for macrophage activation. Hepatic macrophages (Kupffer cells) play a pivotal role in both fibrogenesis and fibrolysis. Macrophages constitutively express TF, which is upregulated during macrophage maturation. Activated macrophages release TGFβ1, which activates HSC, in addition to mitogenic and chemotactic factors for activated HSC[18]. At 8 wk we observed a reduction in the number of activated macrophages (CD68+) in both TF§CT/§CT mice and TF§CT/§CT/PAR-2-/- compared to WT. This may be because the cytoplasmic domain of tissue factor is essential for Factor VIIa induced calcium fluxes in macrophages in vitro. In vivo studies in which TF/Factor VIIa interactions were blocked using a TF antibody led to reduced expression of MHC class II and β2 integrin leucocyte adhesion molecules which are markers of macrophage activation[3].
There is increasing interest in macrophage phenotypes in liver disease as classically activated M1 macrophages are proinflammatory and alternatively activated M2 macrophages are anti-inflammatory. A recent study has shown that TF§CT/§CT mice fed a high fat diet demonstrated reduced inflammatory macrophages in adipose tissue with reduced proinflammatory cytokine production, suggesting that TF signalling is directly involved in regulating macrophage inflammation and may sustain M1 polarisation. Interestingly, there was no additive effect of double deletion of TF and PAR-2 on diet induced obesity, similar to the outcomes we have observed in hepatic fibrosis[8].
In conclusion we have established, for the first time to our knowledge, that deletion of the TF cytoplasmic domain significantly reduces experimental hepatic fibrosis. Furthermore we have shown that deletion of the cytoplasmic domain in addition to PAR-2 deficiency does not lead to more profound protection from fibrosis and in fact that mice lacking the tissue factor cytoplasmic domain phenocopy PAR-2 deficiency. This demonstrates that the tissue factor cytoplasmic domain is involved in pathological TF-PAR-2 signalling. The potential to intervene in this process by inhibiting thrombin or FXa is relevant given the widespread clinical availability of direct inhibitors. For example, administration of argatroban, a direct thrombin inhibitor, to mice fed a Western diet significantly reduced hepatic macrophage accumulation, pro-fibrogenic gene expression and hepatic steatosis suggesting that thrombin inhibition could reduce the risk of fibrosis development in NAFLD patients[9]. It would be prudent to limit therapeutic coagulation inhibition to non-cirrhotic patients at risk of fibrosis progression in order to avoid the risk of bleeding events in cirrhotic patients. This makes the cytoplasmic domain of TF an attractive therapeutic target, as its deletion would not impact on coagulation and thus could be a potentially beneficial anti-fibrotic strategy in patients with advanced liver disease.
Globally, liver cirrhosis is the sixth most common cause of life-years lost to premature mortality. Cirrhosis represents a “wound healing” response to persistent inflammatory injury (commonly due to fat, alcohol or hepatitis viruses) that can lead to excessive collagen deposition and disruption of normal liver architecture. The coagulation system is active in both the acute response to organ injury as well as in inflammatory signaling that perpetuates the wound healing response. The molecular link between inflammation and coagulation occurs through tissue factor (TF) and protease activated receptors (PARs) although the relative contribution of each remains unclear.
Currently no anti-fibrotic agents are available for patients with cirrhosis. Liver transplantation may be the only effective treatment for those who progress to hepatic decompensation or develop hepatocellular carcinoma. In order to develop therapeutic anti-fibrotic agents, the authors must understand the cellular and molecular pathways that cause inflammation and fibrosis.
The authors previously showed that PAR-2 was involved in hepatic fibrosis, since deletion of the PAR-2 gene resulted in decreased fibrosis in mice. Since the TF cytoplasmic domain is a negative regulator of PAR-2 in angiogenesis, they explored this relationship in a model of liver fibrosis. They found that mice with low levels of the cytoplasmic domain of TF had less fibrosis than control mice with fewer activated hepatic stellate cells, the principal liver cells that produce collagen, and fewer activated macrophages, which express cytokines that drive HSC activation.
Inhibition of coagulation, for example, by direct thrombin inhibitors, could prevent fibrosis progression in cirrhotic patients but would increase the risk of bleeding events in a patient group at high risk for such events. However, direct targeting of the cytoplasmic domain of TF, which is involved in intracellular signalling but not in coagulation, could be a potentially beneficial anti-fibrotic strategy in cirrhotic patients.
The paper by Knight et al describes that genetic ablation of the intracellular portion of TF or of PAR2 downstream to TF both reduce in a non-additive fashion fibrosis in the liver induced by treating mice with CCL4. Some insights in the mechanism of action of the two manipulations were provided by histological data indicating that they both reduce the frequency of activated smooth muscle cells and activated macrophages and the levels of TGF-β, a known profibrotic factor, in the liver. The results are interesting in view of the fact that liver fibrosis is an unmet clinical need which involves a large number of patients and that drugs targeting TF have been developed and are currently in clinical trials for treatment of thrombosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee HC, Migliaccio AR S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | de Jonge E, Friederich PW, Vlasuk GP, Rote WE, Vroom MB, Levi M, van der Poll T. Activation of coagulation by administration of recombinant factor VIIa elicits interleukin 6 (IL-6) and IL-8 release in healthy human subjects. Clin Diagn Lab Immunol. 2003;10:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Pawlinski R, Fernandes A, Kehrle B, Pedersen B, Parry G, Erlich J, Pyo R, Gutstein D, Zhang J, Castellino F. Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction. Proc Natl Acad Sci USA. 2002;99:15333-15338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94:3413-3420. [PubMed] |
| 4. | Rivers RP, Hathaway WE, Weston WL. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol. 1975;30:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 250] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Ernofsson M, Siegbahn A. Platelet-derived growth factor-BB and monocyte chemotactic protein-1 induce human peripheral blood monocytes to express tissue factor. Thromb Res. 1996;83:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, Carmeliet P, Mueller BM, Friedlander M, Ruf W. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118:3453-3461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Badeanlou L, Furlan-Freguia C, Yang G, Ruf W, Samad F. Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med. 2011;17:1490-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Kassel KM, Sullivan BP, Cui W, Copple BL, Luyendyk JP. Therapeutic administration of the direct thrombin inhibitor argatroban reduces hepatic inflammation in mice with established fatty liver disease. Am J Pathol. 2012;181:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology. 2012;55:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Melis E, Moons L, De Mol M, Herbert JM, Mackman N, Collen D, Carmeliet P, Dewerchin M. Targeted deletion of the cytosolic domain of tissue factor in mice does not affect development. Biochem Biophys Res Commun. 2001;286:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Lee HS, Shun CT, Chiou LL, Chen CH, Huang GT, Sheu JC. Hydroxyproline content of needle biopsies as an objective measure of liver fibrosis: Emphasis on sampling variability. J Gastroenterol Hepatol. 2005;20:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137-G144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Abdel-Bakky MS, Helal GK, El-Sayed EM, Saad AS. Carbon tetrachloride-induced liver injury in mice is tissue factor dependent. Environ Toxicol Pharmacol. 2015;39:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Van Thiel DH, Farr DE, Mindikoglu AL, Todo A, George MM. Recombinant human factor VIIa-induced alterations in tissue factor and thrombomodulin in patients with advanced liver cirrhosis. J Gastroenterol Hepatol. 2005;20:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Martinelli A, Knapp S, Anstee Q, Worku M, Tommasi A, Zucoloto S, Goldin R, Thursz M. Effect of a thrombin receptor (protease-activated receptor 1, PAR-1) gene polymorphism in chronic hepatitis C liver fibrosis. J Gastroenterol Hepatol. 2008;23:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Wright M, Goldin R, Hellier S, Knapp S, Frodsham A, Hennig B, Hill A, Apple R, Cheng S, Thomas H. Factor V Leiden polymorphism and the rate of fibrosis development in chronic hepatitis C virus infection. Gut. 2003;52:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Marra F, Aleffi S, Galastri S, Provenzano A. Mononuclear cells in liver fibrosis. Semin Immunopathol. 2009;31:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |