Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3928
Peer-review started: January 12, 2017
First decision: March 3, 2017
Revised: March 17, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: June 7, 2017
Processing time: 147 Days and 15.8 Hours
A case of esophageal carcinoma exclusively composed of adenocarcinoma simulating an esophageal gland duct in a 61-year-old man is presented. The tumor arose as a slightly elevated lesion in the middle intrathoracic esophagus. It was almost completely overlaid with non-neoplastic stratified squamous epithelial cells. Beneath the overlying surface epithelium, an adenocarcinoma that was bilayered in structure diffusely invaded both the mucosal and submucosal layers. Although the tumor consisted exclusively of adenocarcinomatous cells, a keratinizing squamous cell carcinoma component was focally observed. The invasive carcinoma was focally continuous with the small area of the surface squamous epithelial layer, which was confirmed to be neoplastic by immunohistochemistry. Morphological and immunohistochemical examinations suggested that the adenocarcinomatous component arose from the esophageal surface epithelium and clearly differentiated into an esophageal gland duct. It is important to consider the possibility of this type of adenocarcinoma when diagnosing a ductal or glandular lesion of the esophagus in small biopsy specimens.
Core tip: We present a case of esophageal carcinoma exclusively composed of adenocarcinoma simulating an esophageal gland duct. Morphologic and immunohistochemical examinations proved that adenocarcinomatous component here arose from the esophageal surface epithelium and showed a clear differentiation toward an esophageal gland duct. It is important to consider the possibility of this type of adenocarcinoma when diagnosing a ductal or glandular lesion of the esophagus in small biopsy specimens.
- Citation: Tamura H, Saiki H, Amano T, Yamamoto M, Hayashi S, Ando H, Doi R, Nishida T, Yamamoto K, Adachi S. Esophageal carcinoma originating in the surface epithelium with immunohistochemically proven esophageal gland duct differentiation: A case report. World J Gastroenterol 2017; 23(21): 3928-3933
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3928
Although the frequency of esophageal adenocarcinoma has dramatically increased in recent years in the United States, squamous cell carcinoma remains predominant and adenocarcinomas are relatively rare in Asian countries. Most tumors develop from Barrett’s esophagus, although they rarely arise from heterotopic gastric mucosa, as a component of adenosquamous cell carcinoma or mucoepidermoid carcinoma[1]. The tumors are similar in appearance to a conventional adenocarcinoma but exhibit various degrees of differentiation. Neoplastic glands or papillae are, if well-differentiated, composed of single-layered epithelium.
In some glandular organs such as mammary and salivary tissues, the cellular lining throughout the ductal-lobular system is bilayered[2,3]. The lining consists of an inner (luminal) epithelial and an outer (basal or myoepithelial) cell layer. Although neoplasm is basically monophasic, rare variants of tumors biphasic in nature, e.g., adenomyoepithelioma and epithelial myoepithelial carcinoma, can develop in these organs[4,5]. In addition, in the esophagus, the esophageal glands normally exhibit a ductal-lobular system with a bilayered structure[6]. Apart from esophageal gland duct adenoma featuring a double-layered epithelium[7], adenocarcinomas that are bilayered in structure are rarely observed as minor components of squamous cell carcinomas[8].
Here we report a case of extremely unique esophageal carcinoma composed exclusively of an adenocarcinoma that was bilayered in structure (i.e., with luminal and basal cell layers) and showed esophageal gland duct differentiation.
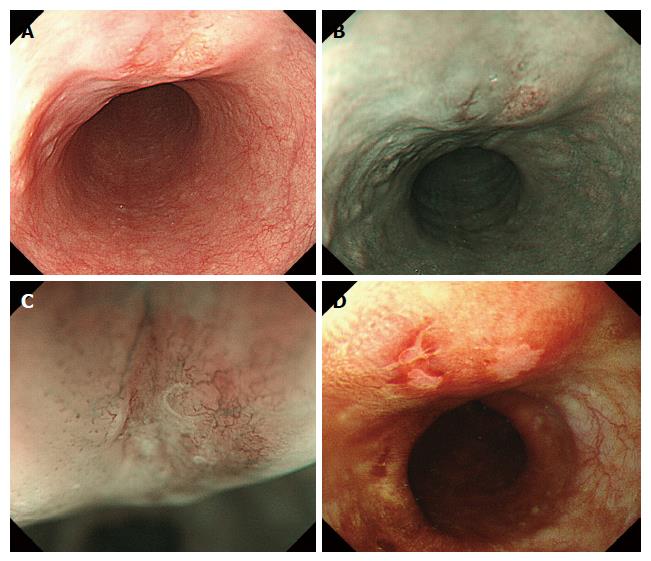
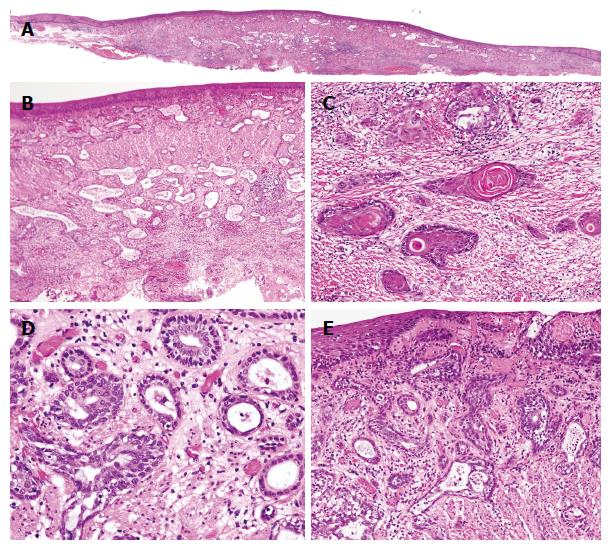
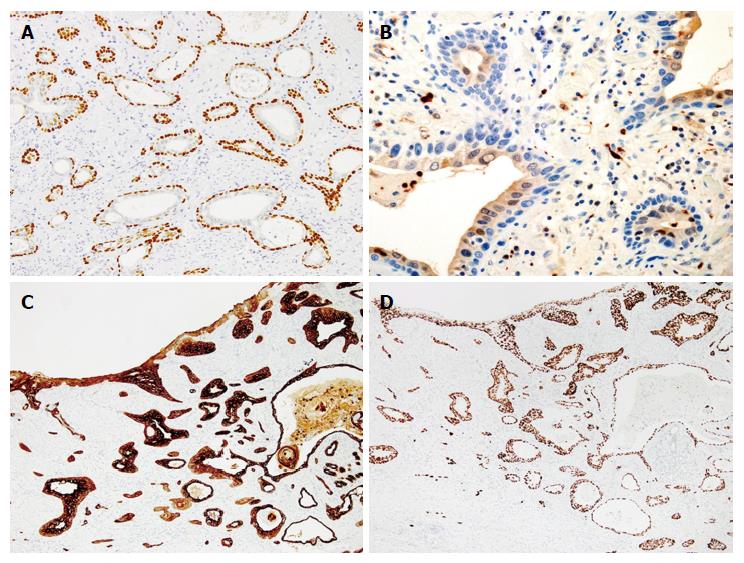
A 61-year-old man, who had been treated for hypertension and diabetes mellitus, was referred to our hospital because of a high level of peripheral CEA (6.9 ng/mL; normal range < 5.0). Endoscopic examinations of the esophagus revealed a slightly elevated and reddish lesion with small depressed areas located 28 cm from the incisors (Figure 1). The central and depressed areas were observed as brownish areas by narrow-band imaging (NBI) and unstained with Lugol’s iodine. Biopsy specimens of the lesion demonstrated atypical glands that were bilayered in structure dispersed, and not lobulated. Endoscopic ultrasonography suggested an infiltrative growth in the submucosal layer. We did not make a diagnosis from the biopsy specimens and sought to endoscopically remove the lesion for thorough examination. After diagnosing an esophageal carcinoma, the patient underwent radiation therapy and chemotherapy because he refused additional surgical treatment of the possible residual tumor. The patient remains in good health without disease after 5 years and 8 mo. The resected specimen (tumor size: 2 cm × 1.5 cm) was slightly elevated with a gentle slope as seen in the endoscopic examination (Figure 2A). The specimen was covered with stratified squamous epithelium except for a small erosive change on the top of the lesion. Neither Barrett’s metaplasia nor ectopic gastric mucosa was observed within the lesion. Variably sized atypical tubules including cystically dilated ones were dispersed in the mucosal and submucosal layers (Figure 2B). In addition, a small number of nests of keratinizing squamous cell carcinoma connecting with the neoplastic tubules were observed, mainly in the deepest region of the invasion (Figure 2C). Some of the neoplastic tubules exhibited relatively smooth contours; others were irregularly dilated. All tubules exhibited a bilayered structure, with inner luminal and outer epithelial layers (Figure 2D). Goblet cell-like mucus cells were rarely observed in the inner layer of the tubules. On top of the lesion, a connection between the invasive carcinoma component and the surface-covering epithelium was observed (Figure 2E). Conventional microscopic examination did not reveal sufficient atypia to support a diagnosis of dysplasia in the surface-covering squamous epithelium. Immunohistochemical examinations demonstrated that the outer layer cells of the neoplastic tubules were reactive for p63 (Figure 3A) and CK5/6, but negative for S100 and SMA. The inner layer cells were immunopositive for S100 (Figure 3B), CK7 (Figure 3C), and CK5/6, but negative for p63 and SMA. Overexpression of p53 was diffusely observed both in the inner luminal and outer layer cells (Figure 3D). Localized, transepithelial, high-level expression of p53 and CK7 was observed in the small region of the surface epithelium that was connected with the invasive carcinoma component (Figure 3C and D, respectively). The results of the examinations are listed in Table 1.
| Antibody | Source | Intralobular | Extralobular | Present case | |||
| Luminal | Myoepithel | Luminal | Basal | Inner | Outer | ||
| Anti-P63 | DAKO (prediluted) | - | ++ | - | ++ | - | ++ |
| Anti-CK5/6 | DAKO (prediluted) | ++ | Focal + | ++ | ++ | ± | ++ |
| Anti-S100 | DAKO (prediluted) | ++ | ++ | + | - | + | - |
| Anti-CK7 | DAKO (prediluted) | ++ | ++ | ++ | ++ | ++ | ++ |
| Anti-SMA | DAKO (prediluted) | - | ++ | - | - | - | - |
| Anti-P53 | DAKO (prediluted) | - | - | - | - | ++ | ++ |
The frequency of esophageal adenocarcinoma has increased in the United States, although squamous cell carcinoma remains predominant in Asian countries. Although most tumors develop as primary adenocarcinomas in the metaplastic glandular tissue of Barrett’s esophagus, an adenocarcinoma can sometimes arise as a metaplastic change in squamous cell carcinoma[8]. These exhibit a conventional tubular/papillary structure with various extents of differentiation; well-differentiated tubular adenocarcinomas feature well-formed neoplastic tubules with a single-layered epithelium. In the present case, the tumor was almost exclusively composed of well-formed tubules of various sizes, all of which exhibited a bilayered structure; i.e., inner luminal and outer cell layers. This unique morphological feature reminded us of the esophageal gland duct that is also composed of inner luminal cells and outer myoepithelial/basal cells. Although, as Takubo et al[9] reported, an adenocarcinomatous component similar to an esophageal gland duct is frequently observed among esophageal carcinomas. Only one case report describing a tumor predominantly composed of adenocarcinoma with a bilayered structure reminiscent of an esophageal gland duct has appeared. Immunohistochemical and morphological examinations clearly revealed that the neoplastic tubules in the present case were differentiating toward an esophageal gland duct. There have been no reports demonstrating immunohistochemical evidence of esophageal gland duct differentiation of a bilayered adenocarcinoma of the esophagus. The present case is the first report of esophageal carcinoma exclusively composed of adenocarcinoma with morphologically and immunohistochemically confirmed differentiation of an esophageal gland duct.
There have been a few case reports of esophageal carcinoma containing a bilayered adenocarcinomatous element. Hishida et al[10] reported a case of esophageal basaloid squamous cell carcinoma that partially contained an adenocarcinomatous component with a bilayered structure. In the cited case, the outer layer cells of the neoplastic tubules had clear cytoplasm and expressed SMA, similar to the outer layer cells seen in adenomyoepithelioma of the breast or epithelial-myoepithelial carcinoma of the salivary gland. The tumor reported by Ohtaka et al[11] was an esophageal carcinosarcoma in which the neoplastic ducts were surrounded by neoplastic myoepithelial cells. These cases suggest that the tubular components of the carcinomas differentiated towards the intralobular ducts of the esophageal gland. Endoh et al[12] also reported a case of esophageal adenocarcinoma differentiating toward an esophageal gland duct. However, unlike the above two cases, the outer layer cells of the neoplastic tubules were immunoreactive for CK34bE12 and CK5/6, but immunonegative for SMA. This immunoprofile suggested that the tumor differentiated towards an extralobular duct of the esophageal gland. In the present case, the tubular component also exhibited differentiation toward an extralobular duct of the esophageal gland.
The adenocarcinomatous components of the former two cases constituted small portions of a basaloid squamous cell carcinoma and carcinosarcoma, respectively. A basaloid squamous cell carcinoma in known to have the capacity to differentiate in different ways[13,14]. It was thus speculated that the component with the bilayered structure was characterized by multidifferentiation of an original squamous cell carcinoma (a basaloid or carcinosarcoma). On the other hand, Endoh et al[12] concluded that the adenocarcinomatous component originated in the esophageal gland duct because the overlying squamous cell epithelium did not exhibit significant atypia. The present case was also exclusively composed of adenocarcinoma without the component reminiscent of basaloid squamous cell carcinoma. The invasive carcinoma component was focally continuous with the overlying squamous epithelial layer. Although this epithelium did not show enough atypia to be diagnosed as dysplasia, the localized epithelial area showed diffuse and strong expression of CK7 and p53. The localized and intense expression of these proteins indicated that the area of the surface epithelium was neoplastic regardless of the relatively bland cytological appearance[15]. Therefore, it follows that the tumor in the present case exhibited bidirectional differentiation towards a novel esophageal gland duct and conventional squamous epithelium. Recent studies have shown that uncommitted stem cells are located in the papillae of the basal layer of the esophageal epithelium[16]. While some reports described that bilayered adenocarcinoma must have arisen in the original gland duct because of its morphological similarity[12], we believe that the origin should not be confused with the differentiation of the tumor. Molecular techniques including next-generation sequencing will aid in elucidating the origin of this unique carcinoma.
Although only a few tumors, including esophageal gland adenomas and salivary gland-type carcinomas, need to be considered in the differential diagnosis of tumors with bilayered structures, precise morphological and immunohistochemical examinations should render a distinction possible[7]. Squamous cell carcinoma sometimes exhibits a pseudoangiomatous pattern resulting from acantholysis and can closely mimic the tumor of the present case. Light microscopy may reveal the presence of a luminal cell layer, and immunohistochemical confirmation of p63 expression only in the outer cell layer can exclude the possibility of acantholytic squamous cell carcinoma.
Although a bilayer-structured adenocarcinoma differentiating towards an esophageal gland duct is not very rare, an esophageal tumor that is exclusively an adenocarcinoma (as in the present case) is exceptionally rare. Awareness of this type of adenocarcinoma in the esophagus can prevent pathologists from being confused when encountering small biopsy specimens of the adenocarcinoma.
Esophageal carcinoma in a 61-year-old man exhibited an endoscopically submucosal tumor-like appearance. The tumor was exclusively composed of an adenocarcinoma with morphologically and immunohistochemically confirmed esophageal gland duct-type differentiation.
A high level of peripheral carcino-embryonic antigen (CEA).
Esophageal carcinoma.
A high level of peripheral CEA.
Esophageal submucosal tumor.
Esophageal carcinoma exclusively composed of adenocarcinoma differentiating into an esophageal gland duct.
Endoscopic submucosal dissection, radiation therapy, and chemotherapy.
There have been reports describing esophageal carcinoma containing a small element of adenocarcinoma similar to an esophageal gland duct. Although one case of a carcinoma entirely comprised of an adenocarcinoma similar to an esophageal gland duct has been reported, neither the origin nor the differentiation pathway was clearly confirmed.
Conventional adenocarcinoma of the esophagus is composed of neoplastic tubules with single-layered epithelium, although the present case is almost exclusively composed of well-formed tubules with bilayered structure reminiscent of an esophageal gland duct.
An esophageal tumor exclusively composed of bilayer-structured adenocarcinoma differentiating towards an esophageal gland duct is exceptionally rare. Awareness of this type of adenocarcinoma in the esophagus can prevent pathologists from being confused when encountering small biopsy specimens of the adenocarcinoma.
This paper is informative and suitable for publication.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen XX, Thota PN S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Lewin KJ, Appelman HD. Barrett’s esophagus, columner dysplasia, and adenocarcinoma of the esophagus. 3rd ed. Rosai J, editor. Tumors of the Esophagus and Stomach. Vol. 18. AFIP Atlas of tumor pathology, Washington DC: Armed Forces of Pathology, 1996: 99-144. . |
| 2. | Collins LC, Schnitt SJ. Breast. 4th ed. Mills SE, editor. Histology for Pathologists, Philadelphia: Lippincott Williams &Wilkins, 2012: 67-81. . |
| 3. | Matrinez-Madrigal F, Bosq J, and Casiraghi O. Major Salivary Glands. 4th ed. Mills SE, editor. Historogy for Pathologists, Philadelphia: Lippincott Williams &Wilkins, 2012: 477-502. . |
| 4. | Tavassoli FA, Eusebi V. Adenomyoepithelioma. 4th ed. Silverberg SG, editor. Tumors of the Mammary Gland. Vol. 10. AFIP Atlas of tumor pathology, Washington DC: Armed Forces of Pathology, 2009: 250-256. . |
| 5. | Ellis GL, Auclair PL. Epithelial-Myoepithelial carcinoma. 4th ed. Silverberg SG, editor. Tumors of the Salivary Glands. Vol. 9, Washinton DC: Armed forces of Pathology, 2008: 309-322. . |
| 6. | Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG. The Nonneoplastic Esophagus. 3rd ed. Fenoglio-Preiser CM, editor. Gastrointestinal pathology, Philadelphia: Lippincott Williams &Wilkins; 2008: 11-84. . |
| 7. | Harada O, Ota H, Katsuyama T, Hidaka E, Ishizaka K, Nakayama J. Esophageal gland duct adenoma: immunohistochemical comparison with the normal esophageal gland and ultrastractural analysis. Am J Surg Pathol. 2007;31:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kuwano H, Ueo H, Sugimachi K, Inokuchi K, Toyoshima S, Enjoji M. Glandular or mucus-secreting components in squamous cell carcinoma of the esophagus. Cancer. 1985;56:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Takubo K, Esaki Y, Watanabe A, Umehara M, Sasajima K. Adenoma accompanied by superficial squamous cell carcinoma of the esophagus. Cancer. 1993;71:2435-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Hishida T, Nakanishi Y, Shimoda T, Igaki H, Tachimori Y, Kato H, Yamaguchi H, Iinuma G. Esophageal basaloid carcinoma with marked myoepithelial differentiation. Pathol Int. 2002;52:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ohtaka M, Kumasaka T, Nobukawa B, Hirai S, Suda K, Ohno Y, Iwazaki R, Ikegami Y, Fukasawa M. Carcinosarcoma of the esophagus characterized by myoepithelial and ductal differentiations. Pathol Int. 2002;52:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Endoh Y, Miyawaki M, Tamura G, Watanabe H, Motoyama T. Esophageal adenocarcinoma that probably originated in the esophageal gland duct: a case report. Pathol Int. 1999;49:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Kobayashi Y, Shimoda T, Nakanishi Y, Sekine S, Taniguchi H, Higetsu Y, Kato H, Yamaguchi H. Wide Histological Spectrum of Basaloid Squamous Cell Carcinoma of the Esophagus. Stomach and Intestine (Tokyo). 2005;40:371-379. [DOI] [Full Text] |
| 14. | Cho KJ, Jang JJ, Lee SS, Zo JI. Basaloid squamous carcinoma of the oesophagus: a distinct neoplasm with multipotential differentiation. Histopathology. 2000;36:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG. The Neoplastic Esophagus. 3rd ed. Fenoglio-Preiser CM, editor. Gastrointestinal pathology. Philadelphia: Lippincott Williams &Wilkins; 2008: 85-134. . |
| 16. | Pan Q, Nicholson AM, Barr H, Harrison LA, Wilson GD, Burkert J, Jeffery R, Alison MR, Looijenga L, Lin WR. Identification of lineage-uncommitted, long-lived, label-retaining cells in healthy human esophagus and stomach, and in metaplastic esophagus. Gastroenterology. 2013;144:761-770. [PubMed] [DOI] [Full Text] |