Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4233
Peer-review started: February 9, 2017
First decision: March 16, 2017
Revised: March 30, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: June 21, 2017
Processing time: 131 Days and 12.4 Hours
To investigate the inhibitory effect of Liuwei Dihuang Pill (LDP) on gastric tumorigenesis induced by N-methyl-N-nitrosourea (MNU) in diabetic mice.
Four-week-old mice were divided into four groups: A, 12 db/m mice treated with MNU and saline, as the non-diabetic control; B, 12 db/db mice treated with MNU and saline, as the diabetic control; C, 12 db/db mice treated with MNU and metformin, as the positive control; and D, 12 db/db mice treated with MNU and LDP. MNU was administrated for 20 wk to induce gastric carcinogenesis. LDP was administrated for 10 wk for improvement of insulin resistance. Body weight and food intake were measured every week. Blood samples were collected for assays of fasting blood glucose, insulin, insulin-like growth factor (IGF)-1, adiponectin and leptin. Stomach tissues were collected for histopathological analysis, immunohistochemical staining of Ki67, quantitative reverse transcription-polymerase chain reaction and western blotting.
The incidence of MNU-induced gastric dysplasia was significantly elevated in diabetic (db/db) mice relative to the control (db/m) mice. The incidence of gastric dysplasia was significantly reduced by LDP with suppression of cell proliferation, as demonstrated by a decrease in Ki67 staining. Hyperglycemia, hyperinsulinemia and serum IGF-1 were inhibited by LDP. Expression of IGF-1 and insulin receptor mRNAs was decreased, phosphorylation of IGF-1 receptor and AKT protein was reduced in the stomach tissues by LDP. In addition, adiponectin was increased and leptin was decreased in the serum by LDP.
LDP decreased risk of gastric dysplasia in type 2 diabetic mice by down-regulation of IGF and insulin activity and correction of adipokines disorders.
Core tip: Type 2 diabetes is reported to increase risk of gastric carcinogenesis, partly by hyperinsulinemia, hyperglycemia, excessive activation of insulin-like growth factor (IGF)-1, and disorders of adipokines. In this study, we demonstrated that Liuwei Dihuang Pill decreased the risk of gastric tumorigenesis in type 2 diabetic mice by alleviating insulin resistance and decreasing IGF-1 and insulin activity, followed by down-regulation of the IGF-1/AKT signaling pathway. The improvement in adiponectin and leptin may also contribute to the effects.
- Citation: Zhuang S, Jian YM, Sun YN. Inhibition of N-methyl-N-nitrosourea-induced gastric tumorigenesis by Liuwei Dihuang Pill in db/db mice. World J Gastroenterol 2017; 23(23): 4233-4242
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4233.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4233
Gastric cancer is one of the most common malignancies, particularly among populations of East Asia. The prevention and treatment of gastric cancer remain a challenge due to the absence of effective strategies. Multiple risk factors, such as Helicobacter pylori infection, high salt intake, smoking and obesity, contribute to the initiation of gastric cancer[1,2]. Recent studies in humans have demonstrated that type 2 diabetes may be a new risk factor for gastric cancer. The incidence of gastric cancer is affected by duration of diabetes and some anti-diabetic drugs. Given the high prevalence of type 2 diabetes and gastric cancer worldwide, control of gastric cancer in diabetic patients has become a new challenge.
The etiology of gastric cancer remains unknown in type 2 diabetic patients. However, hyperinsulinemia and insulin-like growth factor (IGF) may play a central role in the promotion of tumorigenesis. A large Japanese case-control study has demonstrated that hyperinsulinemia and serum C peptide are positively correlated with increased risk of gastric cancer[3]. A high level of insulin increases the bioavailability of serum IGF-1. Both insulin and IGF-1 activate the mitogenic signaling pathways, including the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and mitogen-activated protein kinase pathways[4]. In addition, imbalance of adipokines in diabetes and obesity, such as the high level of leptin and low level of adiponectin, is associated with the risk of cancer[5].
Strategies targeting those factors may decrease the risk of cancer. Metformin is an effective drug in the control of insulin resistance in type 2 diabetes. Population studies have revealed that long-term use of metformin can decrease the risk of gastric cancer[6]. The antitumor effect of metformin may be mediated by activation of the AMP-activated protein kinase pathway or inhibition of the IGF-1 receptor signaling pathway[7,8]. In the present study, metformin was selected as a positive control in the control of gastric tumorigenesis.
The choice of effective and safe drugs remains limited for gastric cancer. Low-grade gastric dysplasia is a precancerous lesion in the early phase of gastric cancer. Inhibition of the dysplasia is able to block advancement of the precancerous lesion into gastric cancer[9]. We propose that this strategy may be used in the control of gastric cancer in diabetes and obesity. Traditional Chinese medicine (TCM) represents a rich resource in the treatment of diabetes and cancer[10,11], and Liuwei Dihuang Pill (LDP) is one of the examples.
LDP is made from a TCM formula that is often prescribed for treatment of diabetes. Studies in rodents have suggested that LDP reduces serum insulin as well as leptin via weight loss and fat reduction[12,13]. LDP also has beneficial effects against certain types of cancers. Several studies have demonstrated that LDP inhibits spontaneous tumorigenesis and suppresses liver tumors[14,15]. In clinical practice, long-term application of LDP prevents epithelial dysplasia of the esophagus[16]. These results suggest that LDP is an effective therapy for both type 2 diabetes and cancer. Despite this knowledge, there is no report on LDP activity in the risk control of gastric cancer in patients with type 2 diabetes.
In the present study, we addressed the issue in diabetic db/db mice, which have a high susceptibility to the carcinogen N-methyl-N-nitrosourea (MNU) for gastric cancer[17-19]. The mice were treated with MNU in drinking water to induce gastric dysplasia. LDP was tested for its ability to inhibit gastric tumorigenesis in the model.
Thirty-six male db/db mice and 12 control (db/m) mice at age 4 wk were obtained from the Nanjing University Animal Laboratories, China (Certification No. SCXK 2015-0001). The animal protocol was designed to minimize pain or discomfort to the animals. The mice were maintained in specific pathogen-free conditions with a 12-h light-dark cycle, room temperature of 25 °C ± 1 °C, and relative humidity of 60% ± 5% in the animal facility of Shanghai Jiao Tong University. The mice had free access to regular chow diet and water. All the experimental protocols were approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University.
LDP was purchased from Tongrentang Group (Beijing, China). LDP is made of six raw medicinal materials (Table 1) and manufactured in accordance with the quality control standards in the Chinese Pharmacopoeia. The bioactive components of LDP were certified according to the high-performance liquid chromatography-fingerprints, which included loganin, paeoniflorin, paeonol, gallic acid, and morroniside[20,21]. The LDP was ground, extracted ultrasonically with distilled water for 30 min, and filtered through a screen with 90 μm aperture. LDP is taken orally at 0.15 g/kg per d of herbal materials in humans, and this dosage is equal to 1.8 g/kg per d in mice. LDP was administrated through the drinking water at a final concentration of 0.09 g/mL (about 9 g/kg per d) for 10 wk. Metformin (Sino-American Shanghai Squibb Co., Shanghai, China) was dissolved in distilled water at a concentration of 5 mg/mL, as described in a previous report[22].
| Chinese name | Latin name | Parts used | Weight, g |
| Shu di huang | Rehmannia glutinosa Libosch | Root | 160 |
| Shan zhu yu | Cornus officinalis Sieb | Fruit | 80 |
| Shan yao | Dioscorea opposite Thunb | Rootstock | 80 |
| Ze xie | Alisma orientale (G. Samuelsson) Juz | Rootstock | 60 |
| Fu ling | Poria cocos (Schw.) Wolf | Sclerotium | 60 |
| Mu dan pi | Paeonia suffruticosa Andrews | Bark | 60 |
MNU (Sigma, St. Louis, MO, United States) was administered to the mice through drinking water 3 times weekly at a concentration of 120 ppm in light-shielded bottles. The administration was performed every other week for 20 wk as described previously[23].
Following 1-wk acclimation, the mice were randomly divided into four groups. Group A, 12 control (db/m) mice were treated with MNU for 20 wk followed by saline treatment for 10 wk to serve as the non-diabetic control. Group B, 12 diabetic (db/db) mice were treated with MNU for 20 wk followed by saline treatment for 10 wk to serve as the diabetic control. Group C, 12 diabetic mice were treated with MNU for 20 wk followed by metformin treatment for 10 wk, as the positive control. Group D, 12 diabetic mice were treated with MNU for 20 wk followed by LDP treatment for 10 wk. At the end of treatment (30 wk), all mice were fasted for 12 h and blood samples were collected from the left ventricle for fasting blood glucose (FBG) measurement.
All animals were euthanized with 1% pentobarbital sodium. Serum was collected and stored at -80 °C for biochemical analysis after centrifugation of the blood at 1000 g for 10 min. The stomach was removed and divided into two parts along the greater curvature. One part was quickly frozen in liquid nitrogen and then stored at -80 °C for quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot analysis. The other part was fixed in neutral-buffered formalin (40 g/L) for 24 h for histological assessment.
Fixed stomach tissue was cut longitudinally into three strips. After dehydration and rinsing in xylol, the strips were embedded in paraffin, sectioned at 4-μm thickness, and stained with hematoxylin and eosin. Gastric lesions were classified as chronic gastritis, gastric dysplasia and gastric carcinoma. These lesions were determined independently by two pathologists. The incidence of gastric lesions in each group was presented as percentage of mice with gastric lesions. If two gastric lesions were found in the same stomach, each lesion was counted.
Paraffin-embedded stomach sections were deparaffinized, rehydrated, and then immersed in 3% hydrogen peroxide to quench endogenous peroxidase. The sections were blocked with 10% goat serum for 20 min followed by incubation with a rabbit anti-Ki67 antibody (1:100; Proteintech, Rosemont, IL, United States) at 4 °C for 12 h. After washing, the tissue sections were incubated with a biotin-conjugated secondary antibody for 60 min and were visualized using diaminobenzidine. Ki67-positive cells were stained brown in the nucleus and were counted in ≥ 10 fields in each slide to obtain the average value.
FBG was measured using a OneTouch glucometer (ACCU-CHEK Performa; Roche, Shanghai, China). Serum IGF-1 and fasting insulin (FINS) were determined with enzyme-linked immunosorbent assay (ELISA) kits (Shibayagi Co. Ltd., Shibukawa, Japan). Serum adiponectin and leptin concentrations were determined with ELISA kits from Crystal Chem (Downers Grove, IL, United States). The homeostatic model assessment of insulin resistance (HOMA-IR) was used to determine insulin resistance by the formula: HOMA-IR = (FBG × FINS)/22.5. The liver function was examined with serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The renal function was evaluated by measuring the levels of blood urea nitrogen (BUN) and creatinine (Cr) using commercial kits (Nanjing Jiancheng Bioengineering, China).
Total RNA was extracted from the stomach tissues with TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and subjected to treatment with DNase I (Takara, Tokyo, Japan) to avoid genomic DNA contamination. RNA (1 μg) was reverse transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Takara). Real-time PCR was conducted with SYBR Green PCR Master Mix (Takara) using ABI 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, United States). Primers sequences are shown in Table 2. Gene expression was normalized to the expression of GAPDH gene using the 2-ΔΔCt method.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| IGF-1 | CTGGACCAGAGACCCTTTGC | GGACGGGGACTTCTGAGTCTT |
| IGF-1R | CATGTGCTGGCAGTATAACCC | TCGGGAGGCTTGTTCTCCT |
| IGF-2 | ACAACTTCGATTTGAACCACATTC | GAGAGCTCAAACCATGCAAACT |
| IGF-2R | TGAATGGTGATCCTTGCCCTC | CCGGTAGCTGTTGGTCTGTC |
| Insulin receptor | TCAAGACCAGACCCGAAGATT | TCTCGAAGATAACCAGGG |
| GAPDH | CCAATGTGTCCGTCGTGGATCT | GTTGAAGTCGCAGGAGACAACC |
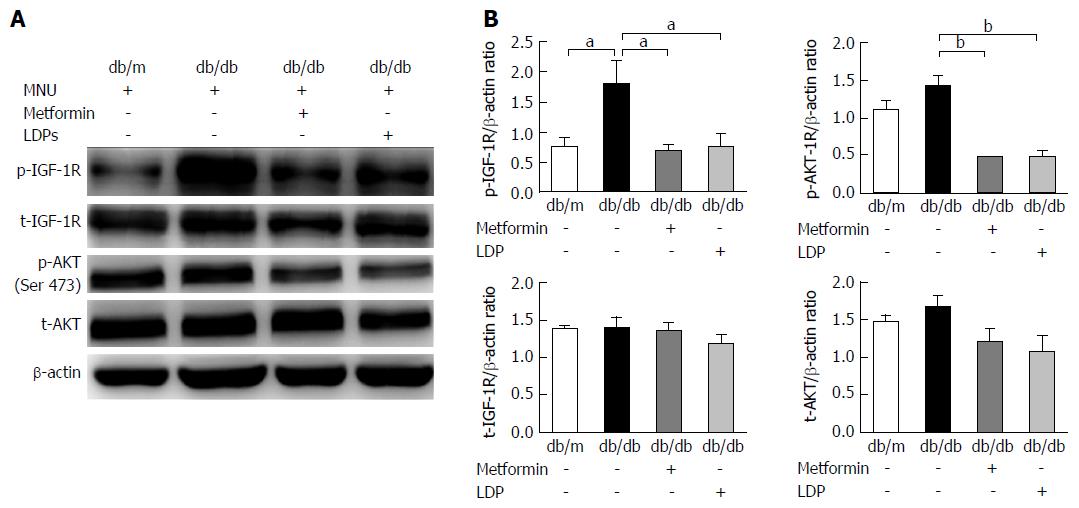
The stomach tissues were ground in liquid nitrogen, homogenized on ice using lysis buffer containing protease inhibitors, and centrifuged at 12000 rpm for 15 min to obtain the supernatant. The concentration of total protein was determined with a BCA protein assay kit (Beyotime Biotechnology Company, Beijing, China). Protein from each mouse was separated at 50 μg/sample using 8% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, United States). The membrane was blocked in tris-buffered saline with 0.1% Tween-20 and 5% fat-free milk, incubated with anti-phospho-IGF-1R (Tyr1135/1136; Cell Signaling Technology, Danvers, MA, United States), anti-IGF-1R (Proteintech), anti-phospho-AKT (Ser473; Cell Signaling Technology), anti-AKT (Proteintech), and β-actin (Cell Signaling Technology) at 4 °C overnight. After being washed in tris-buffered saline with 0.1% Tween-20 3 times, the membranes were incubated with horseradish-peroxidase-conjugated anti-rabbit antibodies at room temperature for 1 h, and then visualized using an enhanced chemiluminescence kit (Millipore). The protein abundance was determined using the intensity of protein bands using Image-J 1.46r software (National Institutes of Health, Bethesda, MD, United States), and normalized to β-actin (loading control).
Data were expressed as mean ± SE and analyzed using SPSS version 19.0 software (IBM Corp., Armonk, NY, United States). Differences among the groups were evaluated with ANOVA, Bonferroni multiple comparisons test, and Fisher’s exact test. Results were considered to be statistically significant at P < 0.05 and highly significant at P < 0.01. The statistical methods were reviewed by Xu-Hong Hou from the Clinical Epidemiology Center of Shanghai Diabetes Research Institution.
The diabetic mice had higher body weights than non-diabetic mice. There was no significant reduction in body weight in the diabetic mice treated with LDP compared to the untreated mice (Table 3). In the positive control, the body weight was significantly reduced by metformin. The diabetic mice had higher level of serum ALT relative to the non-diabetic mice. Moreover, both LDP and metformin significantly reduced serum ALT (Table 3). There was no significant difference in serum AST, BUN and Cr among the four groups, suggesting that LDP did not cause toxicity in the liver and kidney. LDP-treated mice had normal appearance without hair depilation, subcutaneous hematoma, and skin ulcers. There was no difference in mortality between LDP-treated and untreated groups (Table 4). All these data suggest that the mice had good tolerance to LDP without toxicity.
| Group | Body weights at week 20, g | Body weights at week 30, g | ALT, U/L | AST, U/L | BUN, mmol/L | Cr, μmol/L |
| db/m + MNU + saline | 24.80 ± 1.08 | 25.56 ± 1.78 | 8.98 ± 1.78e | 8.91 ± 1.45 | 4.80 ± 0.26 | 13.94 ± 4.17 |
| db/db + MNU + saline | 59.22 ± 5.61 | 61.18 ± 2.10 | 53.31 ± 8.31 | 10.23 ± 0.90 | 4.65 ± 0.52 | 18.44 ± 6.28 |
| db/db + MNU + metformin | 59.44 ± 3.38 | 48.90 ± 4.99b | 23.40 ± 2.37b | 10.79 ± 1.52 | 4.64 ± 0.51 | 9.45 ± 3.07 |
| db/db + MNU + LDP | 62.79 ± 5.03 | 57.96 ± 3.34 | 38.08 ± 4.81a | 11.30 ± 1.14 | 4.85 ± 0.45 | 9.00 ± 1.50 |
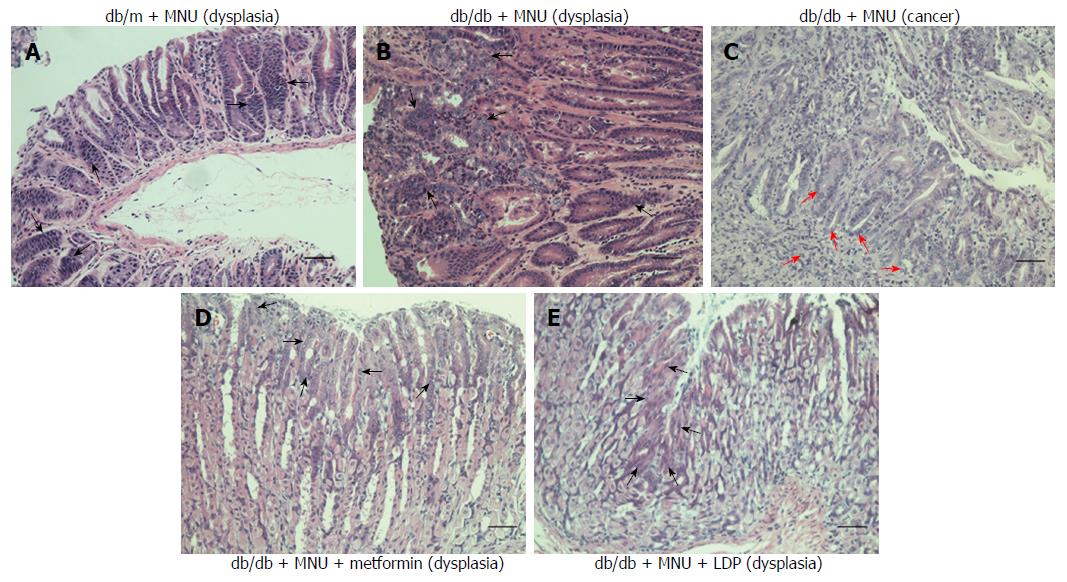
It is reported that diabetes increases and metformin decreases the incidence of gastric cancer[24]. The incidence of gastric dysplasia was significantly increased in the diabetic mice compared to the non-diabetic mice (67% vs 17%; Table 4, Figure 1A and 1B). The incidence was markedly reduced by LDP (10% vs 67%; Table 4 and Figure 1E) or metformin (10% vs 67%; Table 4 and Figure 1D). Metformin was used as the positive control for suppression of gastric tumorigenesis in the diabetic mice. Two diabetic mice had gastric tumors in the untreated group (Figure 1C), while tumors were not observed in the treated groups. However, the difference was not significant among the four groups, which may have been related to the small number of mice in each group. The incidence of chronic gastritis did not differ among the four groups. These data suggest that the incidence of gastric dysplasia was markedly decreased by LDP in the diabetic mice.
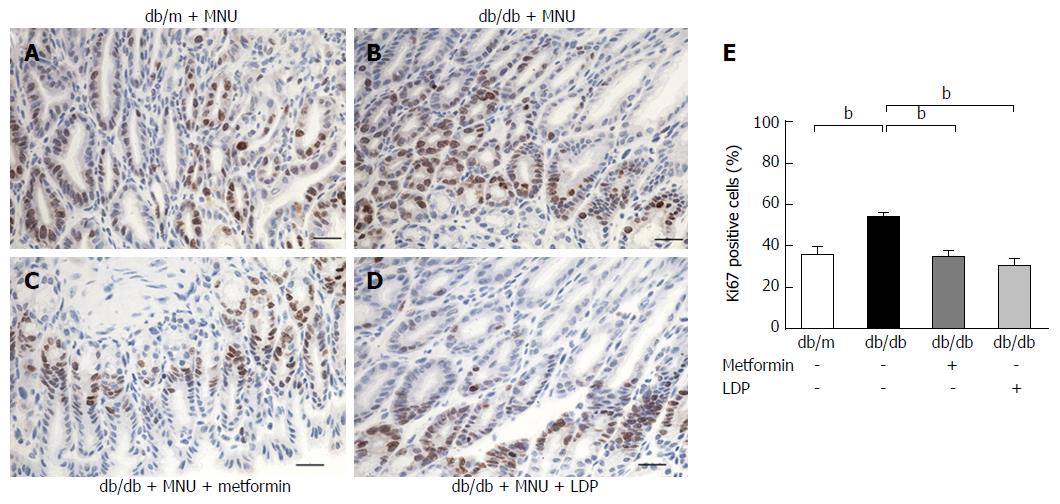
Ki67 is a well-known antigen located in the nucleus and a marker of cell proliferation. We examined the marker to measure dysplasia. The percentage of Ki67-positive cells was significantly increased by the carcinogen MNU. The induction was attenuated by LDP or metformin (Figure 2). These results suggest that diabetes promoted gastric mucosal proliferation in the MNU tumor model. The proliferative activity was inhibited by LDP and metformin.
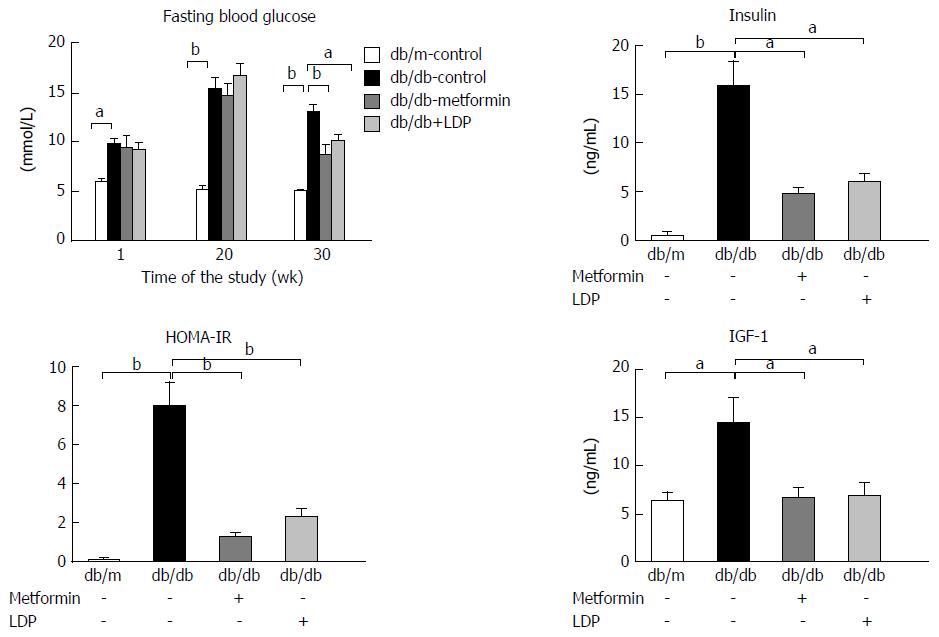
Hyperinsulinemia, hyperglycemia and IGF-1 elevation are important pathological characteristics of type 2 diabetes. These factors have potent activity in the promotion of tumor growth and their activity was significantly elevated in diabetic mice. The elevation was significantly inhibited by LDP or metformin (Figure 3). Insulin resistance was significantly improved by LDP or metformin, as suggested by a reduction in HOMA-IR (Figure 3), indicating that the LDP activity is related to improvement of insulin sensitivity.
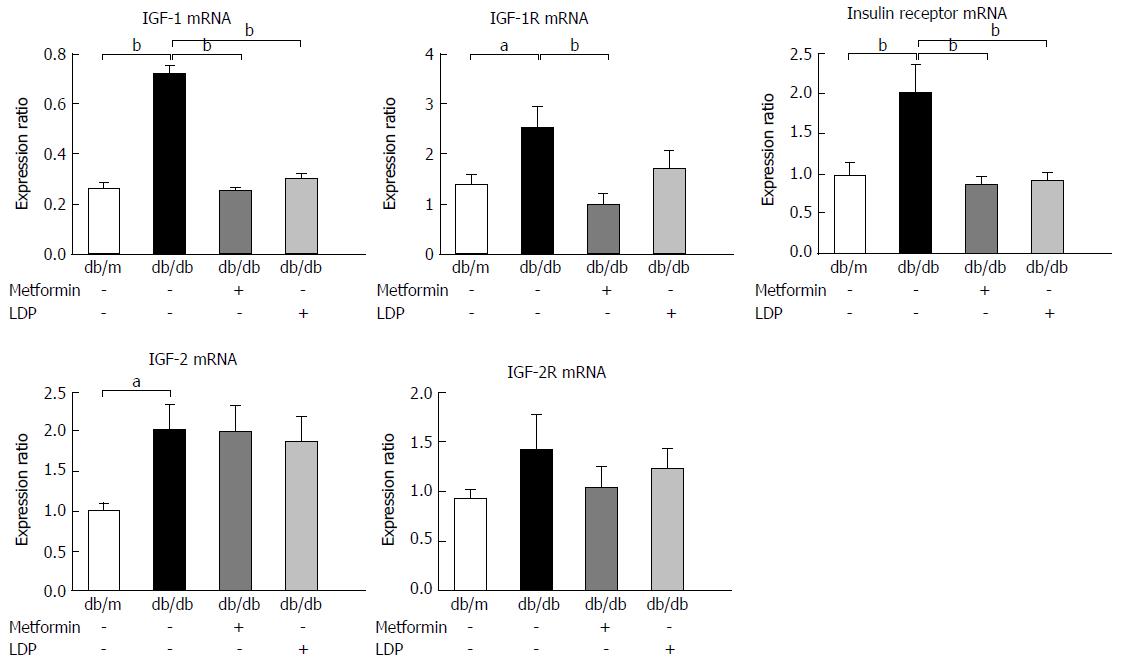
IGF-1 and insulin exert mitogenic effects on cancer cells through activation of their receptors. In this study, their receptor mRNAs were examined by qRT-PCR. The diabetic mice exhibited an increase in the expression of IGF-1, IGF-1 receptor (IGF-1R), insulin receptor, and IGF-2 mRNAs (Figure 4). LDP inhibited the increases in IGF-1 and insulin receptor, but not in IGF-1R, IGF-2 and IGF-2R (Figure 4). A similar activity was observed for metformin in the regulation of gene expression (Figure 4).
Insulin and IGF-1 activate their receptor signaling pathways during promotion of carcinogenesis. As a key signaling molecule, AKT plays a central role in cell growth, cell survival, cancer progression and metastasis. Phosphorylation of AKT and IGF-1R was examined along with total AKT and IGF-1R proteins. Phosphorylation was significantly higher in the diabetic mice than in the non-diabetic mice. The signals were significantly down-regulated by LDP or metformin (Figure 5). These data suggest that LDP inhibits activation of the IGF-1/IGF-1R/AKT signaling pathways in the gastric dysplasia tissue of diabetic mice.
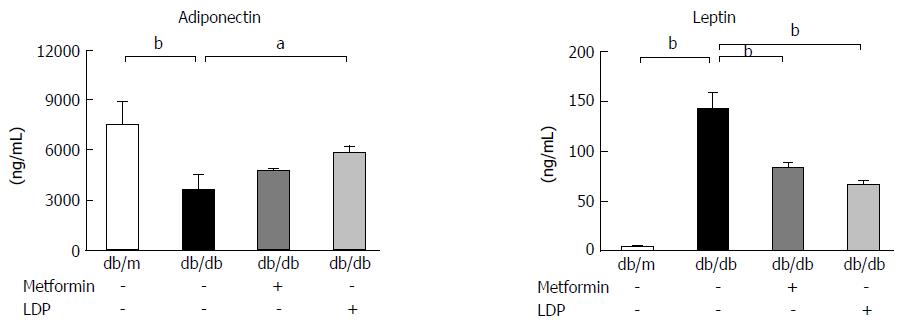
Adipokine change in the context of type 2 diabetes may be associated with gastric carcinogenesis. Serum adiponectin and leptin were examined in this study to investigate adipokine activity. Adiponectin was decreased and leptin was increased in diabetic mice compared with non-diabetic mice (Figure 6). Metformin significantly reduced the level of leptin, but did not markedly increase serum adiponectin. LDP significantly increased adiponectin and decreased leptin in the serum of diabetic mice (Figure 6). These data imply that LDP may significantly improve imbalance of adipokines in the diabetic mice.
In this study, we established a model of gastric dysplasia in diabetic mice by administration of MNU. Gastric dysplasia was enhanced by type 2 diabetes, which was associated with hyperinsulinemia and IGF-1 elevation. The carcinogenic change was significantly inhibited by LDP in the diabetic mice, as indicated by the reduction in Ki67 signaling. The inhibition was associated with a reduction in serum insulin and IGF-1, suggesting improvement of insulin sensitivity by LDP. Insulin sensitivity was improved by LDP as indicated by the HOMA-IR and the pattern of adipokine change in LDP-treated diabetic mice. These data suggest that LDP may reduce the risk of gastric cancer by improvement of insulin sensitivity in diabetic mice.
Our results suggest that LDP may improve insulin sensitivity in the absence of weight loss. Hyperinsulinemia is the most prominent feature of type 2 diabetes, as a result of insulin resistance. The high level of insulin is a risk of gastric cancer for promotion of cell proliferation[3]. This possibility is supported by a study in mice, in which hyperinsulinemia promoted gastric tumor growth in diet-induced obesity[25]. LDP increased insulin sensitivity and reduced hyperinsulinemia through weight loss in one study[12]. However, weight loss was not observed in our study. The discrepancy might be a result of differences in LDP dosage and the animal models. Our data suggest that LDP improves insulin sensitivity without weight loss, although weight loss may contribute to the effect under certain conditions.
A reduction in IGF-1 may have contributed to the cancer preventive effect of LDP in diabetic mice. IGF-1 is a potent growth factor in tumor cells in addition to insulin. IGF-1 promotes cell proliferation and inhibits apoptosis through activation of IGF-1R. Both IGF-1 and IGF-1R are overexpressed in human gastric cancer in most conditions[26]. Inhibition of the signaling pathway is associated with suppression of gastric cancer growth and cancer metastasis[27]. IGF-1 level was elevated in the serum, expression of IGF-1 and its receptor was increased in the lesion tissue of db/db mice. Those alterations were attenuated by LDP in the gastric cancer model. The conclusion was supported by the decrease in phosphorylation of IGF-1R and AKT. IGF-2 and IGF-2R are members of the IGF family. IGF-2 can promote gastric cancer proliferation like IGF-1, while IGF-2R has a tumor-suppressive effect through clearance of IGF-2[28,29]. Our results suggest that expression of IGF-2 and IGF-2R was not changed by LDP, although IGF-2 expression was elevated in the diabetic mice. These results suggest that inhibition of IGF-1 activity contributes to the LDP effect in the suppression of gastric dysplasia.
AKT is activated by both insulin and IGF-1 in their signaling pathways. AKT promotes tumor formation by stimulation of cell proliferation and inhibition of apoptosis. These activities are related to activation of cylin D1 and inhibition of caspase-9, respectively. In gastric cancer, abnormal activation of AKT is widely observed and AKT has become a key target of antitumor agents[30]. Inhibition of phospho-AKT may represent another antineoplastic mechanism of LDP.
In addition to the insulin/IGF signaling, alteration of adipokines may contribute to the chemopreventive effects of LDP in our model. Adiponectin and leptin are two important adipokines that link type 2 diabetes to cancer. Adiponectin has an anti-inflammatory activity in addition to regulation of cell metabolism, proliferation and apoptosis[31]. Contrary to adiponectin, leptin has proinflammatory activity[32], which may increase the cancer risk. Human studies have revealed that a low level of adiponectin and high level of leptin are associated with an increased risk of gastric cancer[33,34]. Correction of the adipokines’ imbalance may contribute to the inhibition of gastric carcinogenesis by LDP. In this study, the effects of LDP on the adipokines are consistent with those reported by Perry et al[12] in obese rats[35]. These findings suggest that restoration of the balance of adiponectin and leptin may contribute to LDP activity.
In our study, LDP did not exhibit toxicity, as determined by body weight, mortality, appearance and physical activity of the mice. In addition, there was no significant increase in other parameters including AST, BUN and Cr in LDP-treated mice, suggesting that our dosage of LDP did not generate any adverse effect on liver and renal function. These results suggest that LDP is safe for treatment of type 2 diabetes and control of gastric tumorigenesis.
In summary, LDP exhibited excellent activity in the risk control of gastric tumorigenesis in diabetic mice. The activity was observed without any toxicity. Inhibition of gastric tumorigenesis was associated with reduction in hyperinsulinemia, serum IGF-1, and local IGF-1 signaling in the gastric tissue. Improvement of adiponectin and leptin imbalance may also contribute to the tumor control effect of LDP. The current study shed light on the potential of LDP in the management of both gastric dysplasia and type 2 diabetes.
The authors thank Professor Jian-Ping Ye very much for providing language help and writing assistance.
Accumulating studies suggest that type 2 diabetes increases the risk of gastric cancer, and some antidiabetic drugs, such as metformin, reduce the incidence of gastric cancer in patients with type 2 diabetes. Traditional Chinese medicine Liuwei Dihuang Pill (LDP) has a history of thousands of years in treating diabetes, and modern pharmacological research shows that LDP also prevents gastrointestinal tumors. However, it remains unknown whether and how LDP inhibits incidence and progression of diabetes-related gastric cancer in vivo.
The increased incidence of gastric cancer in type 2 diabetes may be associated with insulin resistance, hyperinsulinemia, excessive activity of insulin-like growth factor (IGF)-1, chronic inflammation, and abnormal alteration of adipokines. Therefore, targeting these abnormal metabolic alterations may be a promising strategy for reducing risk of gastric cancer in type 2 diabetes. Recent studies suggest that LDP reduces insulin resistance and hyperinsulinemia, and improves imbalance of adipokines. Investigation of the effects of LDP on the insulin/IGF-1 axis and adipokines will facilitate our understanding of the underlying mechanism of LDP in preventing gastric tumorigenesis in type 2 diabetes.
In the present study, LDP inhibited the early phase of gastric carcinogenesis in diabetic and obese mice, partly by alleviating insulin resistance, reducing insulin/IGF-1 activity and restoring adipokine abnormality. To the best of our knowledge, this is the first study to show the chemopreventive effect of LDP on diabetes-related gastric tumorigenesis and the underlying mechanism.
LDP may be a potential candidate for preventing gastric tumorigenesis in type 2 diabetic individuals and has value in clinical applications.
IGF-1 is a peptide hormone with a similar structure to insulin. Both insulin and IGF-1 exert their mitogenic effects by binding to their receptors. The activated insulin receptor and IGF-1 receptor mediate two pivotal signaling transduction pathways, phosphoinositide 3-kinase/protein kinase B and mitogen-activated protein kinase. Both pathways are involved in cancer cell growth, proliferation, apoptosis and angiogenesis.
This paper describes mechanisms of LDP for prevention of development of gastric dysplasia. This paper has several points to be revised before accepted for publication.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shimoyama S S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Tseng CH, Tseng FH. Diabetes and gastric cancer: the potential links. World J Gastroenterol. 2014;20:1701-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Sekikawa A, Fukui H, Maruo T, Tsumura T, Okabe Y, Osaki Y. Diabetes mellitus increases the risk of early gastric cancer development. Eur J Cancer. 2014;50:2065-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Hidaka A, Sasazuki S, Goto A, Sawada N, Shimazu T, Yamaji T, Iwasaki M, Inoue M, Noda M, Tajiri H. Plasma insulin, C-peptide and blood glucose and the risk of gastric cancer: the Japan Public Health Center-based prospective study. Int J Cancer. 2015;136:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Gristina V, Cupri MG, Torchio M, Mezzogori C, Cacciabue L, Danova M. Diabetes and cancer: A critical appraisal of the pathogenetic and therapeutic links. Biomed Rep. 2015;3:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: a review on proposed mechanisms. Cell Biochem Funct. 2016;34:533-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Chae YK, Arya A, Malecek MK, Shin DS, Carneiro B, Chandra S, Kaplan J, Kalyan A, Altman JK, Platanias L. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget. 2016;7:40767-40780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther. 2015;16:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Quinn BJ, Dallos M, Kitagawa H, Kunnumakkara AB, Memmott RM, Hollander MC, Gills JJ, Dennis PA. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res (Phila). 2013;6:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Kato M. Diagnosis and therapies for gastric non-invasive neoplasia. World J Gastroenterol. 2015;21:12513-12518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Zeng JH, Pan HF, Liu YZ, Xu HB, Zhao ZM, Li HW, Ren JL, Chen LH, Hu X, Yan Y. Effects of Weipixiao (胃痞消) on Wnt pathway-associated proteins in gastric mucosal epithelial cells from rats with gastric precancerous lesions. Chin J Integr Med. 2016;22:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Deng X, Liu ZW, Wu FS, Li LH, Liang J. A clinical study of weining granules in the treatment of gastric precancerous lesions. J Tradit Chin Med. 2012;32:164-172. [PubMed] |
| 12. | Perry B, Zhang J, Sun C, Saleh T, Wang Y. Liuwei dihuang lowers body weight and improves insulin and leptin sensitivity in obese rats. Evid Based Complement Alternat Med. 2012;2012:847167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Nair SV, Zhang J, Wang Y. Ethanol extract of Liuwei Dihuang reduces weight gain and visceral fat in obese-prone CD rats fed a high-fat diet. Exp Biol Med (Maywood). 2014;239:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zhao L, Yan S, Jiang T. [Inhibitory effect of liuwei dihuang decoction on induced mutation and spontaneous tumor]. Zhongxiyi Jiehe Zazhi. 1990;10:433-435, 390. [PubMed] |
| 15. | Cai B, Jiang T. Study on preventive and curative effects of liu wei di huang tang on tumors. J Tradit Chin Med. 1994;14:207-211. [PubMed] |
| 16. | Jiang TL, Yan SC, Zhao LF. Preventing effect of “liuwei dihuang decoction” on esophageal carcinoma. Gan To Kagaku Ryoho. 1989;16:1511-1518. [PubMed] |
| 17. | Yoshizawa N, Yamaguchi H, Yamamoto M, Shimizu N, Furihata C, Tatematsu M, Seto Y, Kaminishi M. Gastric carcinogenesis by N-Methyl-N-nitrosourea is enhanced in db/db diabetic mice. Cancer Sci. 2009;100:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kwon HJ, Won YS, Nam KT, Yoon YD, Jee H, Yoon WK, Nam KH, Kang JS, Han SU, Choi IP. Vitamin D3 upregulated protein 1 deficiency promotes N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric carcinogenesis in mice. Gut. 2012;61:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Hayakawa Y, Hirata Y, Nakagawa H, Sakamoto K, Hikiba Y, Kinoshita H, Nakata W, Takahashi R, Tateishi K, Tada M. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci USA. 2011;108:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Liu JP, Feng L, Zhang MH, Ma DY, Wang SY, Gu J, Fu Q, Qu R, Ma SP. Neuroprotective effect of Liuwei Dihuang decoction on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. J Ethnopharmacol. 2013;150:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Xie B, Gong T, Tang M, Mi D, Zhang X, Liu J, Zhang Z. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers. J Pharm Biomed Anal. 2008;48:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Quinn BJ, Dallos M, Kitagawa H, Kunnumakkara AB, Memmott RM, Hollander MC, Gills JJ, Dennis PA. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res (Phila). 2013;6:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Shimizu N, Kobayashi K, Fukushima S, Tatematsu M. N-methyl-N-nitrosourea concentration-dependent, rather than total intake-dependent, induction of adenocarcinomas in the glandular stomach of BALB/c mice. Jpn J Cancer Res. 1998;89:385-391. [PubMed] |
| 24. | Kim YI, Kim SY, Cho SJ, Park JH, Choi IJ, Lee YJ, Lee EK, Kook MC, Kim CG, Ryu KW. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: a nationwide cohort study. Aliment Pharmacol Ther. 2014;39:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Li HJ, Che XM, Zhao W, He SC, Zhang ZL, Chen R, Fan L, Jia ZL. Diet-induced obesity promotes murine gastric cancer growth through a nampt/sirt1/c-myc positive feedback loop. Oncol Rep. 2013;30:2153-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Wang HB, Zhou CJ, Song SZ, Chen P, Xu WH, Liu B, Zhu KX, Yu WH, Wu HL, Wang HJ. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol Res Pract. 2011;207:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Li H, Adachi Y, Yamamoto H, Min Y, Ohashi H, Ii M, Arimura Y, Endo T, Lee CT, Carbone DP. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257-2263. [PubMed] |
| 29. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1567] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 30. | Sasaki T, Kuniyasu H. Significance of AKT in gastric cancer (Review). Int J Oncol. 2014;45:2187-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Wang H, Ye J. Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev Endocr Metab Disord. 2015;16:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466-472. [PubMed] |
| 34. | Capelle LG, de Vries AC, Haringsma J, Steyerberg EW, Looman CW, Nagtzaam NM, van Dekken H, ter Borg F, de Vries RA, Kuipers EJ. Serum levels of leptin as marker for patients at high risk of gastric cancer. Helicobacter. 2009;14:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Perry B, Zhang J, Saleh T, Wang Y. Liuwei Dihuang, a traditional Chinese herbal formula, suppresses chronic inflammation and oxidative stress in obese rats. J Integr Med. 2014;12:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |