Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4615
Peer-review started: February 19, 2017
First decision: March 16, 2017
Revised: March 23, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: July 7, 2017
Processing time: 141 Days and 22.8 Hours
To investigate the characteristic radiologic findings of cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) which can be differentiated from other similar bowel disease and to assess their clinical behavior.
Twenty pathologically and clinically confirmed CMUSE patients (males:females = 8:12; mean age: 40.4 years) between March 2002 and August 2015 from seven academic centers in South Korea were retrospectively reviewed. We evaluated small bowel series (SBS; n = 25), computed tomography (CT) enterography (n = 21), magnetic resonance (MR) enterography (n = 2), and abdominopelvic CT (n = 18) images, focusing on enteric and perienteric manifestations. Any change in radiologic features during follow-up period was recorded. We evaluated clinical data including presenting symptoms, laboratory finding and presence of relapse from electronic medical records. Histopathologic findings were also evaluated.
The main symptoms were abdominal pain (n = 12) and anemia (n = 10). All patients showed small bowel strictures (n = 52, mean: 2.6 per patient) on initial CT/MR, located in the ileum (n = 47) or jejunum (n = 5). Strictures showed short-length (mean: 10.44 mm) and circumferential bowel wall thickening (mean: 5.56 mm) with layered enhancement (n = 48) that were also noted on initial SBS (n = 36) with shallow ulcers (n = 10). Some ulcerative lesions or wall thickening progressed into strictures on follow-up SBS/CT, and some strictures revealed recurrent ulceration on follow-up SBS. There were no penetrating disease features like fistula or abscess and no gastrointestinal tract involvement except the small bowel. Nine patients experienced disease recurrence (median relapse-free period: 32 mo) even post-operatively. Histopathologic features of surgically resected specimens were characterized as multiple superficial ulcerations confined to mucosa or submucosa and multiple strictures.
Under characteristic radiologic findings with multiple short-segmental strictures and/or shallow ulcers of the small intestine, CMUSE should be considered when assessing patients with recurrent abdominal pain and anemia.
Core tip: The patients with multiple unexplained strictures and recurrent abdominal pain are challenging to most physician in daily practice. Because cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) is a rare disease, this disease entity is frequently misdiagnosed as other small bowel diseases and often undergoes unnecessary surgery. Then, we focused on the characteristic radiologic and clinical findings of CMUSE that can differentiate the disease from other similar bowel diseases. In our study, characteristic radiologic features of CMUSE were multiple short strictures and/or shallow ulcers of the small intestine without significant bowel obstruction. Some strictures had recurrent shallow ulceration that might progress to more severe strictures. Under these radiologic findings with relapsing episodes, CMUSE should be considered when assessing patient with recurrent abdominal pain and anemia.
- Citation: Hwang J, Kim JS, Kim AY, Lim JS, Kim SH, Kim MJ, Kim MS, Song KD, Woo JY. Cryptogenic multifocal ulcerous stenosing enteritis: Radiologic features and clinical behavior. World J Gastroenterol 2017; 23(25): 4615-4623
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4615
Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) is a rare disease characterized by chronic and recurrent ileus resulting from multiple unexplained strictures and superficial ulcerations of the small intestine[1,2]. At present, CMUSE is diagnosed mainly based on clinical and macroscopic imaging features of the small intestine. However, CMUSE is frequently misdiagnosed as other ulcerative small bowel diseases, such as Crohn’s disease (CD) or nonsteroidal anti-inflammatory drugs (NSAIDs)-induced enteropathy, because of similar clinical symptoms and nonspecific laboratory findings[3,4].
Although double-balloon endoscopy seems to be the most important diagnostic tool, it is still difficult to diagnose CMUSE even after ruling out the above-mentioned diseases due to limited diagnostic tools and lack of characteristic radiologic features on computed tomography (CT) or small bowel series (SBS). Consequently, most patients with CMUSE have suffered from improper diagnosis and management, including unnecessary surgery.
To our knowledge, most studies about CMUSE have focused on clinical findings and vague radiologic findings such as the presence or absence of strictures and ulcers[3,4]. Hence, this study aims to investigate the characteristic findings of CMUSE that can differentiate the disease from other similar bowel diseases and to assess their clinical behavior through analysis of serial radiologic imagings including SBS, routine abdominopelvic CT (APCT), CT enterography (CTE), and/or magnetic resonance (MR) enterography (MRE) as part of routine abdominal examination in a clinical setting.
Between March 2002 and August 2015, 36 patients with suspected CMUSE were included from seven academic centers in South Korea. The diagnostic criteria for CMUSE were based on previous studies[1,5]: (1) unexplained small bowel strictures; (2) superficial ulcer in the mucosa and submucosa; (3) chronic or relapsing ulcerative stenosis and abdominal pain; (4) no signs of systemic inflammation; and (5) persistent and occult blood loss from the gastrointestinal (GI) tract except during bowel rest or postoperative period. Patients’ records were reviewed to confirm CMUSE as final diagnosis. Twelve patients were excluded due to lack of any pathologic proof suggesting superficial mucosal or submucosal disease. Four patients were also excluded because their final histopathology showed transmural inflammation (n = 2) or panmural inflammation with bowel perforation (n = 2). Patients (8 males, 12 females) were aged 15-64 years (mean, 40.4 ± 14.8) when CMUSE was diagnosed. All patients underwent at least one radiologic examination including SBS, APCT, CTE, or MRE. This study was approved by the IRB of Asan medical center, Seoul, Korea (number, 2016-0235).
Clinical data included sex, age, presenting symptoms, period from onset to hospital visit, the initial diagnosis, and laboratory findings [hemoglobin (Hb), albumin, C-reactive protein (CRP), and white blood cell count (WBC)] at first admission. Moreover, information on follow-up duration, presence of relapse, relapsing interval, treatment methods, treatment outcome, histopathologic findings, total number of each radiologic examination, and total number of surgical resection were collected from the electronic medical records. The type, number, and descriptions of endoscopic inspections were also recorded.
Remission was defined as removal of the presenting symptoms such as bleeding, abdominal pain, or anemia, and relapse as recurrence of bleeding, abdominal pain, or anemia; need for additional medication; or evidence of a recurrent imaging finding by any imaging after remission.
All analysis images were available on a picture archiving and communications system (PACS). SBS were performed in 16 patients, after they fasted for at least 8 h, using a barium and/or methylcellulose solution. A single-contrast examination was performed to evaluate the entire small intestine, followed by a double-contrast examination after peroral administration of an effervescent agent in 9 patients.
APCT and CTE were performed in 11 and 13 patients, respectively, using multidetector CT scanners (Light Speed plus, Light Speed 16 or Light Speed VCT 64, GE Healthcare, Milwaukee, WI, United States; Somatom sensation 16 or Somatom Definition, Siemens Medical Systems, Erlangen, Germany; Toshiba Aquilion 64, Toshiba Medical System, Tokyo, Japan) with intravenous contrast material (Ultravist 370, Schering, Berlin, Germany; Omnipaque, Amersham Health, Inc., Princeton, NJ, United States). APCT consisted of a non-contrast enhanced image and a portal phase image with a fixed 60-70-s delay following the injection of intravenous contrast media.
CTE consisted of a non-contrast image, enteric phase, and portal venous phase image. The enteric- and portal- phase scans were obtained with a 20-25-s delay using a bolus-triggering method or a fixed 40-s delay, and a fixed 70-80-s delay after injection, respectively. CTE or MRE was performed after ingestion of 1200 mL of neutral oral contrast agent using a polyethylene glycol electrolytic solution (Colyte, Taejun Pharmacy, Seoul, South Korea) or 2.5% sorbitol solution.
MRE was acquired with a 3.0-T system (Ingenia, Philips Healthcare, Best, The Netherlands) after intravenous injection of a spasmolytic agent (Buscopan, Boehringer Ingelheim, Ingelheim, Germany). T2-weighted images, diffusion-weighted images (with b-factors of 0 and 900 s/mm2), and dynamic contrast-enhanced images were obtained. Coronal dynamic fat suppressed spoiled gradient-echo T1-weighted sequences, including unenhanced imaging and enteric and portal venous phase sequences, were performed with 0.2 mL/kg body weight gadoterate meglumine (Dotarem; Guerbet, Villepinte, France). Enteric phase was obtained 7 s after the contrast material first arrived at the iliac bifurcation after injection. Subsequent phase imaging was performed continuously after completion of the prior phase with a brief pause to let the patient breathe.
All radiologic images were reviewed by three board-certified radiologists (Kim AY, > 10 years of experience in abdominal imaging; Hwang J, 5 years; and Kim JS, 2 years) in consensus.
In the first stage, all initial radiologic examinations obtained during the initial hospital visit were analyzed, focusing on ulcer, stricture, bowel wall thickening, and other ancillary findings. Radiologic jejunal involvement was regarded as involvement of the proximal two-fifths of the intact small bowel[6].
For strictures, defined as upstream dilatation of small bowel > 3 cm in diameter, the number, location, lesion length, and interval between each lesion were recorded during all radiologic studies. Small bowel obstruction was also evaluated with a 4-grading scale (absent, low-grade, high-grade, or complete obstruction). While a low-grade obstruction was defined as dilatation of proximal small bowel 3-4 cm in diameter, high-grade obstruction was defined as proximal small bowel dilatation of > 4 cm in diameter. Complete obstruction was defined as high-grade obstruction without distal passage of contrast media. Any deep penetrating disease features such as sinus, fistula, or abscess were recorded.
On APCT/CTE/MRE, thickness (one-side wall thickness), enhancement pattern (layered or transmural), enhancement degree (mild, similar enhancement to adjacent normal bowel; moderate, more than normal bowel wall enhancement; strong, marked enhancement compared with normal bowel) of stricture were recorded respectively. In addition, the presence of segmental bowel wall thickening (> 10 cm in length) was recorded, noting the length, thickness, enhancement pattern, and enhancement degree. Mesenteric manifestations such as mesenteric lymphadenopathy, mesenteric hypervascularity or infiltration, and ascites were recorded. In addition, the number, location, lesion length, shape (eccentric, circumferential, linear or aphthoid) of ulcer, and interval between each lesion were evaluated on SBS.
In the second stage, follow-up images (11 SBS, 7 APCT, 13 CTE, and 1 MRE) were assessed focusing on any change from the previous image findings for each patient. Follow-up images without any presenting symptom or image findings changes were excluded from analysis.
Clinical and laboratory findings are summarized in Table 1. The median (range) period from initial onset of symptoms to hospital visit was 8 (0.1-25) years. The main symptoms were abdominal pain (60%, 12/20) and anemia (50%, 10/20); four patients presented with GI bleeding (20%, 4/20). On initial laboratory test, the mean Hb level was 9.9 ± 2.4 g/dL and mean albumin level was 3.4 ± 2.2 g/dL. WBC and CRP levels were within normal ranges for most patients or slightly increased in two and three patients, respectively.
| Patient No./sex/age (yr) | Duration until hospital visit (yr)1 | Presenting symptom at admission | Hb (g/dL) | SBS | APCT/CTE/MRE | DE/CE | Treatment | Treatment response (No. of relapse) | Relapse-free period (yr) | No of surgery | Follow up period (yr)2 |
| 1/M/23 | 9.6 | Abdominal pain | 13.3 | 1 | 3/-/- | 1/- | Surgery | Relapse (1) | 1.1 | 1 | 2 |
| 2/F/56 | 22.9 | Abdominal pain | 11.4 | ND | 1/-/- | -/- | Steroid, 5-ASA, surgery | Remission | 1 | 3 | |
| 3/M/49 | 10.4 | Abdominal pain | 13.7 | ND | 1/1/- | -/1 | Surgery | Remission | 1 | 1.5 | |
| 4/F/61 | 25 | Anemia | 10.5 | 1 | 1/-/- | -/- | Surgery | Remission | 1 | 2.9 | |
| 5/F/29 | 1 | Anemia | 7.4 | 2 | 6/1/- | 1/1 | Steroid, 5-ASA, surgery | Relapse (1) | 1 | 1 | 9.9 |
| 6/F/37 | 8.6 | Anemia | 8 | 2 | 1/-/- | -/- | Steroid, 5-ASA, surgery, others (albumin) | Relapse (2) | 2.7, 6.4 | 1 | 13.4 |
| 7/F/45 | 7.5 | Anemia, GI bleeding | 7.6 | 3 | -/3/- | 2/- | Steroid, surgery | Relapse (2) | 0.5, 3.7 | 1 | 9.4 |
| 8/F/49 | 5.1 | Abdominal pain | 12.9 | ND | -/2/1 | 2/- | Steroid, surgery | Relapse (3) | 1.4, 6, 1.7 | 3 | 9.8 |
| 9/M/54 | 3.5 | Abdominal pain, anemia | 10.4 | 1 | -/1/- | -/- | Surgery | Remission | 1 | 2.5 | |
| 10/M/30 | 4.8 | Abdominal pain | 8.5 | 5 | 1/4/- | -/1 | 5-ASA, surgery | Relapse (2) | 7.9, 0.7 | 2 | 10 |
| 11/F/16 | 14.6 | Abdominal pain | 7.7 | ND | -/1/- | -/- | Surgery | Remission | 1 | 0.5 | |
| 12/M/24 | 0.9 | Abdominal pain, anemia, GI bleeding | 11.3 | 1 | -/2/- | -/- | Surgery, others | Relapse (1) | 2.5 | 1 | 3.6 |
| (conservative treatment) | |||||||||||
| 13/M/41 | 12 | Abdominal pain | 9 | 1 | -/1/- | 1 /- | Steroid | Remission | 0 | 0 | |
| 14/F/64 | 23 | Abdominal pain | 12.3 | ND | -/-/1 | -/- | Surgery | Remission | 1 | 0 | |
| 15/F/39 | 10.3 | Abdominal pain | 9 | 1 | -/3/- | -/- | 5-ASA, surgery | Relapse (1) | 2 | 1 | 6.2 |
| 16/M/60 | 10 | Anemia, GI bleeding | 9.1 | ND | -/1/- | -/1 | Steroid, 5-ASA | Remission | 0 | 1.1 | |
| 17/F/15 | 4 | Abdominal pain, anemia | 7.7 | 4 | 1/-/- | -/- | Steroid, 5-ASA, surgery | Relapse (3) | 3, 4, 4, 3 | 4 | 13.5 |
| 18/F/34 | 0.1 | Anemia | 7.6 | 1 | 1/1/- | 1/- | Steroid, tuberculosis drug | Remission | 0 | 10.6 | |
| 19/M/33 | 1.2 | Anemia | 6.1 | 1 | 1/-/- | 1/1 | Steroid | Remission | 0 | 4.5 | |
| 20/F/48 | 0.2 | GI bleeding | 13.4 | 1 | 1/-/- | -/- | Surgery | Remission | 1 | 4.5 |
The median (range) follow-up period was 4 (0-13.5) years. The initial diagnosis before confirmative diagnosis included CD, intestinal tuberculosis, post-ischemic stricture, CMUSE, vasculitis, protein-losing enteropathy, and non-granulomatous jejunoileitis.
Double-balloon enteroscopy (DBE) or capsule endoscopy (CE) was performed in 10 patients (Table 1). All patients showed multiple ulcers on endoscopic examination: jejunal (n = 1), ileal (n = 6), and both (n = 3). Ulcerative lesions revealed discrete margin and various shapes including linear, circular, geographic, or irregular. Some showed transverse or circular alignment. The intervening mucosa appeared normal. Nine patients also showed multiple strictures: jejunal (n = 2), ileal (n = 6), and both (n = 1). All strictures showed short segmental involvement and were often accompanied by ulcers. Full small intestine evaluation failed in five patients with DBE (5/9, 55.5%) and in two patients with CE (2/5, 40%) due to stricture.
A total of 15 biopsy specimens were available from 11 patients who underwent endoscopic examination. All lesions revealed chronic, nonspecific inflammation with/without ulceration.
Sixteen patients underwent surgical resection of small intestine. Histopathologic features of these resected specimens were characterized as multiple superficial ulcerations confined to mucosa or submucosa and multiple strictures. Some strictures were ulcerated. Ulcerative lesions showed linear or circular configuration and some were associated with submucosal thickening by fibrosis. Some ulcerative lesions were accompanied by chronic crypt inflammation, pyloric gland metaplasia, or lymphoid aggregates. Inflammatory change was not seen in the small bowel segment between ulcers. There was no giant cell granuloma, transmural inflammation, fistula or fissural ulceration, or vasculitis features.
A total of 21 CTE, 18 APCT, 2 MRE, and 25 SBS were finally included in the analysis. Among them, 8 CTE, 11 APCT, 1 MRE, and 14 SBS were performed during the patients’ initial hospital visit.
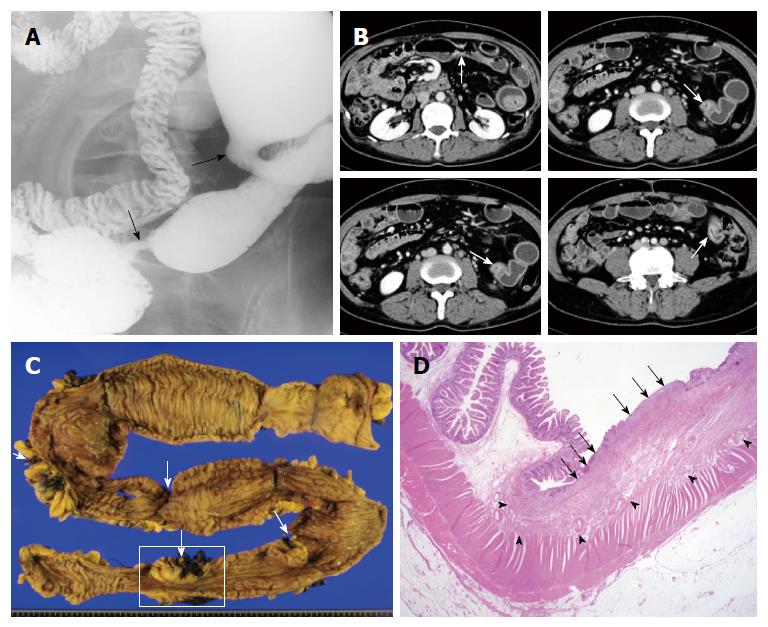
On initial CT or MRI, all patients revealed strictures with a total of 52 (mean number per patient, 2.6; Table 2 and Figure 1) analyzed. Most strictures were located in the ileum (90.4%), followed by jejunum (9.6%). While most patients showed only ileal strictures (17/20, 85%), two had only jejunal strictures and one had both. Mean stricture length and thickness was 10.44 ± 3.95 mm and 5.56 ± 1.58 mm, respectively. On contrast-enhanced CT or MRI, most strictures showed moderate (75%) or strong (9.6%) enhancement with a layered pattern (92.3%; Figure 1). The median (range) interval length between strictures was 4 (1.5-20) cm.
| Stricture | n = 52 |
| Total No. (mean No. per patient, range) | 52 (2.6, 1-6) |
| Location (Jejunum/Ileum) | 5/47 |
| Stricture length (mm), mean ± SD | 10.44 ± 3.95 |
| Stricture thickness (mm), mean ± SD | 5.56 ± 1.58 |
| Enhancement pattern (layered/transmural) | 48/4 |
| Degree of enhancement (mild/moderate/strong) | 8/39/5 |
| Interval between strictures (cm), median (range) | 4 (1.5-20) |
| Segmental bowel wall thickening | n = 4 |
| Location (Jejunum/Ileum/both) | 0/3/1 |
| Wall thickening length (cm), mean ± SD | 36.25 ± 13.77 |
| Wall thickening thickness (mm), mean ± SD | 7.75 ± 1.26 |
| Wall thickening pattern (circumferential/eccentric) | 2/2 |
| Enhancement pattern (layered/transmural) | 3/1 |
| Degree of enhancement (mild/moderate/strong) | 1/2/1 |
| Small bowel obstruction | |
| Absent/low grade/high grade/complete | 13/5/2/0 |
| Mesenteric manifestation | |
| Mesenteric lymphadenopathy | 6 |
| Mesenteric hypervascularity or infiltration | 4 |
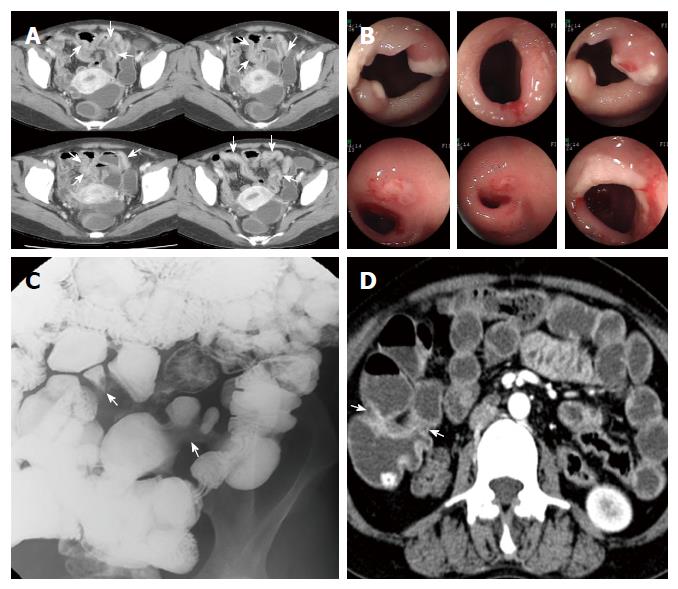
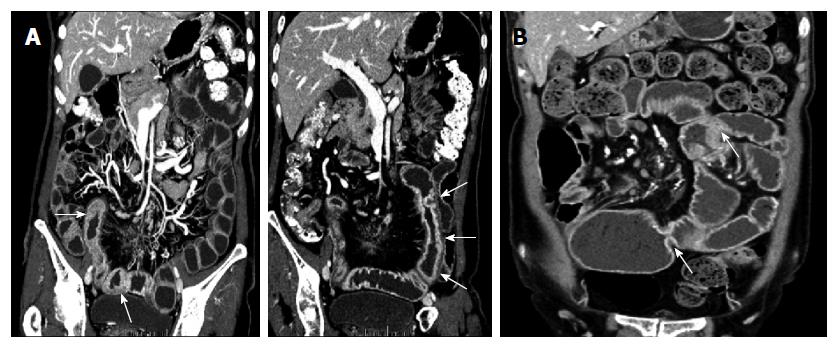
Four out of 20 patients showed segmental bowel wall thickening on the initial CT or MRI (Figures 2 and 3). Mean length and thickness of this bowel wall thickening was 36.25 ± 13.77 cm and 7.75 ± 1.26 mm, respectively (Table 2).
Despite of multiple strictures, high-grade small bowel obstruction was noted in only two patients on CT or MR images. Most patients showed no or low-grade small bowel obstruction.
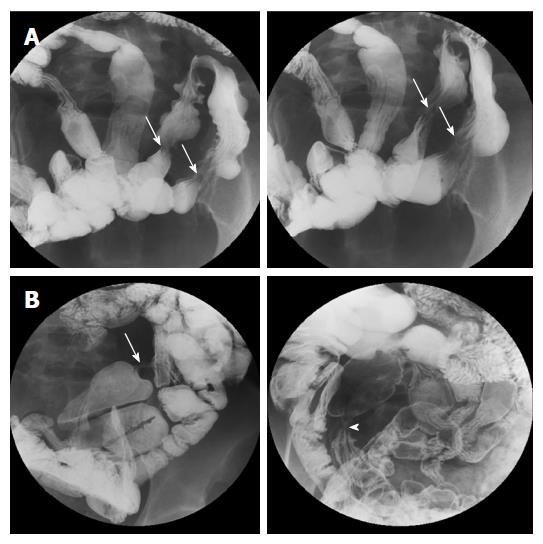
On initial SBS, 36 strictures were detected in 13 patients (mean number of stricture per patient, 1.8; Table 3, Figures 1 and 4). Most strictures were located in the ileum (97.2%), and the mean stricture length was 9.81 ± 4.65 mm. Median (range) interval length between strictures was 4.5 (1.5-20.4) cm. Seven patients also showed 10 ulcers on SBS; nine were located in the ileum and showed eccentric alignment (90%), and six ulcerative lesions were accompanied by stricture (Figure 4).
| Stricture | n = 36 |
| Total No. (mean No. per patient, range) | 36 (1.8, 1-5) |
| Location (Jejunum/Ileum) | 1/35 |
| Stricture length (mm), mean ± SD | 9.81 ± 4.65 |
| Interval between strictures (cm), median (range) | 4.5 (1.5-20.4) |
| Ulcer | n = 10 |
| Total No. (mean No. per patient, range) | 10 (0.5, 1-2) |
| Location (Jejunum/Ileum) | 1/9 |
| Ulcer length (mm), mean ± SD | 11.3 ± 5.58 |
| Ulcer pattern (eccentric/circumferential/linear/ aphthous) | 9/1/0 |
| Small bowel obstruction | |
| Absent/low grade/high grade/complete | 7/5/2/0 |
Follow-up imaging (11 SBS, 7 APCT, 13 CTE, and 1 MRE) was performed in 14 patients. While there was no significant interval change in radiologic features for three of the patients, new strictures and/or ulcers were detected at another site with a progressive nature in seven patients, even post-operatively. On follow-up SBS examinations, ulcers were accompanied with stricture and these ulcerative strictures progressed into more severe strictures over time (Figure 4). Segmental bowel wall thickening newly developed in three patients on follow-up CT. Finally, seven patients had segmental bowel wall thickening on the initial or follow-up CT. In these patients, this radiologic feature completely improved on the next follow-up CT, but eventually, strictures manifested within this thickened bowel segment during the follow-up period (Figures 2 and 3). Disease recurrence at the surgical anastomotic site was noted in only one patient.
There was no penetrating disease features, including fissure, fistula, or abscess, by CT/MRI or SBS. GI involvement other than small intestine was not seen.
Sixteen patients underwent surgical resection (80%), and three received reoperation due to recurred disease (median surgery number, 1). In eight patients, surgery was performed as initial treatment on first admission. Five patients underwent surgery due to ineffective medical therapy during hospitalization. Only four patients showed remission with medical treatment: steroid only (n = 2), steroid with oral 5-aminosalicylic acid (n = 1), and steroid with tuberculosis drugs (n = 1). None of them had recurrence.
As the final outcome, nine patients (45%) revealed disease recurrence (median relapse-free period, 2.6 years; range, 0.5-7.9). The median (range) relapse number was 2 (1-3).
To our knowledge, no study focused on the radiologic features of CMUSE to assist in the differentiation from other similar bowel diseases. Even radiologic information previously reported was relatively simple descriptions and mostly confined to SBS features[1,4,5,7,8].
In this study, the main radiologic manifestations of CMUSE patients were multiple strictures and/or ulcers of the small intestine without significant bowel obstruction. These results were similar to those previously reported. However, bowel wall strictures in this study were short and thin with a layered enhancement pattern (< 2 cm long and < 1 cm thick) with layered enhancement pattern on axial imaging tools such as CT or MRI. Thus, short, thin strictures with layered enhancement may represent relatively superficial disease process, accounting for no or low-grade small bowel obstruction. Histopathologic proof might provide a more convincing explanation as the stricture segment of a surgical specimen revealed fibrosis confined only to submucosa (Figure 1).
The distinctive radiologic finding was the presence of long-segmental bowel wall thickening on CT. Overall, seven patients showed this feature on initial and/or follow-up CT. Three of them underwent DBE at the same time and showed multiple, shallow ulcerations of the small intestine in accordance with CT results. On follow-up CT, this segmental bowel wall thickening progressed to multiple strictures with improved thickening (Figures 2 and 3), confirmed by follow-up endoscopic examination. Thus, this segmental bowel wall thickening on CT might represent the presence of multiple, superficial ulcerations, although there was no direct correlation between these two diagnostic tools in all patients.
One different point of our study results is that ulcers were less frequently depicted on SBS with shallow ulcerations mainly located in the ileum, contrary to previous endoscopic reports of multiple ulcers mainly in jejunum and proximal ileum[5]. We think that the relatively small numbers of ulcers in our radiologic examinations might be due to delayed hospital visits during the healing stage of the ulcer with stricture formation. Early in CMUSE disease progression, more ulcers might be detected and more strictures noted in advanced stages. Considering the long median period from initial onset of symptoms to the hospital visits in our study (8 years), less frequent ulcer depiction can be predicted. Another assumption is the relatively high failure rate of endoscopic examination due to strictures. In this study, the endoscopic failure rate was 55.5% in DBE and 40% of CE; hence, the endoscopic examination failure rate might be relevant to the larger numbers of clinically reported jejunal ulcers.
More importantly, during follow-up studies, these ulcers tended to progress to strictures with ulceration healing (Figure 4). Conversely, strictures accompanied by shallow ulceration during disease recurrence progressed to more severe strictures. Perhaps shallow ulcers and their healing, in the repeated disease process, lead to more severe strictures that required surgical intervention. Furthermore, recurrent ulcers or strictures rarely involved the anastomosis site of the small intestine after surgical resection. Strictures and ulcers showed no consistent direction such as the mesenteric border lining. Like previous reports, there were no penetrating disease features, including fissure, fistula formation, or abscess[1,3,7]. Mesenteric lymphadenopathy or mesenteric infiltration was rarely seen and there was no other GI involvement except the small intestine. Hence, we think that these radiologic features might be helpful to differentiate CMUSE from other inflammatory bowel disease, especially CD.
The main clinical manifestations of the patients in this study were abdominal pain and anemia, resulting from chronic or relapsing subileus episodes associated with small intestinal strictures and small intestinal occult blood loss, respectively. In addition, most patients did not show biological signs of systemic inflammatory reaction. These clinical features largely met the description of previous reports[1,3,5,9,10].
Contrary to previous major reports[1,3,5,9,10], it was difficult to establish the efficacy of steroid therapy in this study. Only four patients were treated only with steroids; others were treated with combination of steroid with oral 5-aminosalicylic acid or tuberculosis drugs. Furthermore, the efficacy of steroid therapy is debatable. Matsumoto et al[5] reported steroid, oral 5-aminosalicylic acid, and oral azathioprine failed to induce mucosal healing or prevent recurrence of small intestine ulcers. Kim et al[11] reported on steroid-refractory disease cases. Similarly, in this study, two out of four patients treated with steroid only required surgery due to disease relapse and were no longer steroid-sensitive.
As a final outcome, the major therapeutic choice of this study population was surgery. Eight patients underwent surgery during the first hospital visit and eight on subsequent visits, with three patients requiring two or more surgical treatments. Another cause of surgical treatment was retention of capsule endoscope in the small intestine due to stricture (n = 2). With the long median pre-diagnostic period (8 years) and high disease relapse rate (45%), higher choice of surgery rather than medicine could be acceptable in this study due to the frequent manifestation of strictures rather than ulcers. Perhaps the conflicting responsiveness to steroid use in CMUSE patients is linked closely to different disease stages, i.e., early stage manifesting as multiple shallow ulcers only and the advanced stage as severe strictures. In addition, some investigators reported significant steroid dependency[1,7]. Hence, further investigation with more collective data is necessary regarding proper CMUSE treatment.
In summary, multiple short strictures and/or shallow ulcers of the small intestine without significant bowel obstruction are characteristic radiologic features of CMUSE on CT, MRI, or SBS. The strictures are short, thin, mainly located in the ileum, and show layered enhancement. Some have recurrent shallow ulceration that may progress to more severe strictures. Considering that routine clinical practice involves the use of CT and SBS abdominal imaging tools, as well as the risk of endoscopic failure due to strictures, comprehensive understanding regarding the characteristic radiologic features on CT/MRI and SBS would be helpful to diagnose CMUSE and prevent unnecessary surgery. Therefore, under these radiologic findings with relapsing episodes, CMUSE should be considered when assessing patient with recurrent abdominal pain and anemia.
The authors thank the Korean Society of Abdominal Radiology for their support in data collection.
Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) is a rare disease characterized by chronic and recurrent ileus resulting from multiple unexplained strictures and superficial ulcerations of the small intestine. At present, CMUSE is diagnosed mainly based on clinical and macroscopic imaging features of the small intestine. However, CMUSE is frequently misdiagnosed as other ulcerative small bowel diseases, because of similar clinical symptoms and nonspecific laboratory findings. Knowledge about characteristic radiologic features of CMUSE and their clinical course would be very useful in differentiating this disease entity from other similar bowel diseases.
Because this disease is very rare, previous studies about CMUSE have mainly focused on clinical manifestations. Even radiologic information previously reported was relatively simple description and mostly confined to small bowel series (SBS) feature such as the presence or absence of stricture.
Multiple short strictures and/or shallow ulcers of the small intestine without significant bowel obstruction are characteristic radiologic features of CMUSE on computed tomography (CT), MRI, or SBS. The strictures are short, thin, mainly located in the ileum, and show layered enhancement. Some have recurrent shallow ulceration that may progress to more severe strictures. This comprehensive understanding regarding the characteristic radiologic features on CT/MRI and SBS would be helpful to diagnose CMUSE and prevent unnecessary surgery.
Under characteristic radiologic findings with multiple short-segmental strictures and/or shallow ulcers of the small intestine, CMUSE should be considered when assessing patients with recurrent abdominal pain and anemia.
CT enterography or MR enterography is, basically, specialized imaging technique for small bowel evaluation, which needs mandatory requirement of optimal bowel distention, proper suppression of active bowel movement, and multiplanar imaging analysis. Bowel enhancement timing is also important in these imaging modalities.
It’s a well-written manuscript, the characteristic radiologic features on CT/MRI and SBS would be helpful to diagnose CMUSE and prevent unnecessary surgery.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Freeman HJ, Wani IA S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Perlemuter G, Guillevin L, Legman P, Weiss L, Couturier D, Chaussade S. Cryptogenetic multifocal ulcerous stenosing enteritis: an atypical type of vasculitis or a disease mimicking vasculitis. Gut. 2001;48:333-338. [PubMed] |
| 2. | Chang DK, Kim JJ, Choi H, Eun CS, Han DS, Byeon JS, Kim JO. Double balloon endoscopy in small intestinal Crohn’s disease and other inflammatory diseases such as cryptogenic multifocal ulcerous stenosing enteritis (CMUSE). Gastrointest Endosc. 2007;66:S96-S98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Chung SH, Park SU, Cheon JH, Kim ER, Byeon JS, Ye BD, Keum B, Shim KN, Jung SA, Kim JO. Clinical Characteristics and Treatment Outcomes of Cryptogenic Multifocal Ulcerous Stenosing Enteritis in Korea. Dig Dis Sci. 2015;60:2740-2745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Kwon SO, Kim YS, Kim SY, Hong SW, Lee HK, Moon JS. A case of cryptogenic multifocal ulcerous stenosing enteritis: differential diagnosis from Crohn’s disease. J Gastrointestin Liver Dis. 2012;21:309-312. [PubMed] |
| 5. | Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007;66:S99-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Park SK, Yang SK, Park SH, Park SH, Kim JW, Yang DH, Jung KW, Kim KJ, Ye BD, Byeon JS. Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol. 2013;47:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Kohoutová D, Bures J, Tycová V, Bártová J, Tachecí I, Rejchrt S, Vacek Z, Repák R, Kopácová M. Severe cryptogenic multifocal ulcerous stenosing enteritis. A report of three cases and review of the literature. Acta Medica (Hradec Kralove). 2010;53:25-29. [PubMed] |
| 8. | Perlemuter G, Chaussade S, Soubrane O, Degoy A, Louvel A, Barbet P, Legman P, Kahan A, Weiss L, Couturier D. Multifocal stenosing ulcerations of the small intestine revealing vasculitis associated with C2 deficiency. Gastroenterology. 1996;110:1628-1632. [PubMed] |
| 9. | Debray C, besancon F, hardouin JP, martin E, marche C, khoury K. [Cryptogenetic plurifocal ulcerative stenosing enteritis]. Arch Mal Appar Dig Mal Nutr. 1964;53:193-206. [PubMed] |
| 10. | Matsumoto T, Iida M, Matsui T, Yao T, Watanabe H, Yao T, Okabe H. Non-specific multiple ulcers of the small intestine unrelated to non-steroidal anti-inflammatory drugs. J Clin Pathol. 2004;57:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Kim CW, Yu CS, Yoon YS, Yoon SN, Lim SB, Kim JC. Steroid-refractory cryptogenic multifocal ulcerous stenosing enteritis. Am J Surg. 2011;202:e48-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |