Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4270
Peer-review started: February 1, 2017
First decision: February 23, 2017
Revised: March 9, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: June 21, 2017
Processing time: 146 Days and 18.9 Hours
To analyze the outcomes of living-donor liver transplantation (LDLT) using left-lobe (LL) or right-lobe (RL) small-for-size (SFS) grafts.
Prospectively collected data of adult patients who underwent LDLT at our hospital in the period from January 2003 to December 2013 were reviewed. The patients were divided into the RL-LDLT group and the LL-LDLT group. The two groups were compared in terms of short- and long-term outcomes, including incidence of postoperative complication, graft function, graft survival, and patient survival. A SFS graft was defined as a graft with a ratio of graft weight (GW) to recipient standard liver volume (RSLV) (GW/RSLV) of < 50%. The Urata formula was used to estimate RSLV.
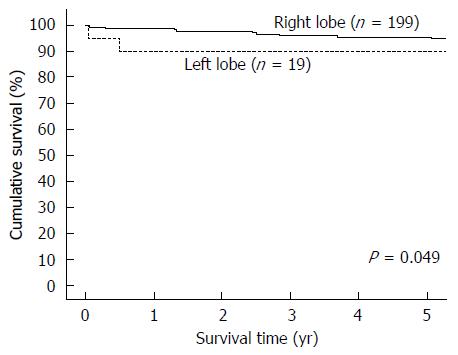
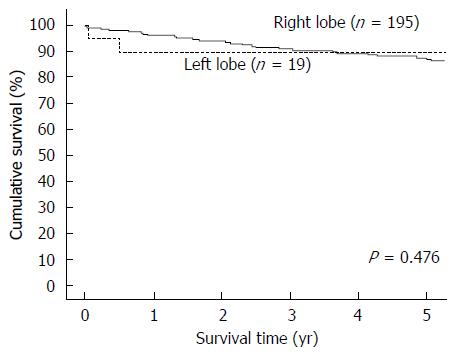
Totally 218 patients were included for analysis, with 199 patients in the RL-LDLT group and 19 patients in the LL-LDLT group. The two groups were similar in terms of age (median, 53 years in the RL-LDLT group and 52 years in the LL-LDLT group, P = 0.997) but had significantly different ratios of men to women (165:34 in the RL-LDLT group and 8:11 in the LL-LDLT group, P < 0.0001). The two groups were also significantly different in GW (P < 0.0001), GW/RSLV (P < 0.0001), and graft cold ischemic time (P = 0.007). When it comes to postoperative complication, the groups were comparable (P = 0.105). Five patients died in hospital, 4 (2%) in the RL-LDLT group and 1 (5.3%) in the LL-LDLT group (P = 0.918). There were 38 graft losses, 33 (16.6%) in the RL-LDLT group and 5 (26.3%) in the LL-LDLT group (P = 0.452). The 5-year graft survival rate was significantly better in the RL-LDLT group (95.2% vs 89.5%, P = 0.049). The two groups had similar 5-year patient survival rates (RL-LDLT: 86.8%, LL-LDLT: 89.5%, P = 0.476).
The use of SFS graft in LDLT requires careful tailor-made surgical planning and meticulous operation. LL-LDLT can be a good alternative to RL-LDLT with similar recipient outcomes but a lower donor risk. Further research into different patient conditions is needed in order to validate the use of LL graft.
Core tip: Liver transplant has become an established treatment for liver failure. The use of living-donor liver graft is one important strategy to expand the donor pool. The use of left lobe graft remains controversial due to the potential problem of small-for-size syndrome. This study illustrates that the use of left lobe graft can produce outcomes similar to right lobe graft. However, the study contains selection bias since most of the recipients of left lobe grafts had relatively lower Model for End-stage Liver Disease scores and were women, who are lighter in weight. Therefore, further study should focus on the establishment of criteria for the use of left lobe graft to allow safe transplant.
- Citation: She WH, Chok KS, Fung JY, Chan AC, Lo CM. Outcomes of right-lobe and left-lobe living-donor liver transplantations using small-for-size grafts. World J Gastroenterol 2017; 23(23): 4270-4277
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4270.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4270
Living-donor liver transplantation (LDLT) was first established as an excellent treatment method for children with end-stage liver disease[1-3]. The lack of cadaveric liver grafts in Asian countries has led to the development of LDLT using the left liver lobe (LL)[4] and the right liver lobe (RL)[5], which has increased the donor pool[6], allowing more patients to be transplanted. The LL is only approximately one-third of the whole liver[7] and thus is generally not adequate for a recipient whose body size is similar to or bigger than that of the donor. Hence the RL is more preferable. Nonetheless, donor right hepatectomy carries a mortality risk of 0.5% whereas it is only 0.1% in donor left hepatectomy[8]. The use of LL was once considered near abandonment because oftentimes it was insufficient for the metabolic demand of recipients, leading to small-for-size (SFS) syndrome. SFS syndrome is caused by a SFS graft, which is defined as a graft with a ratio of graft weight (GW) to recipient standard liver volume (RSLV) (GW/RSLV) of < 50%[9-11]. SFS syndrome describes the constellation of cholestasis, coagulopathy and ascites; it can progress to gastrointestinal bleeding and renal failure. Portal hypertension and sinusoidal injury may lead to graft failure[12,13]. With SFS syndrome, patient survival and graft survival after LDLT would be poor[9,14-16]. However, some studies of LL-LDLT did show promising results[17-21]. Although lowering graft size requirement increases recipient risks, it can improve the applicability of LL-LDLT and, mostly importantly, reduce donor risks[6]. This retrospective study aimed to analyze the outcomes of LDLTs using SFS grafts, be them LLs or RLs.
Data of adult patients who underwent LDLT at our hospital in the period from January 2003 to December 2013 were reviewed. Donor and recipient operations were performed as described elsewhere[22]. The decision of using LL or RL was multifactorial - with donor and recipient factors all considered - but principally depended on Model for End-stage Liver Disease (MELD) score, GW/RSLV, the ratio of GW to recipient body weight, and donor liver anatomy. In general, if the estimated volume of a LL exceeded 35% of the RSLV, LL would be used. However, graft selection was still decided on a case-by-case basis, with consideration of various factors including anatomical variation and recipient condition (MELD score). The bottom line was that the estimated volume of the future liver remnant of the donor must not be < 35% of the donor’s total liver volume. Standard liver volume was calculated using the Urata formula [liver volume (mL) = body surface area (m2) × 706.2 + 2.4][7]. If a RL graft contained the native middle hepatic vein, a single venous cuff would be constructed with a venoplasty of the middle and right hepatic veins[23] to enhance outflow capacity.
LL graft implantation was similar to RL graft implantation. The procedure was done without bypass. The common trunk of the left and middle hepatic veins was first anastomosed to the recipient inferior vena cava. The portal vein was then anastomosed to the recipient portal vein. Clamps were removed to allow revascularization. Hepatic arteries were always reconstructed under the microscope. As to the method of biliary reconstruction, hepaticojejunostomy was preferred in LL-LDLT whereas duct-to-duct anastomosis was preferred in RL-LDLT unless multiple ductal openings were encountered.
A venous cannula was inserted into the inferior mesenteric vein to measure the portal pressure, especially if the GW/RSLV was ≤ 35%. The portal flow was to be maintained at 100-250 mL/min/100 g to prevent SFS syndrome. The use of splenic artery ligation and the use of ligation of spontaneous splenic renal shunt were determined intraoperatively.
Starting from January 2001, 20 mg of basiliximab was given intravenously within 6 h of graft reperfusion and on postoperative day 4. Steroid injection was given intraoperatively with 1 g of hydrocortisone and on postoperative day 1 with 500 mg of hydrocortisone. Immunosuppression was maintained with oral tacrolimus given within 12 h of transplant at a dosage of 0.15 mg/kg body weight/D, and the dose was titrated to achieve a trough level of 5-10 ng/mL. Mycophenolate mofetil at a dosage of 1-1.5 g/d was started within 48 h of transplant and was tapered off within 3 mo. Maintenance steroid was not given routinely. All recipients also orally took 200 mg of fluconazole and 480 mg of cotrimoxazole daily and 400 mg of acyclovir thrice a day for 3 mo for prophylaxis. For patients with renal dysfunction (a creatinine level before administration of tacrolimus twice the normal level), the tacrolimus trough level was kept at 3-5 ng/mL, and prednisolone at 20 mg/d was added. Pentamidine (300 mg) inhalation was given monthly for 3 mo to patients with renal impairment or glucose-6-phosphate dehydrogenase deficiency because the use of acyclovir or cotrimoxazole was prohibited[24]. For hepatitis B virus carriers and recipients of grafts donated by donors who had hepatitis core antibodies, nucleoside analogue monoprophylaxis (entecavir) was administered.
Patient data used in the study were prospectively collected. The patients were divided into the RL-LDLT group and the LL-LDLT group. For further analysis, they were divided into three groups according to GW/RSLV: ≤ 35%, > 35%-40%, and > 40%-< 50%. The RL-LDLT and LL-LDLT groups were compared in terms of short- and long-term outcomes, including incidence of postoperative complication, graft function, graft survival, and patient survival. Recipient conditions were compared on postoperative day 7 and then yearly. Continuous variables were expressed as medians and interquartile ranges and compared by the Kruskal-Wallis test. Categorical variables were compared by Spearman’s test. Survival rates were plotted as Kaplan-Meier curves and compared by log-rank analysis. Statistical significance was defined as P < 0.05. All statistical calculations were performed with SPSS, version 20.0 (SPSS, Chicago, IL, United States), by the statistician at the Department of Surgery, The University of Hong Kong.
From January 2003 to December 2013, 218 adults underwent LDLT with a GW/RSLV < 50%. Nineteen of them had LL-LDLT and 199 had RL-LDLT. The two groups of patients were similar in terms of age (median, 53 years in the RL-LDLT group and 52 years in the LL-LDLT group, P = 0.997) but had significantly different ratios of men to women (165:34 in the RL-LDLT group and 8:11 in the LL-LDLT group, P < 0.0001). The patients’ diagnoses are shown in Table 1 and their perioperative details are shown in Table 2. The two groups of patients were significantly different in GW (P < 0.0001), GW/RSLV (P < 0.0001), and graft cold ischemic time (P = 0.007). Table 3 shows the patients’ survival outcomes and postoperative complications, as well as the reasons for graft losses and the causes of deaths. The only one significant difference between the groups was graft survival (P = 0.049), which can also be viewed in Figure 1. Figure 2 is a Kaplan-Meier plot for patient survival of the series (P = 0.476).
| RL-LDLT (n = 199) | LL-LDLT (n = 19) | P value | |
| Median age (yr) (range) | 53 (17-72) | 52 (17-67) | 0.997 |
| Male:Female | 165:34 | 8:11 | 0.000 |
| Diagnosis | |||
| Liver cirrhosis | |||
| Cryptogenic | 3 | 1 | |
| Hepatitis B virus infection | 85 | 0 | |
| Hepatitis C virus infection | 15 | 5 | |
| Alcohol | 4 | 0 | |
| Infection of hepatitis B and C viruses | 2 | 0 | |
| Alcohol + hepatitis C virus infection | 3 | 0 | |
| Alcohol + hepatitis B virus infection | 1 | 0 | |
| Chronic active hepatitis B | 1 | 1 | |
| Fulminant hepatic failure | |||
| Cryptogenic | 1 | 1 | |
| Hepatitis B | 2 | 0 | |
| Autoimmune | 1 | 0 | |
| Drug-induced | 3 | 1 | |
| Biliary atresia | 2 | 0 | |
| Primary sclerosing cholangitis | 0 | 1 | |
| Primary biliary cirrhosis | 2 | 1 | |
| Graft failure | 4 | 0 | |
| Liver cirrhosis with acute deterioration | |||
| Cryptogenic | 2 | 0 | |
| Hepatitis B | 30 | 3 | |
| Alcohol | 1 | 0 | |
| Autoimmune | 1 | 0 | |
| Wilson's disease | 2 | 0 | |
| Chronic active hepatitis with acute flare | |||
| Hepatitis B | 32 | 1 | |
| Autoimmune | 1 | 0 | |
| Osler-Weber-Rendu syndrome | 1 | 0 | |
| Neuroendocrine syndrome | 0 | 1 | |
| Familial amyloid polyneuropathy | 0 | 1 | |
| Caroli disease | 0 | 1 | |
| Recurrent pyogenic cholangitis | 0 | 1 | |
| Hepatocellular carcinoma | 83 (41.7%) | 5 (26.3%) | 0.191 |
| RL-LDLT (n = 199) | LL-LDLT (n = 19) | P value | |
| Waiting time (d) | 11 (1-1354) | 16 (1-381) | 0.190 |
| Preoperative MELD score | 20 (6-50) | 14 (6-40) | 0.184 |
| GW (g) | 530 (320-715) | 410 (310-585) | 0.000 |
| GW/RSLV (%) | 42.8 (28.4-46.998) | 36.3 (27.3-46.96) | 0.000 |
| GW/RBW (%) | 0.77 (0.46-1.03) | 0.72 (0.49-1.04) | 0.236 |
| Graft cold ischemic time (min) | 105 (53-243) | 85 (69-134) | 0.007 |
| Recipient warm ischemic time (min) | 51 (25-89) | 45 (27-63) | 0.088 |
| Splenic artery ligation | 0 | 0 | - |
| Portosystemic shunt | 0 | 0 | - |
| Ligation of shunt | 2 (1.0%) | 0 | 1.000 |
| Blood transfusion (units) | 4 (0-39) | 2 (0-14) | 0.099 |
| Fresh frozen plasma transfusion (units) | 9 (0-38) | 6 (0-23) | 0.145 |
| Platelet transfusion (units) | 6 (0-32) | 3 (0-22) | 0.341 |
| Operation time (min) | 685 (400-1203) | 670 (485-1273) | 0.738 |
| Intensive care unit stay (d) | 4 (1-124) | 6 (2-16) | 0.055 |
| Hospital stay (d) | 17 (2-128) | 23 (10-68) | 0.072 |
| Follow-up period (d) | 86.9 (0.07-162.9) | 133.7 (0.53-159.2) | 0.054 |
| RL-LDLT (n = 199) | LL-LDLT (n = 19) | P value | |
| In-hospital death | 4 (2) | 1 (5.3) | 0.918 |
| Graft loss | 33 (16.6) | 5 (26.3) | 0.452 |
| Patient status - Alive:Dead | 169:30 | 15:4 | 0.722 |
| Graft survival | 0.049 | ||
| 1-yr | 98.50% | 89.50% | |
| 3-yr | 95.80% | 89.50% | |
| 5-yr | 95.20% | 89.50% | |
| Patient survival | 0.476 | ||
| 1-yr | 95.90% | 89.50% | |
| 3-yr | 90.80% | 89.50% | |
| 5-yr | 86.80% | 89.50% | |
| Postoperative complication1 | 0.105 | ||
| No complication | 92 (46.2) | 7 (36.8) | |
| Grade 1 | 42 (21.1) | 3 (15.8) | |
| Grade 2 | 21 (10.6) | 0 | |
| Grade 3a | 19 (9.5) | 5 (26.3) | |
| Grade 3b | 14 (7.0) | 3 (15.8) | |
| Grade 4a | 7 (3.5) | 0 | |
| Grade 4b | 2 (1.0) | 0 | |
| Grade 5 | 2 (1.0) | 1 (5.3) | |
| Reason for graft loss | |||
| Patient death | 28 | 3 | |
| Hepatic artery thrombosis | 1 | 0 | |
| Portal vein thrombosis | 1 | 0 | |
| PV/IVC thrombosis | 1 | 0 | |
| Recurrent Wilson's disease | 1 | 0 | |
| Biliary complication | 0 | 1 | |
| Reactivation of hepatitis C | 0 | 1 | |
| Rejection | 1 | 0 | |
| Cause of death | |||
| Acute myocardial infarction | 2 | 0 | |
| Chronic rejection | 1 | 0 | |
| Unknown | 2 | 0 | |
| Invasive aspergillosis | 1 | 0 | |
| Malignant cachexia | 16 | 0 | |
| Sepsis/multiorgan failure | 3 | 2 | |
| Subarachnoid hemorrhage | 1 | 0 | |
| Graft failure from PV/IVC thrombosis | 1 | 0 | |
| Lymphoproliferative disease | 1 | 0 | |
| Pulmonary hypertension | 1 | 0 | |
| Respiratory failure | 1 | 0 | |
| Chronic heart failure | 0 | 1 | |
| Reactivation of hepatitis C | 0 | 1 |
Donor details can be found in Table 4. Like the patients, the two groups of donors were similar in terms of age (median, 34 years in the RL-LDLT group and 32 years in the LL-LDLT group, P = 0.847) but had significantly different ratios of men to women (39:160 in the RL-LDLT group and 16:3 in the LL-LDLT group, P < 0.001). When it comes to postoperative complication, the two groups of donors were comparable (16.6% in the RL-LDLT group and 5.3% in the LL-LDLT group, P = 0.905). One donor death occurred and it was in the RL-LDLT group (0.5% vs 0%, P = 1). The death was due to severe peptic ulcer disease resulting in aortoduodenal fistula.
| RL-LDLT (n = 199) | LL-LDLT (n = 19) | P value | |
| Male:Female | 39:160 | 16:3 | 0.000 |
| Median age (yr) (range) | 34 (18-58) | 32 (18-55) | 0.847 |
| Death | 1 (0.5%) | 0 | 1.000 |
| Postoperative complication1 | 0.905 | ||
| No complication | 166 | 18 | |
| Grade 1 | 14 | 1 | |
| Grade 2 | 8 | 0 | |
| Grade 3a | 4 | 0 | |
| Grade 3b | 5 | 0 | |
| Grade 4a | 1 | 0 | |
| Grade 4b | 0 | 0 | |
| Grade 5 | 1 | 0 |
On further analysis, patients with different GW/RSLV (≤ 35% vs > 35%-40% vs > 40%- < 50%) were comparable in terms of graft survival and patient survival. No significant result was shown on multivariate analysis.
Liver transplant has been documented as a life-saving treatment for liver failure and the result is remarkable[25]. However, the scarcity of liver grafts from deceased donors remains a major issue while the number of patients waiting for a liver transplant is rising[26,27]. The problem has led to not only the employment of LDLT but also the use of suboptimal liver grafts - grafts with mild steatosis and grafts from older donors, obese donors, and donors with a borderline small potential residual liver volume[28-31]. At our center, the use of RL for LDLT has been well established with satisfactory long-term graft survival and patient survival[32].
This study reviewed the outcomes of LDLT in patients who received a SFS graft. These patients were prone to SFS syndrome. SFS syndrome is defined by the presence of prolonged cholestasis, coagulopathy and ascites in the absence of ischemia within the first week of liver transplant caused by a partial liver graft that is inadequate to sustain metabolic demand in the recipient. The underlying reason for this is the presence of portal hypertension and graft size mismatch. This condition leads to a reduction in intrahepatic vascular bed with higher portal flow per gram of liver tissue, which leads to a rise in portal pressure and sinusoidal shear stress. Such stress may cause sinusoidal endothelial cell injury and provoke a sequence of hepatocellular damage and cell death[14,33,34], which is manifested by hepatocyte ballooning, steatosis, centrilobular necrosis and parenchymal cholestasis in the histology of the engrafted liver[35-37].
The current study found that SFS RL graft and SFS LL graft did not have much difference when patient survival is concerned - which echoes the results in the literature[38,39] - but the SFS RL grafts had better survival than the SFS LL grafts (P = 0.049). This study is a single-center retrospective study. At our center, RL-LDLT has been the standard for years whereas LL-LDLT, despite the technical similarity, is a relatively unfamiliar procedure, which is also reflected in the small number of patients in the LL-LDLT group. Probably we were still at the learning-curve phase in LL-LDLT. Furthermore, this group of patients underwent transplant for liver failure. In an acute condition (such as acute liver failure or fulminant liver failure), the patient would be very sick with a high MELD score. In the past, patients with high MELD scores were denied LDLT because it would be unwise to put donors in risk for recipient outcomes that would be inferior. However, studies have shown that LDLT can provide patients who have high MELD scores with excellent graft function and patient survival[24,40,41]. The preference for RL graft is due to the shorter cold ischemic time and, in general, greater liver mass. Furthermore, almost all RL grafts we used contained the middle hepatic vein. The inclusion of the middle hepatic vein in a graft allows better venous drainage of the graft and hence ensure good liver function to meet the metabolic demand even if the patient is in critical condition[42]. Therefore, the use of LL graft needs justification, especially in the face of acute liver failure. Having said that, LL graft can still provide reasonable recipient survival. Most importantly, a LL living donor risks much less than a RL living donor. Further comparison between LL and RL grafts can be made when more LL-LDLTs have been performed for different types of patients with different conditions.
In this study, most of the patients in the LL-LDLT group were women. In general, women have a smaller body size. So, although the grafts were lighter and had a smaller GW/RSLV, they fitted well in the patients, who were mostly women. Women with a lower body mass index and a low-to-medium MELD score have been found to be disadvantaged in the “MELD score era” because of the heavy influence of creatinine level on MELD score; they tend to have a lower priority on the liver transplant waitlist and a higher waitlist mortality[43-45]. Balancing donor risks and recipient outcomes, even if there is graft size mismatch, the use of LL graft for LDLT can potentially expand the donor pool to shorten the waiting time and, at the same time, lower the donor risk.
The mortality risk for donors is 0.1% for LL-LDLT and 0.5% for RL-LDLT[8]. The figures are small and at first sight there seems to be not much difference. However, in the long run if a large number of living donors are donating their RLs, the mortality of RL living donors will be significant. In this study, the donor mortality rates - 0.5% in RL-LDLT and 0% in LL-LDLT - were similar to the rates recorded in the literature.
The first successful LL-LDLT was reported in 1994[46]. Generally, a LL graft is 30%-40% of the SLV in adults[7]; as such, SFS syndrome tends to develop. SFS syndrome often results in poor graft survival and patient survival[9,14-16]. However, it has been shown that LL-LDLT can achieve excellent graft survival and patient survival[4] and the results of LL-LDLT can be comparable to those of RL-LDLT[17-21]. The primary goal of LDLT is to minimize donor risks (morbidity and mortality) while maximizing recipient benefits.
Using LL graft instead of RL graft has the advantage of lower donor morbidity[47] and mortality[8], but sometimes a LL graft would not suffice. If the prognosis for a patient is poorer because of a high MELD score or an old donor age, using the larger RL would improve the transplant outcome[19]. In the current study, all the grafts were small for size, and LL-LDLT and RL-LDLT had little difference when long-term patient survival is concerned. In view of this, maybe we can opt for the LL more often. For patients with a low MELD score and relatively stable condition, if a deceased-donor graft is unavailable, the use of LL graft can be advocated after balancing donor risks and potential recipient outcomes.
At our center, if SFS syndrome is expected, flow modulation will be performed. In the operation, portal flow and portal pressure are measured. Additional portal inflow modulations, such as splenic artery ligation[23] and portocaval shunt[48] may be employed[49]. In the current study, although all grafts were small for size, not many patients needed flow modulation. Some patients even required ligation of the spontaneous portosystemic shunt due to inadequate portal flow. All patients had good outcomes.
We wanted to find out the risk for complication and mortality in using SFS LL graft. Unfortunately, due to the small number of cases that used SFS LL graft, it was difficult to run a statistic on it. A multivariate analysis on the relevant data was performed but the result was negative; it was a nonsignificant finding.
The findings of this study may not be universally applicable. Our center is experienced in LDLT, especially RL-LDLT, which might have contributed to the favorable outcomes in the study[32]. Moreover, there were only 19 patients in the LL-LDLT group. The study has a relatively high risk of type-2 error. Furthermore, this is a retrospective cohort study with inevitable selection bias. Although every LDLT center has its LDLT protocol, every LDLT should be individualized, and the selection criteria adopted by a center cannot be universally applied.
The use of SFS graft in LDLT requires careful tailor-made surgical planning and meticulous operation. LL-LDLT can be a good alternative to RL-LDLT with similar recipient outcomes but a lower donor risk. Further research into different patient conditions is needed in order to validate the use of LL graft.
The use of the right lobe graft in living donor liver transplantation has been well established. However the use of the left lobe graft remained cautious, as a lot of patients suffered from complication or mortality due to the small for size graft. This study reviewed the results of the use of small for size grafts (left lobe or right lobe) and analyzed the outcomes of transplantation using left lobe small for size graft.
Donor safety is of paramount importance in living donor liver transplantation. In the past, the mortality rate for right lobe graft donation was around 0.5%, whereas the mortality rate for left lobe graft donation was around 0.1%. The result of using right lobe small for size graft has been well established. However comparison between right lobe small for size graft and left lobe small for size graft is scarce.
This paper focused on the graft outcome and patient outcome of using small for size graft. Although the use of the right lobe and the use of the left lobe achieved similar patient survival, we should analyze the results cautiously, as most of the time recipients using a left lobe graft were relatively less risky.
This study has delivered an important message that allows further enlargement of the donor pool. Left lobe small for size graft can achieve results similar to right lobe small for size graft while reducing donor risk. Further studies can be conducted to find out which subgroup of patients would benefit from left lobe graft the most and to decide the safety limit of using left lobe small for size grafts.
Right lobe graft: A graft that is the right liver lobe, usually containing the middle hepatic vein; Left lobe graft: A graft that is the left liver lobe; Small for size graft: A graft with a ratio of graft weight to recipient standard liver volume of < 40%-50%, or with a ratio of graft to recipient weight of 0.8%-1.0%; Small for size syndrome: Constellation of cholestasis, coagulopathy and ascites. It can progress to gastrointestinal bleeding and renal failure.
Interesting and well written manuscript. Useful for surgeons facing with liver living donor transplantation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fujino Y, Salvadori M S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, Sawada H, Shirahase I, Kim HJ, Yamaoka Y. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 436] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 2. | Emond JC, Heffron TG, Kortz EO, Gonzalez-Vallina R, Contis JC, Black DD, Whitington PF. Improved results of living-related liver transplantation with routine application in a pediatric program. Transplantation. 1993;55:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Broelsch CE, Burdelski M, Rogiers X, Gundlach M, Knoefel WT, Langwieler T, Fischer L, Latta A, Hellwege H, Schulte FJ. Living donor for liver transplantation. Hepatology. 1994;20:49S-55S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, Shiotani S, Harada N, Hideki I, Yonemura Y, Maehara Y. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Liu CL, Fan ST, Lo CM, Wei WI, Chan SC, Yong BH, Wong J. Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann Surg. 2006;243:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Chan SC, Fan ST, Chok KS, Sharr WW, Dai WC, Fung JY, Chan KY, Balsarkar DJ, Lo CM. Increasing the recipient benefit/donor risk ratio by lowering the graft size requirement for living donor liver transplantation. Liver Transpl. 2012;18:1078-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 704] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 8. | Barr ML, Belghiti J, Villamil FG, Pomfret EA, Sutherland DS, Gruessner RW, Langnas AN, Delmonico FL. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Emond JC, Renz JF, Ferrell LD, Rosenthal P, Lim RC, Roberts JP, Lake JR, Ascher NL. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg. 1996;224:544-552; discussion 552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 277] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Park KM, Lee SG, Lee YJ, Hwang S, Nam CW, Choi KM, Nam CH, Choi DN, Kim KH, Choi KT. Adult-to-adult living donor liver transplantation at Asian Medical Center, Seoul, Korea. Transplant Proc. 1999;31:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Kiuchi T, Oike F, Yamamoto H. Small-for-size graft in liver transplantation. Nagoya J Med Sci. 2003;66:95-102. [PubMed] |
| 13. | Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl. 2003;9:S36-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 722] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 15. | Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl. 2001;7:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Tanaka K, Ogura Y. “Small-for-size graft” and “small-for-size syndrome” in living donor liver transplantation. Yonsei Med J. 2004;45:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Soejima Y, Shirabe K, Taketomi A, Yoshizumi T, Uchiyama H, Ikegami T, Ninomiya M, Harada N, Ijichi H, Maehara Y. Left lobe living donor liver transplantation in adults. Am J Transplant. 2012;12:1877-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Taketomi A, Kayashima H, Soejima Y, Yoshizumi T, Uchiyama H, Ikegami T, Yamashita Y, Harada N, Shimada M, Maehara Y. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation. 2009;87:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Kurihara T, Yoshizumi T, Yoshida Y, Ikegami T, Itoh S, Harimoto N, Ninomiya M, Uchiyama H, Okabe H, Kimura K. Graft selection strategy in adult-to-adult living donor liver transplantation: When both hemiliver grafts meet volumetric criteria. Liver Transpl. 2016;22:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Shimada M, Shiotani S, Ninomiya M, Terashi T, Hiroshige S, Minagawa R, Soejima Y, Suehiro T, Sugimachi K. Characteristics of liver grafts in living-donor adult liver transplantation: comparison between right- and left-lobe grafts. Arch Surg. 2002;137:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ikegami T, Masuda Y, Ohno Y, Mita A, Kobayashi A, Urata K, Nakazawa Y, Miwa S, Hashikura Y, Miyagawa S. Prognosis of adult patients transplanted with liver grafts & lt; 35% of their standard liver volume. Liver Transpl. 2009;15:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Lo CM, Fan ST, Liu CL, Wong J. Hepatic venoplasty in living-donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation. 2003;75:358-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Chok KS, Fung JY, Chan AC, Dai WC, Sharr WW, Cheung TT, Chan SC, Lo CM. Comparable Short- and Long-term Outcomes in Living Donor and Deceased Donor Liver Transplantations for Patients With Model for End-stage Liver Disease Scores ≥35 in a Hepatitis-B Endemic Area. Ann Surg. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Au KP, Chan SC, Chok KS, Chan AC, Wong TC, Sharr WW, Lo CM. Durability of small-for-size living donor allografts. Liver Transpl. 2015;21:1374-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS. Organ donation and utilization in the United States, 1996-2005. Am J Transplant. 2007;7:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Eurotransplant. Eurotransplant Annual Reports: Eurotransplant International Foundation. Available from: http://www.eurotransplant.org/cms/index.php?page=annual_reports. |
| 28. | Lo CM, Fan ST, Liu CL, Yong BH, Wong Y, Lau GK, Lai CL, Ng IO, Wong J. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg. 2004;240:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, Yonemura Y, Shimada M, Maehara Y. Feasibility of left lobe living donor liver transplantation between adults: an 8-year, single-center experience of 107 cases. Am J Transplant. 2006;6:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Tanemura A, Mizuno S, Wada H, Yamada T, Nobori T, Isaji S. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Kim SJ, Na GH, Choi HJ, Yoo YK, Kim DG. Surgical outcome of right liver donors in living donor liver transplantation: single-center experience with 500 cases. J Gastrointest Surg. 2012;16:1160-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Chan SC, Lo CM, Ng KK, Fan ST. Alleviating the burden of small-for-size graft in right liver living donor liver transplantation through accumulation of experience. Am J Transplant. 2010;10:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 473] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 34. | Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1729] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 37. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 38. | Ikegami T, Yoshizumi T, Sakata K, Uchiyama H, Harimoto N, Harada N, Itoh S, Nagatsu A, Soejima Y, Maehara Y. Left lobe living donor liver transplantation in adults: What is the safety limit? Liver Transpl. 2016;22:1666-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Braun HJ, Dodge JL, Roll GR, Freise CE, Ascher NL, Roberts JP. Impact of Graft Selection on Donor and Recipient Outcomes After Living Donor Liver Transplantation. Transplantation. 2016;100:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Selzner M, Kashfi A, Cattral MS, Selzner N, McGilvray ID, Greig PD, Levy GA, Renner EL, Grant DR. Live donor liver transplantation in high MELD score recipients. Ann Surg. 2010;251:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Chok KSh, Chan SC, Fung JY, Cheung TT, Chan AC, Fan ST, Lo CM. Survival outcomes of right-lobe living donor liver transplantation for patients with high Model for End-stage Liver Disease scores. Hepatobiliary Pancreat Dis Int. 2013;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Liu CL, Fan ST, Lo CM, Yong BH, Fung AS, Wong J. Right-lobe live donor liver transplantation improves survival of patients with acute liver failure. Br J Surg. 2002;89:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Fink MA, Berry SR, Gow PJ, Angus PW, Wang BZ, Muralidharan V, Christophi C, Jones RM. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007;22:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Axelrod DA, Pomfret EA. Race and sex disparities in liver transplantation: progress toward achieving equal access? JAMA. 2008;300:2425-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Hashikura Y, Makuuchi M, Kawasaki S, Matsunami H, Ikegami T, Nakazawa Y, Kiyosawa K, Ichida T. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994;343:1233-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 278] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Umeshita K, Fujiwara K, Kiyosawa K, Makuuchi M, Satomi S, Sugimachi K, Tanaka K, Monden M; Japanese Liver Transplantation Society. Operative morbidity of living liver donors in Japan. Lancet. 2003;362:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 48. | Troisi R, Ricciardi S, Smeets P, Petrovic M, Van Maele G, Colle I, Van Vlierberghe H, de Hemptinne B. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant. 2005;5:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 49. | Chan SC, Lo CM, Chok KS, Sharr WW, Cheung TT, Tsang SH, Chan AC, Fan ST. Modulation of graft vascular inflow guided by flowmetry and manometry in liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |