Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4311
Peer-review started: February 27, 2017
First decision: April 10, 2017
Revised: April 22, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: June 21, 2017
Processing time: 113 Days and 16.7 Hours
Intrahepatic cholangiocarcinoma (ICC) is a relatively rare form of liver cancer with a poor prognosis. The therapeutic options for patients with advanced ICC are limited and usually ineffective. There is currently no approved targeted therapy for ICC, although accumulating evidence supports inhibition of the PI3K/Akt/mTOR signaling pathway as a promising therapeutic strategy in the treatment of ICC. Here, we report a patient with stage IV ICC harboring a PIK3CA mutation who responded well to the mTOR inhibitor everolimus. Computed tomography and magnetic resonance imaging demonstrated shrinkage of the tumor and maintenance of a partial response for 6.5 mo after everolimus treatment as the best response. To the best of our knowledge, this is the first clinical case report in the literature of clinical benefit from everolimus treatment in an ICC patient with PIK3CA mutation.
Core tip: We report a stage IV intrahepatic cholangiocarcinoma (ICC) patient harboring a PIK3CA mutation who responded well to the mTOR inhibitor everolimus. Computed tomography and magnetic resonance imaging demonstrated shrinkage of the tumor and the maintenance of partial response for 6.5 mo after everolimus treatment as the best response. To the best of our knowledge, this is the first clinical case report in the literature of an ICC patient with PIK3CA mutation deriving benefit from everolimus treatment.
- Citation: Bian JL, Wang MM, Tong EJ, Sun J, Li M, Miao ZB, Li YL, Zhu BH, Xu JJ. Benefit of everolimus in treatment of an intrahepatic cholangiocarcinoma patient with a PIK3CA mutation. World J Gastroenterol 2017; 23(23): 4311-4316
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4311.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4311
Cholangiocarcinoma, the second most common primary malignancy of the liver, is divided into four categories based on the anatomic location of origin within the biliary system as follows: intrahepatic, hilar, distal biliary, and ampullary[1,2]. Complete surgical resection remains the only potentially curative option for patients with intrahepatic cholangiocarcinoma (ICC). However, because most cases are diagnosed at advanced stages, only one-third of ICC tumors are amenable for surgical resection with a 5-year survival rate of 20%-40%[3,4], and unresectable ICC carries a dismal prognosis. Systemic chemotherapy, conventional external beam radiation, and brachytherapy are established standard treatments but show limited success and are associated with toxicity[2].
Doublet gemcitabine and cisplatin therapy is currently proposed as the standard first-line therapy for patients with an advanced disease; however, the efficiency is limited[5]. Locoregional therapy appears to have a better effect against ICC[6], but more data are needed to define its role. To date there has been no approved targeted molecular therapy for ICC and identification of a definitive treatment remains an unmet need. Recently, the use of next-generation sequencing (NGS) technologies has enabled the identification of frequently observed actionable molecular alterations that hold the promise of improving the management of advanced ICC patients.
The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway plays an essential role in regulating cell survival and proliferation[6]. Mutation in PIK3CA has been reported in 8% of ICC patients according to The Cancer Genome Atlas (TCGA) database, and activating mutations of PIK3CA that are related to tumorigenesis and cancer progression have been identified in a broad spectrum of malignant tumors[7,8]. Therefore, inhibition of the mTOR pathway represents a promising therapeutic strategy in the treatment of ICC. Everolimus is a novel macrolide derivative of rapamycin that inhibits mTOR and was approved by the Food and Drug Administration (FDA) for the treatment of advanced renal cell carcinoma[9] and other cancer types[10]. However, whether everolimus is effective against ICC is unknown. In studies of ICC-related cancers, in vitro and in vivo results demonstrated that everolimus exhibits cytotoxic and antimetastatic effects in a cholangiocarcinoma cell line[11]. These results suggest that everolimus may be a potential therapeutic agent for the treatment of patients with ICC possessing an aberrant PI3K/Akt/mTOR signaling pathway.
Here, we report a patient with stage IV ICC harboring a PIK3CA mutation who responded well to the mTOR inhibitor everolimus, demonstrating that inhibition of the PI3K/Akt/mTOR signaling pathway is a promising therapeutic avenue for ICC. To the best of our knowledge, this is the first clinical case report in the literature of an ICC patient with PIK3CA mutation deriving benefit from everolimus treatment.
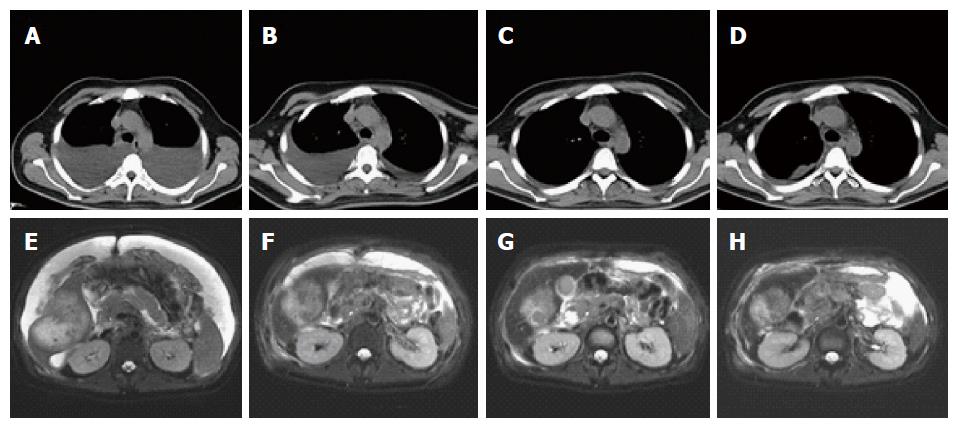
A 31-year-old Chinese man presented with 1-mo history of progressive abdominal distension and was admitted to hospital. A computed tomography (CT) scan revealed a space-occupying mass in the liver with massive peritoneal effusion and some pleural effusion in both sides of the chest. The patient had no history of alcohol abuse, hepatitis, or cirrhosis, and denied any family history of cancers and other hereditary diseases. He was transferred to our hospital in December 2015. Physical examination revealed a distended abdomen with tenderness and muscle guarding, and his abdominal girth was measured at 105 cm. No icteric sclera or xanthochromia was detected, and the Murphy’s sign was negative. Liver function test indicated that the levels of total protein and albumin were 57.6 g/L and 32.4 g/L, respectively, which were below normal and indicated malnutrition and hypoalbuminemia, whereas bilirubin and aminotransferase were within the normal range. The tumor markers carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), cancer antigen 19-9 (CA19-9), and carbohydrate antigen 72-4 (CA72-4) were all within the normal range. Further magnetic resonance imaging (MRI) of the liver showed a 9.9 cm × 7.4 cm mass at the posterior right lobe of the liver with multiple swollen retroperitoneal lymph nodes and massive peritoneal effusion (Figure 1A and E). The Eastern Cooperative Oncology Group (ECOG) performance score was 2-3. Abdominal paracentesis was performed repeatedly to relieve abdominal distension, which was also used to collect exfoliated tumor cells from the ascites for cytological diagnosis; however, no tumor cells were detected. Consequently, core needle biopsy of the liver mass was performed and the specimens were sent for pathological evaluation, which indicated a poorly differentiated adenocarcinoma. Immunohistochemical staining showed that the cells were positive for CK7, CK19, CK8, and CEA and negative for glypican-3, hepatocyte marker, and vimentin. These results suggested a diagnosis of stage IV ICC (cT3N1M1).
There is currently no standard treatment for ICC, and chemotherapy is generally ineffective. This patient was not suitable for chemotherapy due to his poor physical condition, therefore he was recommended for NGS to identify possible therapeutic targets. His liver biopsy specimen and matched blood sample were sent for NGS panel analysis after consent was obtained from the patient himself and his family. We detected all genomic alteration types, including base substitutions, insertions and deletions, copy number alterations, and rearrangements, for more than 390 genes commonly associated with cancers. While we were waiting for the NGS results, intraperitoneal chemotherapy with cisplatin plus Endostar was initiated for the control of ascites. The patient was perfused with three cycles of cisplatin 30 mg in 250 mL of normal saline (NS) and Endostar 60 mg in 250 mL of NS every 5 d. Amino acids, fat emulsion, and albumin were added during the treatment for nutritional support. Before the first intraperitoneal chemotherapy the patient felt aggravated chest stuffiness and a CT scan demonstrated increased pleural effusion. After three cycles of treatment, the patient’s abdominal girth decreased from 105 cm to 85 cm and the CT scan indicated decreased peritoneal effusion (Figure 1B and F).
In January 2016, the genomic profile of the patient revealed three somatic mutations, including E545G mutation of the PIK3CA gene (NM_006218), R132C mutation of the IDH1 gene (NM_005896) and c.714+1G>T mutation of the PBRM1 gene (NM_018313). As preclinical data suggest that activating mutations in PIK3CA may predict a sensitivity to inhibitors of the PI3K/AKT/mTOR pathway[11], the patient received everolimus (10 mg orally daily), provided off-label with insurance approval. CT scans showed a notable decrease in pleural effusion and tumor shrinkage after everolimus treatment for 2 mo (Figure 1C and G). One month after everolimus treatment, the levels of total protein and albumin increased to 76.2 g/L (normal range, 63-82 g/L) and 42.9 g/L (normal range, 35-50 g/L), respectively, and the ECOG performance score was evaluated as 1. MRI showed shrinkage of the tumor from 9.9 cm × 7.4 cm to 6.4 cm × 4.3 cm, which was considered a partial response (PR) according to Response Evaluation Criteria in Solid Tumors criteria.
In May 2016, the patient suddenly displayed icterus, icteric sclera, and xanthochromia. On May 8, the levels of total bilirubin, direct bilirubin, indirect bilirubin, and ammonia increased to 145, 122, 23, and 64 μmol/L, respectively. Serum levels of alanine transaminase (ALT) and aspartate aminotransferase (AST) levels were 404 and 321 U/L and those of total protein and albumin were 76 and 42 g/L, respectively. His abdominal girth increased from 85 cm to 87 cm, and color Doppler ultrasound demonstrated a slight increase in pleural and peritoneal effusion (Figure 1D and H). The Child-Pugh score of the patient was classified as Class B, therefore the everolimus dosage was decreased to 5 mg orally once daily (half the standard dose) from May 10 with consideration of his liver dysfunction. Magnetic resonance cholangiopancreatography showed a high-position biliary obstruction, and local three-dimensional conformal radiotherapy (3DCRT) was started on May 16. However, the 3DCRT was ineffective because the icteric index and liver function showed continued aggravation with no improvement in biliary obstruction. On May 30, surgical biliary drainage was performed to reduce icterus. The patient continued to receive everolimus (5 mg once daily) to the present time. At the latest follow-up on July 16, the tumor response remained as stable disease and the progression-free survival (PFS) had lasted for more than 6.5 mo from the initial treatment with everolimus.
Genomic profiling of the patient revealed three somatic mutations: E545G mutation of PIK3CA and mutations of IDH1 and PBRM1 genes. Activating mutations in the region encoding the p110α subunit of PI3K (PIK3CA) have been identified in a broad spectrum of malignant tumors. Codon 545 is a hotspot for PIK3CA mutations that are known to activate the PI3K/Akt/mTOR signaling pathway[12,13]. However, the E545K substitution is much more common in cancers than the E545G mutation; E545K represents 25% of all PIK3CA mutations[14] whereas E545G has only been reported in a few carcinomas[15]. In vitro research studies comparing the activity of mutant PIK3CA proteins have shown that the E545G substitution displays strong transforming activity in chicken embryo fibroblasts, although its effect is lower than that of the more common E542K and E545K substitutions[16].
Both the IDH1 and PBRM1 genes are recurrently mutated in ICC with frequencies of 4.9% and 18%, respectively[17]. Mutations in IDH1 and IDH2 are confined to the active site and result in the production of a neomorphic metabolite 2-hydroxyglutarate (2HG), which is normally found in scarce amounts, through NADPH-dependent reduction of 2-OG to the R enantiomer of 2HG. Frequent somatic hotspot mutations in IDH1 have been identified in gliomas, chondrosarcomas, myeloid leukemias, and other cancers. Suppression of endogenous mutant IDH1 expression was recently reported in HT180, a fibrosarcoma cell line with a native IDH1 R132C heterozygous mutation[18]. PBRM1 is associated with chromatin remodeling and is crucial for the suppression of aggressive clear cell renal cell carcinoma (ccRCC) tumors[19]. However, there are no preclinical or early clinical data implicating IDH1 and PBRM1 as biomarkers for targeted cancer therapy in ICC.
The mTOR inhibitor everolimus has been approved by the FDA for the treatment of advanced RCC, subependymal giant cell astrocytoma (SEGA), and progressive neuroendocrine tumors (PNET) of pancreatic origin as monotherapy, and of advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane. The effect of everolimus is clearly proven in many cancers, especially those with PIK3CA mutations. Phase III clinical trials suggested that patients with HER2-positive advanced breast cancer with PIK3CA mutations could derive a PFS benefit from everolimus[20]. Recent research demonstrated that mTOR pathway activating mutations confer sensitivity to everolimus regardless of cancer type[21]. A case report supported the use of everolimus monotherapy in a patient with refractory metastatic gastric cancer harboring PIK3CA and pS6 aberrations[22]. In our case study, everolimus exhibited good efficacy in an ICC patient. The adverse effects of everolimus on liver dysfunction may be a cause for concern. In May 2011 the FDA approved everolimus for the treatment of PNET of pancreatic origin. The approval was based on a randomized controlled trial of everolimus 10 mg/d (n = 207) vs placebo (n = 203) in patients with unresectable, locally advanced, or metastatic pancreatic neuroendocrine tumors. The median PFS for patients treated with everolimus was 11.0 mo vs 4.6 mo for patients treated with placebo. However, deaths occurred in seven patients treated with everolimus and one patient treated with placebo[23]. The causes of death in patients treated with everolimus included one case with hepatic failure. Although there is no direct evidence that everolimus is related to hepatic failure, hepatobiliary patients should be kept under strict surveillance when taking everolimus.
Generally speaking, ICC is a relatively rare cancer, accounting for 3% of gastrointestinal malignancies, and has a poor prognosis. The limited number of patients leads to a lack of clinical trials conducted specifically in ICC patients, which precludes the generation of clinical practice guidelines establishing a “standard of care” for these patients. At present, the prognosis for patients diagnosed with unresectable ICC is poor, with a life expectancy of approximately 1 year and actuarial probability of survival of 5% at 5 years with traditional chemotherapy[2,24]. To date, no molecular targeted therapy has been proven effective for ICC. To the best of our knowledge, this is the first clinical case report in the literature of benefit from everolimus treatment in an ICC patient with a PIK3CA mutation. This patient is still considered progression-free with good quality of life at the latest follow-up, highlighting the potential of the PI3K/Akt/mTOR signaling pathway as a therapeutic target in ICC.
To our knowledge, this case represents the first report of an ICC patient with a PIK3CA mutation who derived benefit from everolimus treatment. However, whether the presence of mutation of IDH1 and PBRM1 contributed to the patient’s response to targeted therapy is unclear, and the reason for the patient’s response to everolimus and prolonged survival has yet to be elucidated.
A 31-year-old Chinese man presented with 1-mo history of progressive abdominal distension and was admitted to hospital.
Physical examination revealed a distended abdomen with tenderness and muscle guarding, and his abdominal girth was measured at 105 cm.
Hepatocellular carcinoma, intrahepatic cholangiocarcinoma, stage IV intrahepatic cholangiocarcinoma (cT3N1M1).
Liver function test indicated that the levels of total protein and albumin were 57.6 g/L and 32.4 g/L, respectively, which were below normal and indicated malnutrition and hypoalbuminemia, whereas bilirubin and aminotransferase were within the normal range. The tumor markers carcinoembryonic antigen, alpha-fetoprotein, cancer antigen 19-9, and carbohydrate antigen 72-4 were all within the normal range.
A computed tomography (CT) scan revealed a space-occupying mass in the liver with massive peritoneal effusion and some pleural effusion in both sides of the chest.
Pathological examination revealed intrahepatic cholangiocarcinoma (ICC).
The genomic profile of the patient revealed three somatic mutations, including E545G mutation of the PIK3CA gene, R132C mutation of the IDH1 gene and c.714+1G>T mutation of the PBRM1 gene. Based on the gene alteration testing report and the clinical trial studies, the patient received everolimus (10 mg orally daily), provided off-label with insurance approval.
The mTOR inhibitor everolimus has been approved by the FDA for the treatment of advanced RCC, subependymal giant cell astrocytoma, and progressive neuroendocrine tumors of pancreatic origin as monotherapy, and of advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane. To the best of our knowledge, this is the first clinical case report in the literature of an ICC patient with a PIK3CA mutation deriving benefit from everolimus treatment.
Results observed in this case encourage further research on the activity of everolimus in ICC, based on the presence of PIK3CA mutation. This could lead to a selection of ICC patients to be treated with this drug, and could help identify a novel treatment strategy for PIK3CA-mutated ICC patients.
Very interesting case report. In this study, the authors report a stage IV ICC patient harboring a PIK3CA mutation who responded well to the mTOR inhibitor everolimus.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Imai K, McHenry L, Shimizu Y S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 2. | Mahadevan A, Dagoglu N, Mancias J, Raven K, Khwaja K, Tseng JF, Ng K, Enzinger P, Miksad R, Bullock A. Stereotactic Body Radiotherapy (SBRT) for Intrahepatic and Hilar Cholangiocarcinoma. J Cancer. 2015;6:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1069] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 4. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 557] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 5. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3157] [Article Influence: 210.5] [Reference Citation Analysis (1)] |
| 6. | Vogl TJ, Schwarz W, Eichler K, Hochmuth K, Hammerstingl R, Jacob U, Scheller A, Zangos S, Heller M. Hepatic intraarterial chemotherapy with gemcitabine in patients with unresectable cholangiocarcinomas and liver metastases of pancreatic cancer: a clinical study on maximum tolerable dose and treatment efficacy. J Cancer Res Clin Oncol. 2006;132:745-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Schneck H, Blassl C, Meier-Stiegen F, Neves RP, Janni W, Fehm T, Neubauer H. Analysing the mutational status of PIK3CA in circulating tumor cells from metastatic breast cancer patients. Mol Oncol. 2013;7:976-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Dunlap J, Le C, Shukla A, Patterson J, Presnell A, Heinrich MC, Corless CL, Troxell ML. Phosphatidylinositol-3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Res Treat. 2010;120:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2374] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 10. | Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 722] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 11. | Moolthiya P, Tohtong R, Keeratichamroen S, Leelawat K. Role of mTOR inhibitor in cholangiocarcinoma cell progression. Oncol Lett. 2014;7:854-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Hafner C, López-Knowles E, Luis NM, Toll A, Baselga E, Fernández-Casado A, Hernández S, Ribé A, Mentzel T, Stoehr R. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc Natl Acad Sci USA. 2007;104:13450-13454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2599] [Cited by in RCA: 2759] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 15. | López-Knowles E, Hernández S, Malats N, Kogevinas M, Lloreta J, Carrato A, Tardón A, Serra C, Real FX. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66:7401-7404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Gymnopoulos M, Elsliger MA, Vogt PK. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci USA. 2007;104:5569-5574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Prensner JR, Chinnaiyan AM. Metabolism unhinged: IDH mutations in cancer. Nat Med. 2011;17:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Jin G, Pirozzi CJ, Chen LH, Lopez GY, Duncan CG, Feng J, Spasojevic I, Bigner DD, He Y, Yan H. Mutant IDH1 is required for IDH1 mutated tumor cell growth. Oncotarget. 2012;3:774-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Pawłowski R, Mühl SM, Sulser T, Krek W, Moch H, Schraml P. Loss of PBRM1 expression is associated with renal cell carcinoma progression. Int J Cancer. 2013;132:E11-E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | André F, Hurvitz S, Fasolo A, Tseng LM, Jerusalem G, Wilks S, O’Regan R, Isaacs C, Toi M, Burris H. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J Clin Oncol. 2016;34:2115-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Lim SM, Park HS, Kim S, Kim S, Ali SM, Greenbowe JR, Yang IS, Kwon NJ, Lee JL, Ryu MH. Next-generation sequencing reveals somatic mutations that confer exceptional response to everolimus. Oncotarget. 2016;7:10547-10556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Park JH, Ryu MH, Park YS, Park SR, Na YS, Rhoo BY, Kang YK. Successful control of heavily pretreated metastatic gastric cancer with the mTOR inhibitor everolimus (RAD001) in a patient with PIK3CA mutation and pS6 overexpression. BMC Cancer. 2015;15:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2114] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 24. | Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861-4870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |