Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5669
Peer-review started: May 19, 2017
First decision: June 22, 2017
Revised: June 30, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: August 21, 2017
Processing time: 92 Days and 18.9 Hours
To evaluate the measurement of zonulin level and antibodies of zonulin and other tight junction proteins in the blood of controls and celiac disease patients.
This study was conducted to assess the variability or stability of zonulin levels vs IgA and IgG antibodies against zonulin in blood samples from 18 controls at 0, 6, 24 and 30 h after blood draw. We also measured zonulin level as well as zonulin, occludin, vinculin, aquaporin 4 and glial fibrillary acidic protein antibodies in the sera of 30 patients with celiac disease and 30 controls using enzyme-linked immunosorbent assay methodology.
The serum zonulin level in 6 out of 18 subjects was low or < 2.8 ng/mL and was very close to the detection limit of the assay. The other 12 subjects had zonulin levels of > 2.8 ng/mL and showed significant fluctuation from sample to sample. Comparatively, zonulin antibody measured in all samples was highly stable and reproducible from sample to sample. Celiac disease patients showed zonulin levels with a mean of 8.5 ng/mL compared to 3.7 ng/mL in controls (P < 0.0001). Elevation of zonulin level at 2SD above the mean was demonstrated in 37% of celiac disease patients, while antibodies against zonulin, occludin and other tight junction proteins was detected in up to 86% of patients with celiac disease.
Due to its fluctuation, a single measurement of zonulin level is not recommended for assessment of intestinal barrier integrity. Measurement of IgG and IgA antibodies against zonulin, occludin, and other tight junction proteins is proposed for the evaluation of the loss of intestinal barrier integrity.
Core tip: We studied possible variability in zonulin levels vs measuring antibodies against zonulin and other tight junction proteins in blood. We found that fluctuations in zonulin level from hour-to-hour and day-to-day were too great to recommend it for assessing intestinal permeability. Measurement of IgG and IgA antibodies against tight junction proteins in controls and in celiac disease patients proved to be very stable and reproducible, and we recommend this method for such an assessment in future studies.
- Citation: Vojdani A, Vojdani E, Kharrazian D. Fluctuation of zonulin levels in blood vs stability of antibodies. World J Gastroenterol 2017; 23(31): 5669-5679
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5669.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5669
The intestinal barrier is one of the most continuously challenged body barriers. Its importance for maintaining health cannot be overestimated. Protected by outer layers of gut microbiota and mucus, the intestinal epithelial layer is the last defensive barrier. This one-cell-thick, picket fence-like structure both absorbs nutrients and blocks the entry of immunogenic molecules from infiltrating the body. If the barrier is breached, inflammation, autoimmunity or even cancer may follow[1,2]. A broken barrier, therefore, is a serious matter and a therapeutic target for disease amelioration[3,4].
Intestinal barrier structures include epithelial cells and a system of junctions linking them together. This structure prevents pathogens, endotoxins and undigested dietary proteins from reaching the underlying lamina propria. Many factors can alter intestinal barrier structures, causing increased barrier permeability. These factors include physical and/or emotional stress[5], gut microbiota modifications[6-8], dust mite allergen[9], long-term use of non-steroidal anti-inflammatory drugs[10-12], diet[8,13], alcohol[14] and autoimmune reactivity against barrier structures[15-17].
Lacing the epithelial cells to one another are complex cellular junctions: tight junctions, gap junctions, adherens junctions and desmosomes[4]. Making up the tight junctions are occludins and claudins with zonulin anchoring them to the actomyosin network within the epithelial cells. Together these cells regulate the intestinal paracellular pathway.
Occludin and claudin are intra-membrane proteins, which regulate water ion flow and electrolyte loss[3]. Occludin has also been linked to the regulation of intermembrane and paracellular diffusion of small molecules[18].
Zonula occludens (ZO-1, ZO-2, ZO-3) are intracellular tight junction proteins that bind directly the C-terminal 146 amino acids of occludin to the cable cytoskeletal protein actomyosin[19]. Zonula occludens are members of a family of membrane-associated signaling proteins known as the membrane-associated guanylate kinase homologs (MAGUKs)[20]. MAGUKs are suggested to be involved in signal transduction pathways controlling growth and differentiation[21,22]. Zonulin modulates small-intestinal tight junction permeability through a protein kinase C-alpha-mediated actin polymerization[23].
Vinculin is a cytoskeletal protein that is found in both focal contacts and adherens junction[24,25], and contributes to the mechanical link of the contractile actomyosin cytoskeleton to the extracellular matrix through integrin receptors. Vinculin is capable of binding to alpha-actinin, actin, talin[26] and to itself[27-29]. Vinculin also plays a role in the establishment, or regulation, of cadherin-based cell adhesion[30,31].
Beneath the intestinal epithelial cells reside astrocyte-like cells, known as enteric glia cells (EGCs). The expression marker, glial fibrillary acidic protein (GFAP), of EGCs is identical to the astrocytes of the central nervous system[32,33]. GFAP is an intermediate filament that is closely related to its non-epithelial family members, vimentin, desmin, and peripherin, which are all involved in the structure and function of the cell’s cytoskeleton. GFAP is thought to help to maintain astrocyte mechanical strength[34]. The mucosal EGC population are in close proximity to the epithelial cells of the colonic crypts and their terminal foot processes often extend to the epithelial basement membrane and blood capillaries in the intestinal mucosa[33,35]. EGCs are the major constituent of the enteric nervous system and outnumber enteric neurons by a factor of 4 to 10[35].
Aquaporin 4 (AQP4) is a class of water channels found in many cells of the body including the stomach, brain, lung, and skeletal muscle. It is the predominant water channel in the central nervous system. In the brain, AQP4 is believed to have a role in maintaining and regulating the brain’s functions. These same water channel cells are found in plants, which studies indicate may be involved in the development of some neural autoimmune diseases through molecular mimicry[36]. Other studies also suggest the involvement of intestinal aquaporins in early stage inflammatory bowel disease and intestinal barrier integrity impairment[37-39].
A variety of tests have been introduced for the assessment of intestinal epithelial cell damage, intestinal tight junction integrity and increased intestinal permeability to macromolecules. This includes the measurement of fatty acid binding proteins, glutathione S-transferase, claudins, the absorption of polyethylene glycols, circulating bacterial endotoxins, anti-endotoxin antibodies, zonulin level in blood, and others. These methods for the assessment of barrier integrity and function were reviewed by Grootjans et al[40].
During the past 3 years, a few published studies have shown elevations of zonulin levels in the blood of subgroups of patients with type 1 diabetes[41], metabolic syndrome[42], polycystic ovary syndrome[43], and type 2 diabetes[44]. Based on these publications, several clinical laboratories are now offering measurement of zonulin levels in blood as a biomarker of gut barrier assessment and autoimmunities.
This is despite indications that molecules such as zonulin and occludin have molecular sizes from 45000-65000 Da[45-47]. Molecules greater than 5000 Da in size are immunogenic and thus, incite immune cells into action. That is why levels of molecules such as zonulin and similar molecules, in a single individual, fluctuate from non-detectable to upregulated within minutes to hours[41,48-50]. When these immunogenic molecules enter the submucosa and then into circulation, immune system macrophages will take up the molecules, or they are processed by liver Kupffer cells. Because of this innate immune response, molecule levels will fluctuate in the blood stream. The half-life of these molecules, in the blood stream, ranges from 4 min to 4 h[48,50,51]. Indeed, this fluctuation in blood zonulin level was studied for a period of 6 d in ICU patients with sepsis, and values were varied by a factor of 2-10 from day to day[52].
The first goal of this study was to measure zonulin level at 0-, 6-, 24- and 30-h blood-draw intervals. On the other hand, because the half-life of antibodies is about 21 d, the assessment of antibodies against zonulin provides a better clinical picture with one blood draw. Consequently we decided to measure IgA and IgG antibodies against zonulin in the same blood specimens. Finally, zonulin levels in blood have not been measured in celiac disease (CD). We decided to measure both zonulin level and zonulin antibody as well as antibodies against other tight junction proteins in patients with CD.
Antigens such as zonulin, occludin, vinculin, AQP4, and GFAP were purchased from Abcam (Abcam, Cambridge, MA, United States). A zonulin enzyme-linked immunosorbent assay (ELISA) kit was purchased from MyBioSource (MyBioSource, Inc., San Diego, CA, United States). Seventy-two blood samples were obtained from 18 volunteers at intervals of 0, 6, 24, and 30 h. We did not test the 18 volunteers for allergies, diabetes, CD, NCGS, other possible GI complaints, autoimmune disorders or any other general conditions. Blood samples were then centrifuged and the separated sera were kept at -20 °C for 48 h and then used for the measurement of zonulin concentration as well as IgG and IgA levels to zonulin and other tight junction proteins. Sera from 30 random human donors aged 18-65 were purchased from Innovative Research Inc. (Southfield, MI, United States). The samples were registered as healthy human subjects. Before shipping, each blood sample was tested according to FDA guidelines for the detection of hepatitis B surface antigen, antibodies to HIV, antibodies to hepatitis C, HIV-1 RNA, and syphilis. All units yielded non-reactive/negative results for each test performed. Additionally, commercially available sera from 30 patients with CD were purchased from the Binding Site (San Diego, CA, United States), Inova (San Diego, CA, United States), Diamedix (Miami Lakes, FL, United States), Innovative Research (Novi, MI, United States), and Trina International Nanikon (Switzerland). The samples from patients with CD were confirmed by degree of positivity for IgA against both deamidated alpha gliadin peptide as well as IgA to transglutaminase-2 antibody using kits purchased from Inova Diagnostics. Zonulin levels as well as levels of IgG and IgA antibodies to zonulin and other tight junction proteins were measured in these specimens.
Determination of zonulin levels in serum was done using sandwich ELISA technique. In this kit the pre-coated antibody is human zonulin monoclonal antibody, and the detecting antibody is polyclonal antibody labeled with biotin. The standard curve ranged from 1.5-100 ng/mL with a sensitivity starting at 3 ng/mL.
Zonulin, occludin, vinculin, AQP4, and GFAP at a concentration of 100 ng/mL were each dissolved in 0.01 mol/L phosphate buffer saline with a pH of 7.4. These proteins were then diluted 1:100 in 0.1 mol/L carbonate buffer, pH 9.5, and 100 μL were added to wells of microtiter plates and incubated for 8 hours at room temperature followed by incubation at 4 °C for 16 h. The plates were washed three times with 200 μL of Tris-buffered saline (TBS) containing 0.05% Tween 20 (pH 7.4). The non-specific binding of immunoglobulins was prevented by adding 200 μL of 2% bovine serum albumin (BSA) in TBS, and incubated overnight at 4 °C. Plates were washed as previously described and then serum samples (diluted 1:50 for IgA, 1:100 for IgG) in 1% BSA in TBS containing 0.05% Tween 20 (pH 7.4) were added to duplicate wells and incubated for 1 h at room temperature.
Plates were washed, and then alkaline phosphatase goat antihuman IgG or IgA F(ab)2 fragments (KPI, Gaithersburg, MD) optimal dilution of 1:400 for IgA and 1:800 for IgG in 1% BSA-TBS were added to each appropriate well; plates were incubated for an additional hour at room temperature. After washing five times with TBS-Tween buffer, the enzyme reaction was started by adding 100 μL of 1 mg/mL paranitrophenylphosphate in diethanolamine buffer containing 1 mmol/L MgCl2 and sodium azide (pH 9.8). The reaction was stopped 45 mins later with 50 μL of 2 mol/L NaOH. The optical density (OD) was read at 405 nm by a microtiter plate reader. To exclude nonspecific binding, the ODs of the control wells coated with dry milk or human serum albumin were subtracted from all other wells. Sera from patients with CD with known high titers of IgG and IgA against various tight junction proteins were used as positive controls. Also assay normalizer positive and negative controls were used for additional levels of quality control and the calculation of the ELISA indices. For each assay the ELISA index was calculated using the following formula: ELISA index = (mean OD of sample - background)/(mean OD of serum normalizer - background)
Statistical analysis was performed to study the relationships of zonulin level, zonulin antibody, occludin antibody, vinculin antibody, AQP4 antibody, and glial fibrillary acid protein antibody. The determination of the presence of statistically significant correlative relationships was conducted with Pearson’s correlation coefficients, Kendall’s tau and Spearman’s rho. These measures are invariant to any monotonic transformation. A standard P-value of 0.05 and a confidence interval of 95% were used. Correlative analysis and the magnitude of relationship were reported. STATA software package was used to conduct all inferential and descriptive analysis.
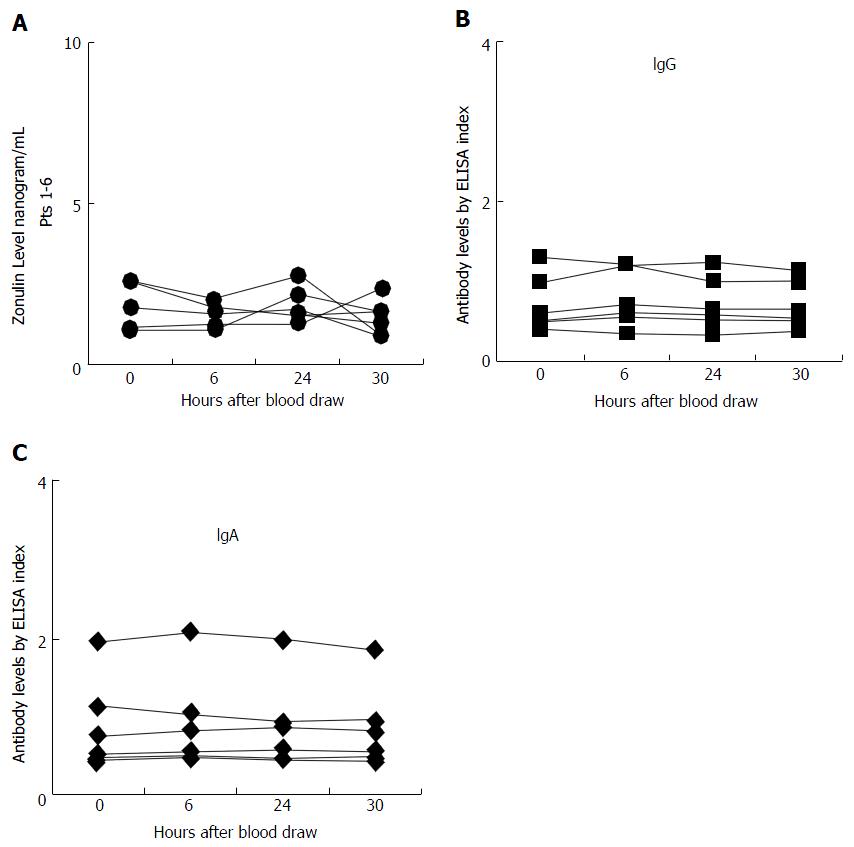
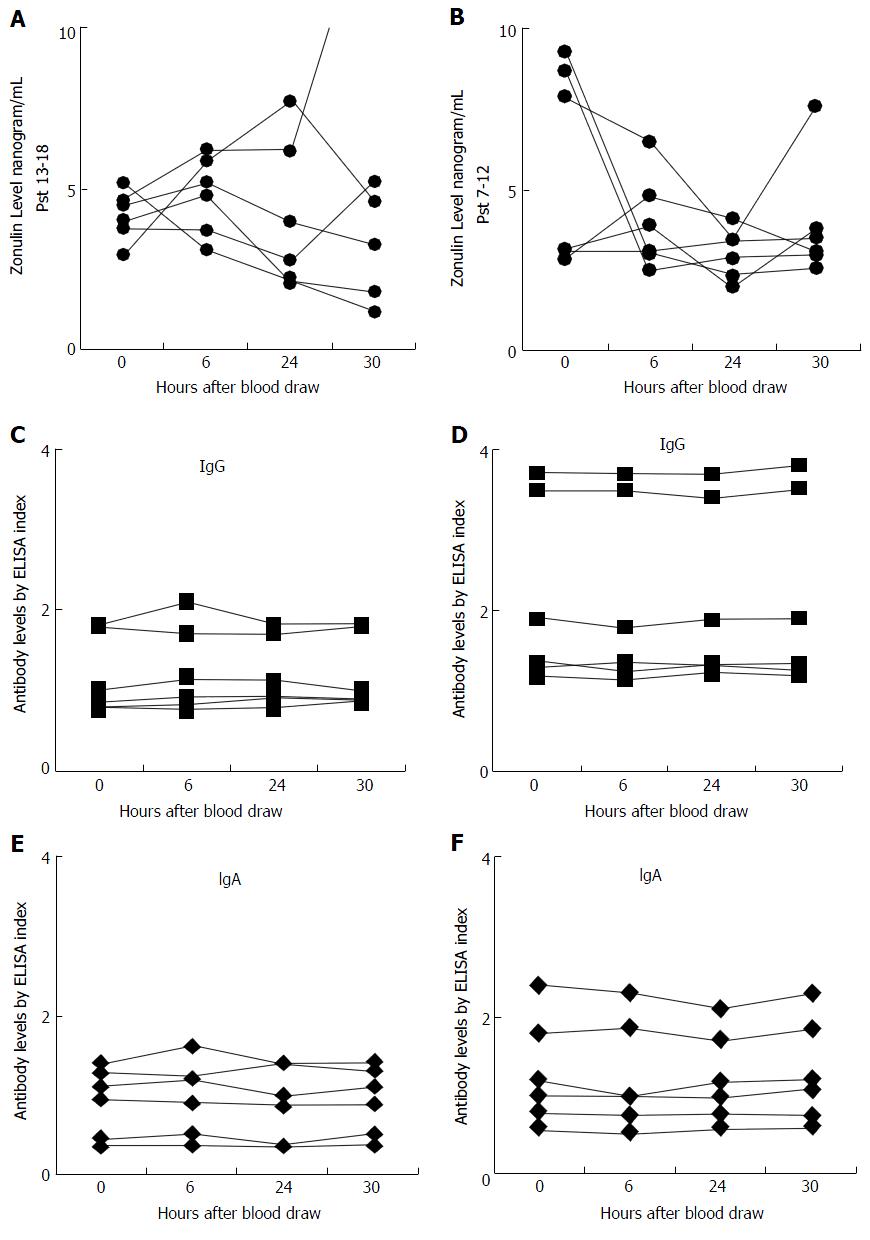
Serum zonulin level was measured in the sera of 18 control subjects at different intervals as well as in sera from 30 patients with known CD. The zonulin assay r² for the standard curve was 0.998, and the reproducibility of the duplicates varied less than 10% for samples with zonulin concentration of 2.8 ng/mL or greater. This indicated that the analytical sensitivity of the assay for serum zonulin levels was 2.8 ng/mL; for samples with zonulin levels < 2.8 the reproducibility of duplicates was not good. Of the 18 control subjects, 6 had serum zonulin levels measuring less than 2.8 ng/mL, and due to lack of sensitivity those levels did not significantly fluctuate, as shown in Figure 1. However, for the other 12 subjects who had serum zonulin levels measuring greater than 2.8 ng/mL at time 0 (Figure 2), significant fluctuation in zonulin levels was observed in almost all 12 of these subjects at the 6-, 24- or 30-h blood draws (Figure 2). Since zonulin is a relatively large molecule with the approximate size of 55000 Da, it may enter the systemic circulation after its release from the tight junctions where an immune response against it can result in the production of zonulin-specific antibodies. To demonstrate their stability, IgG and IgA antibodies to zonulin were measured in the same 18 subjects along the 30-h time course. Data presented in Figures 1B and C and 2B and C showed that both IgG and IgA antibody levels from blood obtained at 0, 6, 24, and 30 h were highly stable with variations of less than 10%.
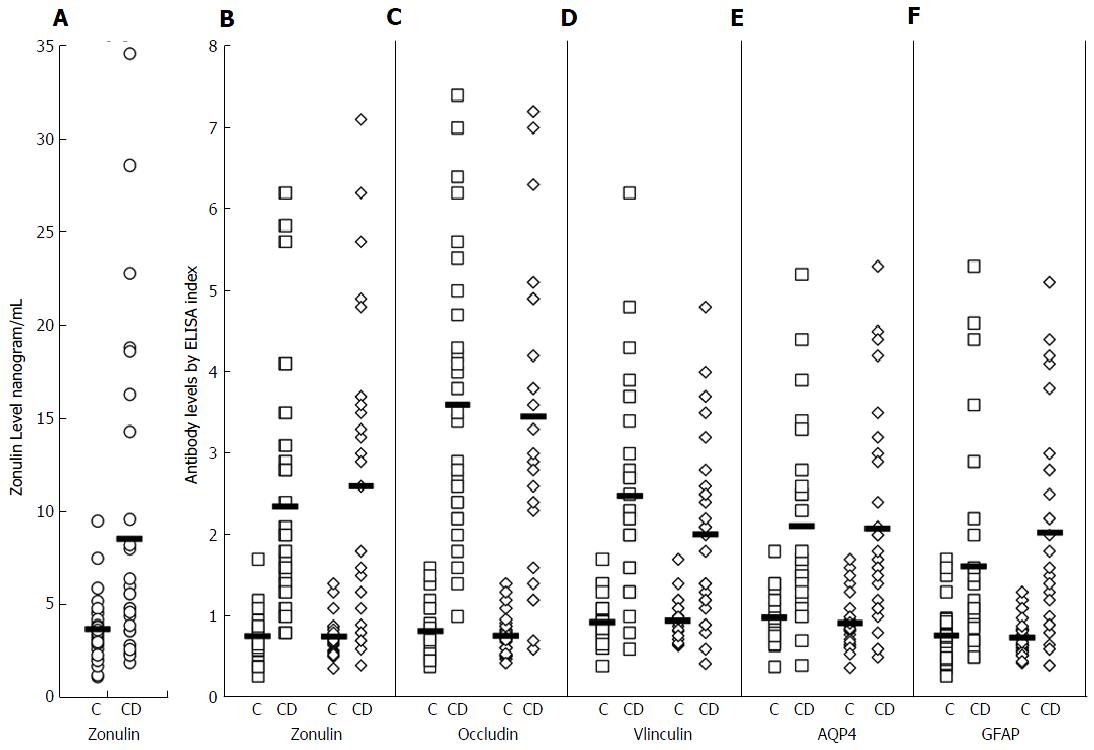
Serum zonulin levels were then measured in 30 healthy controls along with 30 patients with known celiac disease. The CD patients showed statistically significant higher serum zonulin levels with a mean of 8.5 ng/mL compared to healthy control subjects with a mean of 3.7 ng/mL (P < 0.0001). At 2 standard deviations above the mean of control subjects, 10% or 33% of the 30 CD patients demonstrated elevation of serum zonulin levels (Figure 3A).
To establish whether serum zonulin levels correlate with zonulin and other tight junction protein antibodies, zonulin, occludin, vinculin, AQP4 and GFAP antibody levels were measured in the same 30 patients and 30 controls. The distribution of IgG and IgA antibodies against tight junction proteins in these patients is shown in Figure 3B-F. At three standard deviations above the mean of healthy controls, 63% and 67%, 87% and 77%, 70% and 57% respectively had serum IgG and IgA antibody reactivity against zonulin, occludin, and vinculin. The elevation of both IgG and IgA antibodies against AQP4 was 43%; against enteric glial cells expressing GFAP it was 23% for IgG and 53% for IgA (Figure 3). Statistical analysis was performed using Ecxel to compare controls and CD patients; for zonulin level P value ≤ 0.002, while for tight junction proteins antibody levels P value ≤ 0.001. Since not all patients with celiac disease had serum zonulin elevation, we examined the correlation between serum zonulin levels and tight junction protein antibodies. Overall, we found three subgroups. Subgroup 1 consists of 9% or 30% of the CD patients with significant elevations in both zonulin level and antibodies against zonulin and other tight junction proteins when compared to the mean control levels shown in Figure 3. Subgroup 2 consists of 13% or 43% of the CD patients with a normal serum zonulin level but a significant elevation in IgG or IgA antibodies against zonulin, occludin, and other tight junction proteins. Subgroup 3 is composed of 8% or 27% of the CD patients with low or normal serum zonulin levels and low or normal IgG and IgA antibody levels to tight junction proteins. This direct or indirect correlation between serum zonulin levels and various tight junction protein antibodies is shown in Table 1, where the “r” is the best indication of correlation between serum zonulin level and antibodies against zonulin and other tight junction proteins. This table summary of the two-way scatter plot evaluation for zonulin levels and antibody measurements of the tight junction proteins demonstrates a strong positive monotonic relationship for all correlations (Table 1). Statistical analysis using Pearson’s correlations coefficient were statistically significant for 9 of the 10 correlations, showing a positive relationship, with the exceptions of zonulin level with AQP4 IgA (r = 0.24, P = 0.197).
| Tight junction protein | IgG | IgA | ||
| r | P value | r | P value | |
| Zonulin | 0.34 | 0.064 | 0.55 | 0.002 |
| Occludin | 0.60 | < 0.001 | 0.50 | 0.005 |
| Vinculin | 0.40 | 0.032 | 0.52 | 0.003 |
| Aquaporin 4 | 0.42 | 0.020 | 0.24 | 0.197 |
| Glial fibrillary acidic protein | 0.55 | 0.002 | 0.60 | < 0.001 |
Zonulin levels had statistically significant correlations with zonulin IgA and IgG antibodies; for IgG the relationship with zonulin levels and zonulin IgG was r = 0.34, P = 0.064, but the magnitude of the relationship was much stronger with zonulin IgA antibodies (r = 0.55, P = 0.002). Zonulin levels also had statistically significant correlations with vinculin IgA and IgG antibodies, and the magnitude of the relationship was likewise better with vinculin IgA antibodies (Table 1).
Due to the availability of methods for the measurement of serum zonulin levels, several laboratories in the United States have begun to offer zonulin level analysis for the detection of increased intestinal permeability in patients with autoimmune disease or other chronic inflammatory illnesses. The majority of these laboratories analyze serum zonulin levels from a single blood draw without consideration for the variability of serum zonulin levels during the time course of a single day or from day to day. The scientific basis for offering serum zonulin levels as a diagnostic indicator mainly stems from a paper published by Sapone et al[41] in 2006 which demonstrated a correlation with zonulin upregulation and increased gut permeability in a subgroup of patients with type 1 diabetes. While this study contains several key and landmark discoveries with which we agree wholeheartedly, it is our opinion that the data on which the conclusions were drawn merit further analysis before they become the sole basis for future diagnostic utility.
One of the many points used by Sapone to demonstrate a correlation between zonulin upregulation and increased gut permeability in his study is an overall elevated serum zonulin level detected in a subgroup of patients with type 1 diabetes[41]. Careful examination of the data reveals that only 42% of the patients have an elevated serum zonulin level (defined by two standard deviations above the control group mean). Furthermore, in correlating serum zonulin levels and leaky gut, the data was plotted against lactulose/mannitol levels in the same patients. These revealed a correlation coefficient of 0.36, which is very weak. In fact, it was concluded by the authors that “While these numbers are very useful in the setting of research analysis, they are insufficiently correlated to be applied to diagnostic medical use[41]”.
Another point of interest is the importance of intra-day or day-to-day variability of serum zonulin levels and their effect on use as a diagnostic marker. In an article published by Klaus et al[52] in 2013, plasma zonulin levels were measured and analyzed in ICU patients with sepsis and compared to healthy controls. Analysis of the day-to-day serum zonulin levels for septic patients presented a significant day-to-day fluctuation. In the present study we decided to extrapolate this idea further with a timed blood draw protocol in which serum was drawn at 0-, 6-, 24-, and 30-h intervals in 18 control volunteers to uncover any significant intra-day fluctuation of serum zonulin levels. Analysis of the data demonstrates significant variability of serum zonulin levels in 12 out of 18 controls with baseline zonulin level of > 2.8 ng/mL, but not in 6 samples with zonulin levels around the detection limit of the zonulin ELISA kit.
It is important to point out that our 18 volunteer subjects were controls in the sense that they had not been definitely confirmed to be positive for CD, unlike the 30 purchased samples. We did not test the 18 volunteers for allergies, diabetes, CD, NCGS, other possible GI complaints, autoimmune disorders or any other general conditions. In light of this, and given the plurality of the individual, it is not surprising that a subgroup of 6 of the 18 had lower levels than the other 12. In fact, the whole point of the study is to show the widely varying fluctuations in measuring zonulin levels, even among so-called controls.
Zonulin is a protein the size of 47000 Da. Due to many environmental factors, it is released from the lamina propria and is presented to the submucosal gut immune system, where an immune response against it results in the production of zonulin-specific antibodies[53]. This immune response against zonulin and other large molecules may be an explanation for zonulin fluctuation from sample to sample in the 12 control specimens with relatively elevated zonulin. Although the half-life in serum of zonulin has yet to be determined, it is reasonable to assume zonulin’s half-life based on the known half-lives of other similarly-sized proteins. For example, LPS, which, along with zonulin, is involved in the induction of inflammation in type-2 diabetes, has a known half-life of 2-4 min in blood[50].
This fluctuation is not unique to zonulin. For example, the presence of circulating autoantibodies directed against U1 nuclear antigen and elevated blood levels of U1 antigen were shown to be the hallmark of systemic lupus erythematosus. While antibody levels against U1 antigens was demonstrated to be highly stable, the level of U1 antigens varied from day to day[54]. This fluctuation in antigen level in the blood could be associated with antibody-antigen complex formation in the circulation, where it is expected to exit transiently[55].
Based on this mucosal and possible systemic immune response against zonulin, we measured both IgA and IgG antibodies to zonulin in the 72 specimens from the 18 control subjects. In contrast to zonulin level and its fluctuation, we observed a significant stability in antibody levels in all four blood specimens obtained at different intervals from each of the 18 subjects. The variability for both IgA and IgG antibodies against zonulin was 10% or less which is similar to inter-assay variation of the ELISA methodology used in this study (Figures 1A-C, 2A-F).
Celiac disease is an autoimmune disease in which exposure to dietary gluten peptides results in villous atrophy and crypt hypertrophy[56]. During the acute phase of CD when the tight junctions are open, zonulin expression in intestinal specimens was shown to be increased by three-fold[53]. The increased expression of zonulin due to tight junction breakdown allows zonulin presentation to the immune system and production of antibodies against zonulin in a subgroup of patients with CD[53]. In one study that followed 7 patients with CD longitudinally, the raised anti-zonulin IgA returned to normal after 3-6 mo on a gluten-free diet[57]. While zonulin upregulation in CD has been shown in several studies[48,53,57], to the best of our knowledge and throughout our literature search, zonulin elevation in the sera of patients with CD has not been examined. For this reason, we measured serum zonulin levels as well as serum antibody levels to several tight junction proteins in 30 patients with CD. At 2 standard deviations above the mean of control or a serum zonulin level of 7.1 ng/mL, 10 out of the 30, or 33% of the CD patients exhibited elevations in zonulin levels (P < 0.0001) as well as in zonulin IgA and IgG antibody levels. However, we found that an additional 10 CD patients or a total of 20 (67%) produced antibodies to zonulin without having a significant elevation in serum zonulin levels (Figure 3A and B). The detection of antibodies against zonulin in 67% of patients with CD while zonulin level elevations were detected in only 33% may be related to the zonulin fluctuation in the blood and its removal by the immune system.
Because intestinal tight junctions consist of several protein complexes including occludin, zonulin, vinculin, talin, claudin, actin, alpha-actinin, desmoglein, and others, we decided to test for antibody production to additional tight junction proteins including occludin and vinculin. We found a significant elevation in antibody production against both occludin and vinculin in addition to zonulin. In fact, levels of these antibodies were the highest against occludin. Further analysis of the data reveals that occludin IgG actually has a stronger positive correlation to serum zonulin levels (r = 0.6) than either zonulin or vinculin antibody measurement (r = 0.34-0.55 and r = 0.40-0.52 respectively). The reason for this is not immediately clear though it appears from our data that the inclusion of antibody measurement to other tight junction proteins such as occludin may enhance the diagnostic utility of these antibodies.
Recent research by Spadoni et al[58] has shed light on the existence and function of the gut vascular barrier, which in healthy individuals functions to protect the systemic blood from pathogenic microbiota. In their article they discuss the importance of the expression of intermediate GFAP by enteric glial cells in their formation and function of a gut vascular unit. In addition, AQP4 is a known component of the blood brain barrier, and is also a resident water channel in human stomach tissue, as published by Laforenza[38]. We therefore decided to measure for potential antibody formation against these two proteins in our CD group in an attempt to further understand the pathogenic process of inflammatory and autoimmune disease in the gastrointestinal (GI) tract. We were not surprised to detect elevated antibody levels to both AQP4 and GFAP in these patients because of their structural presence and utility in the gut. Antibody levels to both proteins (except AQP4 IgA) positively correlated with serum zonulin levels as demonstrated in Table 1 with “r” values ranging from 0.42-0.60. This indicates a promising area of future study into what appears to be a clear indicator that reactivity to proteins outside of the tight junction plays a role in inflammatory pathogenesis of CD and possibly other GI disorders.
Although this was a small study, the data shows significant hourly fluctuation of serum zonulin levels in control subjects, making its clinical utility questionable. In comparison, both IgG and IgA zonulin antibodies measured in the sera of the same individuals showed excellent stability over the course of the blood draw. Moreover, measuring antibodies to zonulin and other tight junction proteins revealed significant elevation in a large percentage of patients with CD, which suggests that they play a significant role in the pathogenesis of CD and possibly other autoimmune disorders. This indicates a need for further research about the role of antibodies against tight junction proteins in patients with inflammatory and autoimmune disorders.
Acknowledgment is given to Joel Bautista for the preparation of this manuscript for publication as well as the creation of the figures.
In recent times newly available technology has led to laboratories offering testing for serum levels of zonulin, a gut barrier tight junction protein, for the detection of increased intestinal permeability in patients with autoimmune disease or other chronic inflammatory disorders. However, studies demonstrate that the testable half-life of zonulin and similar molecules is extremely brief, so that the reliability of tests based just on zonulin levels is arguable. A more stable, more reliably accurate methodology is required.
The scientific basis for offering serum zonulin levels as a diagnostic indicator comes mainly from a 2006 paper by Sapone et al which demonstrated a correlation between zonulin upregulation and increased gut permeability in a subgroup of patients with type 1 diabetes. However, Sapone et al themselves concluded that their numbers were “insufficiently correlated to be applied to diagnostic medical use.”
This study shows that due to the size of zonulin and similar molecules, the normal functions of the immune system may cause zonulin levels in the blood to rise and fall from day to day, and even from hour to hour. This may cast doubt on their reliability as biomarkers, whereas the measurement of antibodies against zonulin and other tight junction proteins is comparatively much more stable, accurate and reliable. This was demonstrated in a large percentage of patients with celiac disease who showed significant elevations in antibodies against zonulin and other tight junction proteins as opposed to measurements of just zonulin level.
Measuring antibodies against zonulin and other tight junction proteins may be an important diagnostic tool in the detection of intestinal hyperpermeability in patients with inflammatory disorders and autoimmune disease.
The intestinal or gut barrier is one of the body’s most important defenses against the entry of disease-causing pathogens and food antigens. It is a complex wall of layered and inter-laced proteins. Zonulin is one of the foremost of these tight junction proteins.
Th paper is well done and helps to clarify some questions about zonulin and related molecules. It is practically very relevant.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Esposito O S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 635] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 2. | Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 3. | Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1218] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 4. | Yu YB, Li YQ. Enteric glial cells and their role in the intestinal epithelial barrier. World J Gastroenterol. 2014;20:11273-11280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994;267:G794-G799. [PubMed] |
| 6. | Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 480] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 7. | Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113 Pt 24:4435-4440. [PubMed] |
| 8. | Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (1)] |
| 9. | Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 530] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Bjarnason I, Zanelli G, Smith T, Prouse P, Williams P, Smethurst P, Delacey G, Gumpel MJ, Levi AJ. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987;93:480-489. [PubMed] |
| 11. | Fries JF, Miller SR, Spitz PW, Williams CA, Hubert HB, Bloch DA. Toward an epidemiology of gastropathy associated with nonsteroidal antiinflammatory drug use. Gastroenterology. 1989;96:647-655. [PubMed] |
| 12. | Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sáinz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683-689. [PubMed] |
| 13. | Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100-1101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 14. | Massey VL, Arteel GE. Acute alcohol-induced liver injury. Front Physiol. 2012;3:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Clemente MG, Musu MP, Frau F, Brusco G, Sole G, Corazza GR, De Virgiliis S. Immune reaction against the cytoskeleton in coeliac disease. Gut. 2000;47:520-526. [PubMed] |
| 16. | Pimentel M, Morales W, Pokkunuri V, Brikos C, Kim SM, Kim SE, Triantafyllou K, Weitsman S, Marsh Z, Marsh E. Autoimmunity Links Vinculin to the Pathophysiology of Chronic Functional Bowel Changes Following Campylobacter jejuni Infection in a Rat Model. Dig Dis Sci. 2015;60:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Das KM, Dasgupta A, Mandal A, Geng X. Autoimmunity to cytoskeletal protein tropomyosin. A clue to the pathogenetic mechanism for ulcerative colitis. J Immunol. 1993;150:2487-2493. [PubMed] |
| 18. | Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031-1049. [PubMed] |
| 19. | Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 717] [Cited by in RCA: 726] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 20. | Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745-29753. [PubMed] |
| 21. | Fanning AS, Lapierre LA, Brecher AR, Itallie CMV, Anderson JM. Protein interactions in the tight junction: the role of MAGUK proteins in regulating tight junction organization and function. Curr Top Membr. 1996;43:211-235. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 295] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Otto JJ. Vinculin. Cell Motil Cytoskeleton. 1990;16:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Geiger B, Ginsberg D. The cytoplasmic domain of adherens-type junctions. Cell Motil Cytoskeleton. 1991;20:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308:744-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 324] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Bendori R, Salomon D, Geiger B. Identification of two distinct functional domains on vinculin involved in its association with focal contacts. J Cell Biol. 1989;108:2383-2393. [PubMed] |
| 28. | Johnson RP, Craig SW. An intramolecular association between the head and tail domains of vinculin modulates talin binding. J Biol Chem. 1994;269:12611-12619. [PubMed] |
| 29. | Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 299] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448-32453. [PubMed] |
| 31. | Mierke CT, Kollmannsberger P, Zitterbart DP, Diez G, Koch TM, Marg S, Ziegler WH, Goldmann WH, Fabry B. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J Biol Chem. 2010;285:13121-13130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 32. | Gershon MD, Rothman TP. Enteric glia. Glia. 1991;4:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Cullen DK, Simon CM, LaPlaca MC. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 2007;1158:103-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:G231-G241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Vojdani A, Mukherjee PS, Berookhim J, Kharrazian D. Detection of Antibodies against Human and Plant Aquaporins in Patients with Multiple Sclerosis. Autoimmune Dis. 2015;2015:905208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Aspects Med. 2012;33:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Zhang W, Xu Y, Chen Z, Xu Z, Xu H. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett. 2011;585:3113-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Morgan E, Peplowski M, MacNaughton W. Aquaporin 3 promotes intestinal epithelial proliferation and inhibits cytokine-induced apoptosis. FASEB. 2015;29:766. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Grootjans J, Thuijls G, Verdam F, Derikx JPM, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg. 2010;2:61-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 137] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 42. | Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7:e37160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 43. | Zhang D, Zhang L, Yue F, Zheng Y, Russell R. Serum zonulin is elevated in women with polycystic ovary syndrome and correlates with insulin resistance and severity of anovulation. Eur J Endocrinol. 2015;172:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 45. | Vanuytsel T, Vermeire S, Cleynen I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1:e27321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 46. | Fasano A. Intestinal zonulin: open sesame! Gut. 2001;49:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [PubMed] |
| 48. | Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D’Agate C. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 49. | Sapone A, de Magistris L, Caravelli G, Familiar V, Riegler G, Zampini L, Catassi C, Fasano A. Serum zonulin and intestinal permeability before and after a gluten-containing meal in both type 1 diabetes and in their relatives. Dig Liver Dis. 2006;38:S75. [DOI] [Full Text] |
| 50. | Yao Z, Mates JM, Cheplowitz AM, Hammer LP, Maiseyeu A, Phillips GS, Wewers MD, Rajaram MV, Robinson JM, Anderson CL. Blood-Borne Lipopolysaccharide Is Rapidly Eliminated by Liver Sinusoidal Endothelial Cells via High-Density Lipoprotein. J Immunol. 2016;197:2390-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | Li C, Gao M, Zhang W, Chen C, Zhou F, Hu Z, Zeng C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci Rep. 2016;6:29142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, Krenn CG, Roth GA. Increased plasma zonulin in patients with sepsis. Biochem Med (Zagreb). 2013;23:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 436] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 54. | Doedens JR, Jones WD, Hill K, Mason MJ, Gersuk VH, Mease PJ, Dall’Era M, Aranow C, Martin RW, Cohen SB. Blood-Borne RNA Correlates with Disease Activity and IFN-Stimulated Gene Expression in Systemic Lupus Erythematosus. J Immunol. 2016;197:2854-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Davies KA, Peters AM, Beynon HL, Walport MJ. Immune complex processing in patients with systemic lupus erythematosus. In vivo imaging and clearance studies. J Clin Invest. 1992;90:2075-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1272] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 57. | Duerksen DR, Wilhelm-Boyles C, Parry DM. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet. Dig Dis Sci. 2005;50:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |