Published online Aug 21, 2017. doi: 10.3748/wjg.v23.i31.5645
Peer-review started: May 28, 2017
First decision: June 23, 2017
Revised: July 4, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: August 21, 2017
Processing time: 85 Days and 8.5 Hours
Hepatitis C virus (HCV) infection induces steatosis and is accompanied by multiple metabolic alterations including hyperuricemia, reversible hypocholesterolemia and insulin resistance. Total cholesterol, low-density lipoprotein-cholesterol and triglyceride levels are increased by peginterferon and ribavirin combination therapy when a sustained virologic response (SVR) is achieved in patients with HCV. Steatosis is significantly more common in patients with HCV genotype 3 but interferon-free regimens are not always effective for treating HCV genotype 3 infections. HCV infection increases fatty acid synthase levels, resulting in the accumulation of fatty acids in hepatocytes. Of note, low-density lipoprotein receptor, scavenger receptor class B type I and Niemann-Pick C1-like 1 proteins are candidate receptors that may be involved in HCV. They are also required for the uptake of cholesterol from the external environment of hepatocytes. Among HCV-infected patients with or without human immunodeficiency virus infection, changes in serum lipid profiles are observed during interferon-free treatment and after the achievement of an SVR. It is evident that HCV affects cholesterol metabolism during interferon-free regimens. Although higher SVR rates were achieved with interferon-free treatment of HCV, special attention must also be paid to unexpected adverse events based on host metabolic changes including hyperlipidemia.
Core tip: Eradication of hepatitis C virus (HCV) decreases the rate of complications, including liver-related and liver-unrelated death, and improves patient quality of life. Individuals infected with HCV have an increased risk of cardiovascular diseases and intracerebral hemorrhage, which are both associated with lipid metabolism. HCV infection causes abnormal host lipid metabolism. Treatment with interferon-based and interferon-free regimens has an impact on the eradication of HCV, as well as lipid abnormalities, during treatment and after treatment. Further observations are needed to determine the long-term effects on lipid metabolism caused by HCV and by eradication of the virus.
- Citation: Kanda T, Moriyama M. Direct-acting antiviral agents against hepatitis C virus and lipid metabolism. World J Gastroenterol 2017; 23(31): 5645-5649
- URL: https://www.wjgnet.com/1007-9327/full/v23/i31/5645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i31.5645
Hepatitis C virus (HCV) encodes at least 10 viral proteins, which include structural (core, E1, E2 and p7) and non-structural (NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins[1]. HCV is a leading cause of cirrhosis and hepatocellular carcinoma in the United States and Japan. Eradication of HCV is important for preventing death due to these liver diseases.
Associations of HCV with host lipoproteins have been reported[2]. Hepatocytes take up low-density lipoproteins (LDLs) and very low-density lipoproteins through LDL receptors. Antibodies to the HCV envelope may disrupt the HCV lipid-containing envelope[3]. These antibodies could provide an efficient mode of viral entry into liver cells[2]. HCV core protein colocalizes with apolipoprotein AII at the surface of lipid droplets, suggesting a relationship between the expression of HCV core protein and cellular lipid metabolism[4]. HCV infection or core protein expression also increases the expression of sterol regulatory element binding protein 1c and its target, fatty acid synthase (FASN), which are both involved in lipid synthesis[5].
Although interferon-free regimens could result in higher sustained virologic response (SVR) rates, Endo et al[6] reported that serum cholesterol levels were significantly increased during combination treatment with the HCV NS5B inhibitor sofosbuvir and the HCV NS5A inhibitor ledipasvir, compared with those during interferon-included regimens[7]. Of interest, the authors also observed that regardless of the regimens, total cholesterol, LDL cholesterol and high-density lipoprotein (HDL) cholesterol levels increased post-treatment[6].
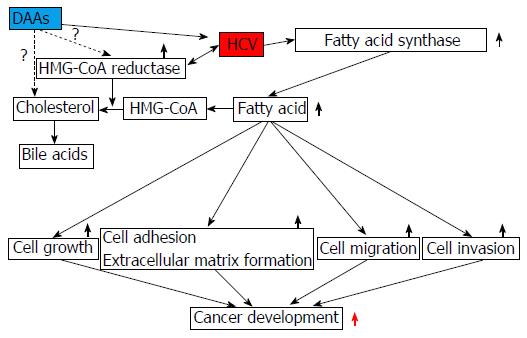
HCV increases FASN levels[5], resulting in the accumulation of fatty acids in hepatocytes (Figure 1). Fatty acids are needed for cell growth, cell adhesion, extracellular matrix formation, cell migration and cell invasion, which are essential for cancer development. Synthesis of cholesterol requires 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Among HMG-CoA reductase inhibitors, fluvastatin is an anti-HCV reagent that is used in combination with interferons[8]. Steatosis and abnormal lipid metabolism caused by HCV infection may enhance lipid droplet formation in hepatocytes[9-11]. Lipid droplets, which store neutral lipids, are required for the formation of infectious HCV particles[11].
In most cells, the major source of new sterol is endogenous synthesis from acetyl-CoA[12]. HMG-CoA is formed from acetyl-CoA and acetoacetyl-CoA[12]. There are at least 4 mechanisms for acquiring cholesterol: (1) de novo synthesis within the cells and uptake of unesterified or esterified cholesterol from the external environment via; (2) the LDL receptor (LDLR); (3) scavenger receptor class B type I (SR-BI); or (4) Niemann-Pick C1-like 1 protein (NPC1L1)[13]. HDL particles containing apoA-I can be bound by SR-BI in hepatocytes and endocrine cells[13]. Interestingly, LDLR, SR-BI and NPC1L1 are candidate receptors that may be involved in HCV[14-16].
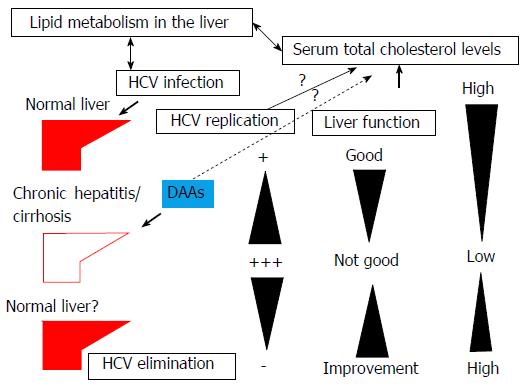
The total cholesterol pool in a human is 2.2 g/kg body weight. There is a continuous flow of cholesterol from the endoplasmic reticulum to the cell membrane and then from the plasma membrane to the liver and intestine[13]. In humans, the flux of cholesterol through the whole body is approximately 10 mg/d per kilogram body weight[17], although the half-life of plasma cholesterol is only a few days[13]. The serum lipids, total cholesterol, cholesteryl ester, LDL cholesterol and HDL cholesterol levels were significantly lower in HCV-related cirrhosis patients than in controls. HCV-related cirrhosis severely impairs liver lipid metabolism[18] (Figure 2). The serum total cholesterol level is an independent predictor of significant fibrosis[19]. When evaluating serum total cholesterol levels, liver function should also be checked (Figure 2).
Total cholesterol, LDL cholesterol and triglyceride levels are increased by peginterferon and ribavirin combination therapy when an SVR is achieved in patients with HCV genotype 1[20]. HCV eradication is closely related to lipid metabolism in patients treated with interferon-based regimens. Lange et al[21] examined the serum lipid profiles of 575 European HCV genotype 1-infected patients before, during and after treatment with peginterferon-α-2a (180 μg/wk) and ribavirin (1000-1200 mg/d) for 48 wk. The authors found substantial pretreatment hypocholesterolemia with a nonresponse to interferon-α-based therapy, and lower pretreatment cholesterol levels were an independent predictor of not attaining an SVR[21]. After treatment-induced HCV eradication, the median cholesterol levels increased above baseline. Kuo et al[22] reported that chronic HCV infection is associated with hypocholesterolemia and hypotriglyceridemia, and these conditions can be reversed by successful antiviral therapy.
Serfaty et al[23] reported that hypobetalipoproteinemia is prevalent and associated with steatosis, especially in patients infected with HCV genotype 3. It has been reported that HCV, particularly genotype 3, is associated with steatosis. Poynard et al[24] reported that an SVR, achieved with interferon-based regimens, is associated with a reduction in steatosis in HCV genotype 3 patients, as well as a correction of serum cholesterol levels at baseline. Steatosis is significantly more common in HCV genotype 3 patients than in those with other HCV genotypes, and in patients treated with peginterferon alpha-2a plus ribavirin, an SVR is associated with reduction of steatosis[25]. New treatments using HCV NS3/4A protease inhibitors have limited activity against HCV genotype 3[26]. HCV NS5B and HCV NS5A inhibitors have also performed poorly in HCV genotype 3 patients[26].
Endo et al[6] studied 276 patients with chronic HCV genotype 1b infection who were treated with interferon-free regimens. Of these 276 patients, 141 were treated with the HCV NS5A inhibitor daclatasvir plus the HCV NS3/4A inhibitor asunaprevir for 24 wk[27,28] and 135 were treated with sofosbuvir plus ledipasvir for 12 wk[6].
In the daclatasvir plus asunaprevir-SVR group, the total cholesterol levels were significantly increased throughout the observation period, and the total cholesterol levels were significantly increased at 4 wk after treatment and 12 wk after treatment, compared with those at the end of treatment (EOT)[6]. Serum LDL cholesterol levels increased after the EOT. HDL cholesterol was significantly increased throughout the treatment period, but there were no significant changes in serum triglyceride levels[6].
In the sofosbuvir plus ledipasvir-SVR group, the total cholesterol levels were markedly increased from the early stage of therapy and lasted until the EOT[6]. The total cholesterol levels were sharply decreased after the EOT (P < 0.001). Changes in the LDL cholesterol levels were quite similar to those found in the total cholesterol levels. After the EOT, the HDL cholesterol levels were decreased compared to those during therapy (P < 0.001), but there were no significant changes in triglyceride levels[6]. Hashimoto et al[29] also reported that the increase in cholesterol levels during treatment was much greater in the sofosbuvir plus ledipasvir-SVR group than in daclatasvir plus asunaprevir-SVR group. The authors also observed that a rapid increase in the serum LDL cholesterol concentration during the interferon-free treatment was associated with the type of regimen and decrease in the HCV core protein level. Morales et al[30] also reported that there was a significant increase in the LDL and total cholesterol levels after treatment, compared to the pre- and post-treatment laboratory data from 52 patients receiving sofosbuvir-based regimens, but there was no change in body mass index between pre-and post-treatment. Among HIV/HCV coinfected patients, an increase in LDL cholesterol was observed after an SVR was achieved with interferon-free treatment[31].
HCV infection induces steatosis and is accompanied by multiple metabolic alterations, such as hyperuricemia, reversible hypocholesterolemia, insulin resistance, arterial hypertension and visceral adipose tissue expansion[32-34]. Eradication of HCV with interferon-free regimens increases total cholesterol levels. Because of the worsening nutritional status as an adverse event of interferon-based regimens, it is difficult to examine the effects of HCV on serum lipid profiles[6]. It is evident that HCV affects cholesterol metabolism during interferon-free regimens because these regimens have no influence on the nutritional status of the host[6]. The increase in cholesterol levels during treatment was much greater in the sofosbuvir plus ledipasvir-SVR group than in the daclatasvir plus asunaprevir-SVR group[6,29]. Although higher SVR rates were achieved with interferon-free treatment of HCV, special attention must also be paid to unexpected adverse events based on host metabolic changes.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin:
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guerrieri F, Ikura Y S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
| 1. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Prince AM, Huima-Byron T, Parker TS, Levine DM. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J Viral Hepat. 1996;3:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Hijikata M, Shimizu YK, Kato H, Iwamoto A, Shih JW, Alter HJ, Purcell RH, Yoshikura H. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J Virol. 1993;67:1953-1958. [PubMed] |
| 4. | Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 499] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Bose SK, Kim H, Meyer K, Wolins N, Davidson NO, Ray R. Forkhead box transcription factor regulation and lipid accumulation by hepatitis C virus. J Virol. 2014;88:4195-4203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Endo D, Satoh K, Shimada N, Hokari A, Aizawa Y. Impact of interferon-free antivirus therapy on lipid profiles in patients with chronic hepatitis C genotype 1b. World J Gastroenterol. 2017;23:2355-2364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Ooka Y, Ogasawara S, Chiba T, Saito T, Haga Y. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. Int J Mol Sci. 2017;18:906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 470] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Nakamura M, Kanda T, Nakamoto S, Miyamura T, Jiang X, Wu S, Yokosuka O. No correlation between PNPLA3 rs738409 genotype and fatty liver and hepatic cirrhosis in Japanese patients with HCV. PLoS One. 2013;8:e81312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 981] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 12. | Spady DK, Dietschy JM. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983;24:303-315. [PubMed] |
| 13. | Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 800] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 14. | Monazahian M, Böhme I, Bonk S, Koch A, Scholz C, Grethe S, Thomssen R. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J Med Virol. 1999;57:223-229. [PubMed] |
| 15. | Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017-5025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 891] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 16. | Sainz B Jr, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, Uprichard SL. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18:281-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 17. | Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Vere CC, Streba CT, Streba L, Rogoveanu I. Lipid serum profile in patients with viral liver cirrhosis. Med Princ Pract. 2012;21:566-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, Lin R, Samarasinghe D, Liddle C, McCaughan GW. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology. 2004;39:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Tada S, Saito H, Ebinuma H, Ojiro K, Yamagishi Y, Kumagai N, Inagaki Y, Masuda T, Nishida J, Takahashi M. Treatment of hepatitis C virus with peg-interferon and ribavirin combination therapy significantly affects lipid metabolism. Hepatol Res. 2009;39:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Lange CM, von Wagner M, Bojunga J, Berg T, Farnik H, Hassler A, Sarrazin C, Herrmann E, Zeuzem S. Serum lipids in European chronic HCV genotype 1 patients during and after treatment with pegylated interferon-α-2a and ribavirin. Eur J Gastroenterol Hepatol. 2010;22:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Kuo YH, Chuang TW, Hung CH, Chen CH, Wang JH, Hu TH, Lu SN, Lee CM. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Serfaty L, Andreani T, Giral P, Carbonell N, Chazouillères O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 431] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 25. | Reddy KR, Govindarajan S, Marcellin P, Bernstein D, Dienstag JL, Bodenheimer H Jr, Rakela J, Messinger D, Schmidt G, Ackrill A, Hadziyannis SJ. Hepatic steatosis in chronic hepatitis C: baseline host and viral characteristics and influence on response to therapy with peginterferon alpha-2a plus ribavirin. J Viral Hepat. 2008;15:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Ampuero J, Romero-Gómez M, Reddy KR. Review article: HCV genotype 3 - the new treatment challenge. Aliment Pharmacol Ther. 2014;39:686-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 28. | Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Haga Y, Sasaki R, Wu S, Nakamoto S, Imazeki F. Daclatasvir plus Asunaprevir Treatment for Real-World HCV Genotype 1-Infected Patients in Japan. Int J Med Sci. 2016;13:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Hashimoto S, Yatsuhashi H, Abiru S, Yamasaki K, Komori A, Nagaoka S, Saeki A, Uchida S, Bekki S, Kugiyama Y. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS One. 2016;11:e0163644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Morales AL, Junga Z, Singla MB, Sjogren M, Torres D. Hepatitis C eradication with sofosbuvir leads to significant metabolic changes. World J Hepatol. 2016;8:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Townsend K, Meissner EG, Sidharthan S, Sampson M, Remaley AT, Tang L, Kohli A, Osinusi A, Masur H, Kottilil S. Interferon-Free Treatment of Hepatitis C Virus in HIV/Hepatitis C Virus-Coinfected Subjects Results in Increased Serum Low-Density Lipoprotein Concentration. AIDS Res Hum Retroviruses. 2016;32:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, Loria P. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | González-Reimers E, Quintero-Platt G, Rodríguez-Gaspar M, Alemán-Valls R, Pérez-Hernández O, Santolaria-Fernández F. Liver steatosis in hepatitis C patients. World J Hepatol. 2015;7:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Chang ML. Metabolic alterations and hepatitis C: From bench to bedside. World J Gastroenterol. 2016;22:1461-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |