Published online May 28, 2017. doi: 10.3748/wjg.v23.i20.3721
Peer-review started: February 16, 2017
First decision: March 14, 2017
Revised: March 27, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: May 28, 2017
Processing time: 100 Days and 8.1 Hours
to investigate the expression of proliferating cell nuclear antigen (PCNA) and E-cadherin in gastric carcinoma and to analyze their clinical significance.
A total of 146 patients were selected for this study, including 38 patients with intestinal metaplasia, 42 with dysplasia, and 66 with primary gastric cancer. In addition, 40 patients with normal gastric tissues were selected as controls. The expression of PCNA and E-cadherin was detected by immunohistochemistry. Differences in PCNA and the E-cadherin labeling indexes among normal gastric mucosa, intestinal metaplasia, dysplasia, and gastric carcinoma were compared. Subjects with normal gastric tissues were assigned to a normal group, while gastric cancer patients were assigned to a gastric cancer group. The difference in PCNA and E-cadherin expression between these two groups was compared. The relationship between expression of PCNA and E-cadherin and clinicopathological features was also explored in gastric cancer patients. Furthermore, prognosis-related factors, as well as the expression of PCNA and E-cadherin, were analyzed in patients with gastric cancer to determine the 3-year survival of these patients.
The difference in PCNA and the E-cadherin labeling indexes among normal gastric mucosa, intestinal metaplasia, dysplasia, and gastric carcinoma was statistically significant (P < 0.05). During the transition of normal gastric mucosa to gastric cancer, the PCNA labeling index gradually increased, while the E-cadherin labeling index gradually decreased (P < 0.05). The PCNA labeling index was significantly higher and the E-cadherin labeling index was significantly lower in gastric cancer than in dysplasia (P < 0.05). The expression of PCNA was significantly higher in the gastric cancer group than in the normal group, but E-cadherin was weaker (P < 0.05). There was a negative correlation between the expression of PCNA and E-cadherin in gastric carcinoma (r = -0.741, P = 0.000). PCNA expression differed significantly between gastric cancer patients with and without lymph node metastasis and between patients at different T stages. E-cadherin expression also differed significantly between gastric cancer patients with and without lymph node metastasis (P < 0.05). High T stage and positive PCNA expression were risk factors for the prognosis of patients with gastric cancer (RR > 1), while the positive expression of E-cadherin was a protective factor (RR < 1). The sensitivity, specificity, and accuracy of PCNA positivity in predicting the 3-year survival of patients with gastric cancer were 93.33%, 38.89%, and 0.64, respectively; while these values for E-cadherin negativity were 80.0%, 41.67%, and 0.59, respectively. When PCNA positivity and E-cadherin negativity were combined, the sensitivity, specificity, and accuracy were 66.67%, 66.67%, and 0.67, respectively.
Combined detection of PCNA and E-cadherin can improve the accuracy of assessing the prognosis of patients with gastric cancer.
Core tip: The expression of proliferating cell nuclear antigen (PCNA) and E-cadherin was detected by immunohistochemistry in gastric tissues of 186 patients. During the transition of normal gastric mucosa to gastric cancer, the PCNA labeling index gradually increased, while the E-cadherin labeling index gradually decreased (P < 0.05). There was a negative correlation between the expression of PCNA and E-cadherin in gastric carcinoma. High T stage and positive PCNA expression were risk factors for the prognosis of patients with gastric cancer (RR > 1), while the positive expression of E-cadherin was a protective factor (RR < 1). Combined detection of PCNA and E-cadherin can improve the accuracy of assessing the prognosis of patients with gastric cancer.
- Citation: Hu L, Li HL, Li WF, Chen JM, Yang JT, Gu JJ, Xin L. Clinical significance of expression of proliferating cell nuclear antigen and E-cadherin in gastric carcinoma. World J Gastroenterol 2017; 23(20): 3721-3729
- URL: https://www.wjgnet.com/1007-9327/full/v23/i20/3721.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i20.3721
Gastric cancer is a common digestive system malignancy that progresses rapidly[1-3]. Its occurrence and development are a very complex process that involves the dysregulation of a variety of oncogenes and tumor suppressor genes[4-7]. At present, an increasing number of scholars have focused their attention on exploring protein and gene markers, in order to help clinicians early and accurately diagnose gastric cancer and assess its prognosis.
E-cadherin has been known as an epithelial cell adhesion molecule. A decrease in E-cadherin expression allows tumor cells to easily transfer and invade. Hence, E-cadherin has been identified as a metastatic suppressor of cancer cells[8,9]. Gastric cancer is a malignant tumor that originates from gastric epithelial cells. It has been reported that E-cadherin expression decreases in gastric cancer tissues, and that decreased E-cadherin expression correlates with high degree of malignancy and poor prognosis in patients with gastric cancer[8].
Proliferating cell nuclear antigen (PCNA) is a cell proliferation-associated protein. PCNA expression is associated with metastases of breast cancer, liver cancer and other malignancies, as well as tumor infiltration[10-13]. However, the expression of PCNA in gastric cancer and its clinical significance remain to be further studied.
In the present study, we detected the expression of PCNA and E-cadherin in gastric tissues of patients with gastric precancerous lesions or gastric cancer. We also evaluated the correlations of PCNA and E-cadherin expression with clinicopathological features and survival in patients with gastric cancer, with an aim to determine their clinical and prognostic significance in this malignancy.
One hundred and forty-six patients who underwent gastric surgery at our hospital from March 2012 to September 2013 were included in this observational study. These patients were pathologically diagnosed with intestinal metaplasia (n = 38), dysplasia (n = 42), or primary gastric cancer (n = 66). Forty patients with normal gastric tissues, who underwent gastrectomy during the same period, were included as controls. The inclusion criteria were: (1) patients who did not receive preoperative radiotherapy, chemotherapy, or other anti-cancer treatments; (2) patients with a clear pathological diagnosis; (3) patients without other malignancies; (4) patients who were followed for > 3 years (the deadline for the follow-up was the time of death) and had complete medical records. Among the patients with primary gastric cancer, 50 were male and 16 were female, with a mean age of 61.1 ± 11.2 years (range: 32-83 years). Among patients with intestinal metaplasia, 28 were male and 10 were female, with a mean age of 62.3 ± 10.6 years (range: 33-84 years). Among patients with dysplasia, 31 were male and 11 were female, with a mean age of 60.8 ± 10.9 years (range: 30-82 years). Among control subjects with normal gastric tissues, 29 were male and 11 were female, with a mean age of 61.4 ± 11.2 years (range: 32-81 years). There was no significant difference in age, gender or other demographic data between these four groups (P > 0.05). Informed consent was obtained from all patients enrolled in this study.
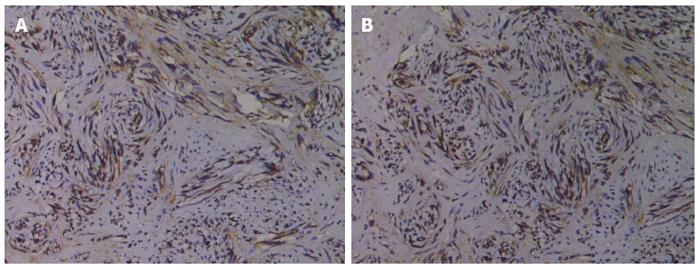
Tissue specimens were fixed in 10% formalin, embedded in paraffin, and sectioned into 3-μm thick sections. The sections were then dewaxed in xylene and hydrated in graded ethanol solutions (100%, 95% and 75%). After antigen retrieval with citrate buffer and inactivation of endogenous peroxidase with hydrogen peroxide, the slides were incubated with a primary antibody overnight at 4 °C, followed by incubation with a secondary antibody at 37 °C for 30 min. Sections were visualized using DAB solution, counterstained with hematoxylin, mounted with neutral gum, and observed under a microscope.
Immunohistochemical staining was evaluated by two pathologists in a double-blind manner. Using a high-power microscope, five fields of vision were randomly selected from each slice, with 100 cells counted in each field. The number of positive cells and the intensity of staining were then scored. The number of positive cells was scored as follows: 0 points, < 5% of stained cells; 1 point, 5%-20%; 2 points, 11%-50%; 3 points, 51%-75%; and 4 points, > 75%. The calculated percentage of positive cells was referred to as the labeling index. Staining intensity was scored as: 0 points, no staining; 1 point, light yellow; 2 points, brown yellow; and 3 points, tan. Protein expression was graded based on the product of scores for the percentage of stained cells and staining intensity: 1-3 points, negative (-); 4-5 points, weakly positive (+); 6-7 points, positive (++); ≥ 8 points, strongly positive (+++).
Differences in E-cadherin and PCNA labeling indexes were compared among normal gastric mucosa, intestinal metaplasia, dysplasia, and gastric cancer tissues. The expression of PCNA and E-cadherin was compared between subjects with normal gastric tissues (normal control group) and patients with gastric cancer (gastric cancer group). Association of E-cadherin and PCNA expression with clinicopathological features in patients with gastric cancer, including gender, age, degree of differentiation, lymph node metastasis, and T stage, were also analyzed. Factors that may affect the survival of patients were assessed, in order to identify whether PCNA and E-cadherin expression influences the prognosis of patients with gastric cancer. The survival curve of gastric cancer patients was drawn, and the accuracy of PCNA and E-cadherin in predicting 3-year survival of patients with gastric cancer was also assessed.
SPSS18.0 software was used for statistical analyses. Analysis of variance was used to analyze the difference in PCNA and E-cadherin labeling indexes among normal gastric mucosa, intestinal metaplasia, dysplasia, and gastric cancer tissues, and pairwise comparisons were performed using the Student-Newman-Keuls test. The expression of PCNA and E-cadherin between the gastric cancer group and normal control group was compared using the Mann-Whitney rank sum test, and Spearman’s correlation analysis was used for correlation assessment. The relationship between PCNA and E-cadherin expression and clinicopathological features of patients was assessed using the χ2-test (Fisher’s exact test). Log-rank analysis and Cox regression model were used to identify the factors that influence the survival of patients with gastric cancer, and the survival curve of gastric cancer patients was plotted. P-values < 0.05 were considered statistically significant.
Analysis of variance was used to compare the PCNA and E-cadherin labeling indexes in normal gastric mucosa, intestinal metaplasia, dysplasia, and gastric cancer tissues, and significant differences in the PCNA and E-cadherin labeling indexes were observed among these groups (P < 0.05). During the transition from normal gastric mucosa to intestinal metaplasia, dysplasia, and gastric cancer, the PCNA labeling index gradually increased and the E-cadherin labeling index gradually decreased. The PCNA labeling index was significantly higher and the E-cadherin labeling index was significantly lower in gastric cancer than in dysplasia (P < 0.05; Table 1, Figure 1).
| Tissue type | Number of cases | PCNA labeling index | E-cadherin labeling index |
| Normal gastric tissue | 40 | 1.37 ± 0.32 | 22.34 ± 6.23 |
| Intestinal metaplasia | 38 | 11.53 ± 3.38 | 13.92 ± 4.34 |
| Dysplasia | 42 | 14.34 ± 4.71 | 7.84 ± 2.08 |
| Gastric cancer | 66 | 44.50 ± 9.85 | 1.68 ± 0.47 |
| F-value | - | 4.170 | 5.181 |
| P-value | - | 0.018 | 0.002 |
| Comparison of gastric cancer and dysplasia | |||
| Q-value | -18.519 | 23.204 | |
| P-value | 0.000 | 0.000 | |
The expression of PCNA in gastric cancer was significantly higher than that in normal gastric tissues (Z = -5.231, P = 0.000), while the expression of E-cadherin was significantly lower in gastric cancer than in normal gastric tissues (Z = -4.982, P = 0.000) (Table 2). Spearman’s correlation analysis showed that PCNA expression was negatively correlated with E-cadherin expression in gastric cancer (r = -0.741, P = 0.000).
| Group | No. of cases | PCNA | E-cadherin | ||||||
| - | + | ++ | +++ | - | + | ++ | +++ | ||
| Normal group | 40 | 29 | 6 | 4 | 1 | 5 | 15 | 11 | 9 |
| Gastric cancer group | 66 | 16 | 11 | 17 | 22 | 45 | 11 | 7 | 3 |
| Z-value | - | -5.231 | -4.982 | ||||||
| P-value | - | 0.000 | 0.000 | ||||||
Among the 66 patients with gastric cancer, PCNA expression was positive in 50 cases and negative in 16 cases, while E-cadherin expression was positive in 21 cases and negative in 45 cases. The expression of PCNA was not significantly associated with gender, age, or degree of differentiation (P > 0.05), but was significantly correlated with lymph node metastasis and T stage (P < 0.05). E-cadherin expression was not significantly correlated with gender, age, degree of differentiation, or T stage (P > 0.05), but was significantly associated with lymph node metastasis (P < 0.05) (Table 3).
| Clinicopathological characteristic | No. of cases | PCNA | E-cadherin | ||
| Positive | P value | Positive | P value | ||
| Gender | 0.073 | 0.340 | |||
| Male | 45 | 37 | 16 | ||
| Female | 21 | 13 | 5 | ||
| Age (yr) | 0.319 | 0.140 | |||
| ≥ 60 | 40 | 32 | 10 | ||
| < 60 | 26 | 18 | 11 | ||
| Lymph node metastasis | 0.039 | 0.000 | |||
| Yes | 42 | 36 | 7 | ||
| Non | 24 | 14 | 14 | ||
| Degree of differentiation | 0.278 | 0.065 | |||
| Medium and low differentiation | 42 | 30 | 10 | ||
| High differentiation | 24 | 20 | 11 | ||
| T stage | 0.003 | 0.568 | |||
| T1/T2 | 25 | 14 | 9 | ||
| T3/T4 | 41 | 36 | 12 | ||
Log-rank analysis was performed to identify factors that influence the survival of patients with gastric cancer, with gender, age, lymph node metastasis, degree of differentiation, T stage, PCNA expression, and E-cadherin expression analyzed. It was found that lymph node metastasis, T stage, PCNA expression, and E-cadherin expression were correlated with the prognosis of patients (P < 0.05). These indexes were then included in the Cox regression model multivariate analysis, which revealed that T stage and the expression levels of E-cadherin and PCNA were independent prognostic factors in gastric cancer (P < 0.05). Among these factors, high T stage and positive PCNA expression were risk factors for the prognosis of patients with gastric cancer (RR > 1), while the positive expression of E-cadherin was a protective factor (RR < 1) (Table 4).
| Variable | Log-rank univariate analysis | Cox regression multivariate analysis | |
| P value | P value | RR (95%CI) | |
| Gender | 0.285 | - | - |
| Age | 0.128 | - | - |
| Lymph node metastasis | 0.000 | 0.055 | 4.369 (0.967-19.733) |
| Degree of differentiation | 0.268 | - | - |
| T stage | 0.004 | 0.000 | 17.556 (5.343-57.680) |
| PCNA | 0.003 | 0.000 | 28.786 (5.088-162.853) |
| E-cadherin | 0.021 | 0.005 | 0.174 (0.051-0.598) |
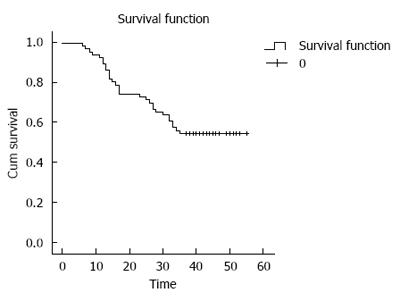
The 3-year survival rate of the 66 patients with gastric cancer was 60.1% (40/66), and the survival curve is shown in Figure 2. The significance of E-cadherin and PCNA expression in predicting 3-year survival rate of gastric cancer patients was then assessed. It was found that PCNA positivity had a sensitivity, specificity, and accuracy of 93.33%, 38.89%, and 0.64, respectively, while the sensitivity, specificity, and accuracy of E-cadherin negativity were 80.0%, 41.67%, and 0.59, respectively. When combining these two indexes (PCNA positivity and E-cadherin negativity), the sensitivity, specificity, and accuracy were 80.0%, 66.67%, and 0.73, respectively (Table 5).
| Indicators | Survival time <3 yr | Survival time >3 yr | Sensitivity | Specificity | Accuracy |
| PCNA | 93.33% | 38.89% | 0.64 | ||
| Positive | 28 | 22 | |||
| Negative | 2 | 14 | |||
| E-cadherin | 80.0% | 41.67% | 0.59 | ||
| Positive | 6 | 15 | |||
| Negative | 24 | 21 | |||
| The combination of both (PCNA[+] and E-cadherin[-]) | 80.0% | 66.67% | 0.73 | ||
| Positive | 24 | 12 | |||
| Negative | 6 | 24 |
The development of gastric cancer is a gradual process of evolution controlled by a variety of oncogenes and tumor suppressor genes, and this multistep and sequential process evolves from normal gastric mucosa to intestinal metaplasia, dysplasia, and gastric cancer[14-18]. At present, TNM staging of gastric cancer has been applied clinically to assess the prognosis of patients. However, the TNM stage does not fully reflect the prognosis of patients with gastric cancer[19-24]. For some gastric cancer patients with the same TNM stage, their response to treatment and prognosis are different[25,26]. The wide use of endoscopy and other technologies has allowed to obtain lesion samples from patients at an earlier stage. Simultaneously, these tissues can also be sent for more molecular testing to assess the nature of these lesions and evaluate the prognosis. Therefore, more experts and scholars have focused on the study of molecular changes in gastric cancer and the prognostic value of TNM staging in patients with gastric cancer[27-32].
Strong proliferation is an important characteristic of malignant tumors. PCNA, as a cell cycle-related protein, is closely related to DNA synthesis. PCNA is rarely expressed in the G0 phase of the cell cycle, but begins to increase in the G1 phase, reaches a peak in the S phase, and decreases in the G2-M phase. Thus, PCNA can be a good indicator of cellular proliferation and be used to assess invasive lesions. However, its expression and clinical significance in the development of gastric cancer remains to be further studied[33-35]. The invasion and metastasis of malignant tumors involve the tumor cell itself and the interaction between tumor cells and their microenvironment, in which cell adhesion changes play an important role. E-cadherin, as a marker of epithelial cells, can mediate the adhesion between cells. A decline in E-cadherin expression would cause cells to lose its polarity, decrease cell junction stability, and contribute to the invasion and metastasis of tumor cells[36,37]. Previous studies have confirmed that E-cadherin is a cancer metastasis inhibitory molecule, and that decreased expression of E-cadherin may be used as a molecular marker to evaluate the malignant degree of gastric cancer[8,36]. Therefore, in this study, the expression of PCNA and E-cadherin in gastric cancer tissues was detected, with E-cadherin as a malignancy assessment indicator, in order to analyze changes in PCNA expression in the occurrence and development of gastric cancer, and determine its prognostic significance in patients with gastric cancer.
The degree of malignancy in the progression of normal gastric tissues to gastric cancer gradually increased. In order to understand the changes in PCNA expression during this process, we detected the expression of PCNA and E-cadherin in normal gastric mucosa, intestinal metaplasia, dysplasia, and gastric cancer, and significant differences in the E-cadherin and PCNA labeling indexes were found among these four phases. The expression of PCNA had a gradually increasing trend and that of E-cadherin exhibited a decreasing trend, and the differences were statistically significant between dysplasia and gastric cancer. In order to clarify whether the expression of PCNA and E-cadherin in gastric cancer is different from that in normal gastric tissues, we further conducted a detailed analysis on the expression of E-cadherin and PCNA in gastric carcinoma and normal gastric tissues. The expression of PCNA in the gastric cancer group was stronger than that in the normal group, while the expression of E-cadherin was weaker. Furthermore, there was a negative correlation between the expression of PCNA and E-cadherin in gastric carcinoma. With the gradual evolution of normal gastric mucosa toward gastric cancer, the degree of malignancy increased. During this progression, the proliferation rate of malignant cells was significantly higher than that in normal tissues[38-40]. Previous studies have demonstrated that p53 is associated with the progression of gastric cancer, while PCNA is a downstream regulatory target of p53, suggesting that the expression of PCNA is associated with the progression of gastric cancer[41-43]. The occurrence and development of gastric cancer and gastric epithelial hyperplasia are correlated, and malignant cell proliferation also enables the number of cells entering the cell cycle to significantly increase. In the G1 and S phases, cells express large amounts of PCNA. Therefore, we found that as the degree of malignancy increased in tissues, PCNA expression gradually increased[44-47]. We also found that changes in the expression of PCNA and E-cadherin exhibited a contradictory trend, and there was a negative correlation between them. E-cadherin is known as a tumor suppressor. It is important in maintaining the number of cells and the interconnection between normal cells. The occurrence of tumor suppressor gene mutations and other changes affect the expression of E-cadherin, which thus weakens the connection between tumor cells and promotes cancer cell activity and invasion[48,49]. Therefore, we speculate that PCNA may reflect the degree of malignancy in the occurrence and development of gastric cancer, and increased expression of may PCNA suggest the increased malignancy of tissues.
In order to further understand whether PCNA has good value in assessing the malignancy and prognosis of gastric cancer, we further analyzed the relationship of PCNA and E-cadherin expression with the clinicopathological characteristics of gastric cancer. We found that there were significant differences in the expression of PCNA between patients with and without lymph node metastasis, and between patients at different T stages. In addition, the expression of E-cadherin in patients with and without lymph node metastasis was also significantly different. Since T stage and lymph node metastasis are important prognostic factors and are closely related to the prognosis of patients with gastric cancer, we hypothesized that the expression of PCNA in patients who present with these prognostic factors may also be affected. Therefore, we analyzed the survival time of gastric cancer patients. Results revealed that high T stage and positive PCNA expression are risk factors for the prognosis of patients with gastric cancer, while the positive expression of E-cadherin was a protective factor. Thus, high PCNA expression may be associated with tumor proliferation and invasion ability, and is a risk factor for the prognosis of patients with gastric cancer[50,51]. Since E-cadherin is an inhibitor of cancer cell metastasis, the normal expression of E-cadherin reflects the good adhesion between cells, and in this condition cancer cells from tumor tissues could not easily metastasize. Therefore, E-cadherin expression is a protective factor for the prognosis of patients with gastric cancer[8].
In order to further understand the value of PCNA and E-cadherin expression in evaluating the survival of patients with gastric cancer, we analyzed the accuracy of PCNA and E-cadherin expression in predicting the 3-year survival of patients with gastric cancer. It was found that PCNA expression had a high sensitivity but a low specificity in predicting the 3-year survival. This may be associated with the presence of non-cancerous cells in such cases of benign proliferation. In addition, the proliferation of normal cells also produces PCNA protein. The expression of E-cadherin had a slightly lower sensitivity but a higher specificity than that of PCNA. Taking into account the use of tissue immunohistochemical detection for conducting multiple molecular tests, it is feasible to evaluate the degree of malignancy by combining multiple molecules. We further combined both PCNA positivity and E-cadherin negativity to evaluate the prognosis of gastric cancer patients, and found that although the sensitivity of the combined detection was slightly lower than that of PCNA alone, the specificity and accuracy were higher than those of PCNA alone. Therefore, we believe that when assessing the prognosis of patients with gastric cancer, PCNA can be first detected, and the positive of E-cadherin can be further detected, in order to help improve the accuracy of prognostic evaluation.
However, the development of gastric cancer is the result of a variety of genetic variations and abnormal proteins. In this study, only PCNA and E-cadherin were detected and analyzed. Future research may consider combining Ki67, Oct4, and other molecular indicators of tumor invasion and metastasis, in order to evaluate the prognosis of patients with gastric cancer. Furthermore, extending the follow-up time may also be considered, in order to obtain a more comprehensive understanding of their prognostic value for patients.
In summary, PCNA expression in gastric cancer tissue increases, and the expression of E-cadherin decreases. The detection of both indicators can help assess tumor proliferation and metastasis activity. Furthermore, the combined application of these two indicators can improve the accuracy of assessing the prognosis of patients with gastric cancer.
Gastric cancer is a common digestive system malignancy that progresses rapidly. Its occurrence and development are a very complex process that involves the dysregulation of a variety of oncogenes and tumor suppressor genes. At present, an increasing number of scholars have focused their attention on exploring protein and gene markers, in order to help clinicians early and accurately diagnose gastric cancer and assess its prognosis.
It has been reported that E-cadherin expression decreases in gastric cancer tissues, and that decreased E-cadherin expression correlates with high degree of malignancy and poor prognosis in patients with gastric cancer. Proliferating cell nuclear antigen (PCNA) is a cell proliferation-associated protein. PCNA expression is associated with metastases of breast cancer, liver cancer and other malignancies, as well as tumor infiltration. However, the expression of PCNA in gastric cancer and its clinical significance remain to be further studied. Therefore, this study investigated the clinical significance of expression of PCNA and E-cadherin in gastric carcinoma, with an aim to help explore more molecular markers for assessing gastric cancer.
The wide use of endoscopy and other technologies has allowed to obtain lesion samples from patients at an earlier stage. The use of immunohistochemical method has made it simple and convenient to detect the expression of PCNA and E-cadherin. PCNA, as a cell cycle-related protein, is closely related to DNA synthesis. It can be a good indicator of cellular proliferation and be used to assess invasive lesions. E-cadherin is a cancer metastasis inhibitory molecule, and decreased expression of E-cadherin may be used as a molecular marker to evaluate the malignant degree of gastric cancer. Therefore, detecting the expression of PCNA and E-cadherin in gastric cancer tissues can help evaluate the prognosis of patients with gastric cancer.
This study demonstrated that as normal gastric mucosa transitioned into gastric cancer, the PCNA labeling index gradually increased, while the E-cadherin labeling index gradually decreased. There was a negative correlation between the expression of PCNA and E-cadherin in gastric carcinoma. Combined detection of PCNA and E-cadherin improves the accuracy of assessing the prognosis of patients with gastric cancer. Therefore, combined detection of PCNA and E-cadherin is recommended to evaluate the tissue malignancy and the prognosis of patients.
This is an interesting study about the expression and detection value of PCNA and E-cadherin in gastric carcinoma. This study is well designed and the results are very interesting. In this study, the authors investigated the expression and detection value of PCNA and E-cadherin in gastric carcinoma. Approximately 146 patients were selected for this study, including 38 cases with intestinal metaplasia, 42 with severe atypical hyperplasia, and 66 with primary gastric cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ominami M, Park SJ, Yamaoka Y S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Abreu Velez AM, Howard MS, Kim J, Googe PB. Markers for Sebaceoma Show a Spectrum of Cell Cycle Regulators, Tumor Suppressor Genes, and Oncogenes. N Am J Med Sci. 2015;7:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Akrami H, Aminzadeh S, Fallahi H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumour Biol. 2015;36:3237-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Martín-Núñez GM, Cabrera-Mulero A, Alcaide-Torres J, García-Fuentes E, Tinahones FJ, Morcillo S. No effect of different bariatric surgery procedures on LINE-1 DNA methylation in diabetic and nondiabetic morbidly obese patients. Surg Obes Relat Dis. 2017;13:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Zhu B, Wu JR, Zhou XP. A Retrospective Comparison of Trastuzumab Plus Cisplatin and Trastuzumab Plus Capecitabine in Elderly HER2-Positive Advanced Gastric Cancer Patients. Medicine (Baltimore). 2015;94:e1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Choi HJ, Rha SY. Palliative prognostic tools for the terminally ill patients with gastric cancer. J Clin Oncol. 2014;32:120. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Yao Y, Feng S, Xiao M, Li Y, Yang L, Gong J. MTA1 promotes proliferation and invasion in human gastric cancer cells. Onco Targets Ther. 2015;8:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Yie SM, Ye SR, Ma XL, Xie K, Zhang JB, Cao M, He X, Hu ZB, Yang CL, Zhang J. A protein fragment derived from DNA-topoisomerase I as a novel tumour-associated antigen for the detection of early stage carcinoma. Br J Cancer. 2016;115:1555-1564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Khouzam RA, Molinari C, Salvi S, Marabelli M, Molinaro V, Orioli D, Saragoni L, Morgagni P, Calistri D, Ranzani GN. Digital PCR identifies changes in CDH1 (E-cadherin) transcription pattern in intestinal-type gastric cancer. Oncotarget. 2017;8:18811-18820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Mellman I, Yarden Y. Endocytosis and cancer. Cold Spring Harb Perspect Biol. 2013;5:a016949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Hamzaoui L, Bouassida M, Kilani H, Medhioub M, Chelbi E. Metastatic Squamous Cell Carcinoma of the Stomach. J Clin Diagn Res. 2015;9:OD05-OD06. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Wang F, Sun GP, Zou YF, Zhong F, Ma T, Li XQ, Wu D. Helicobacter pylori infection predicts favorable outcome in patients with gastric cancer. Curr Oncol. 2013;20:e388-e395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Özmen T, Javadov M, Yeğen CS. Factors affecting surgical site infection rate after elective gastric cancer surgery. Ulus Cerrahi Derg. 2016;32:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Poosarla C, Ramesh M, Ramesh K, Gudiseva S, Bala S, Sundar M. Proliferating Cell Nuclear Antigen in Premalignancy and Oral Squamous Cell Carcinoma. J Clin Diagn Res. 2015;9:ZC39-ZC41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yang XW, Gao F, Chen YJ, Teng FM. The Clinical Study of Urokinase-Type Plasminogen Activator and Vascular Endothelial Growth Factor in Gastric Cancer. Cell Biochem Biophys. 2015;72:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Sajeevan TP, Saraswathi TR, Ranganathan K, Joshua E, Rao UD. Immunohistochemical study of p53 and proliferating cell nuclear antigen expression in odontogenic keratocyst and periapical cyst. J Pharm Bioallied Sci. 2014;6:S52-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yamaguchi H, Kitayama J, Ishigami H, Kazama S, Nozawa H, Kawai K, Hata K, Kiyomatsu T, Tanaka T, Tanaka J. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: Intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol. 2015;7:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lee JC, Lee SY, Kim CY, Yang DH. Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer. J Korean Surg Soc. 2013;85:283-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Kitajima Y, Miyazaki K. The Critical Impact of HIF-1a on Gastric Cancer Biology. Cancers (Basel). 2013;5:15-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Shady MM, Fathy HA, Ali A, Galal EM, Fathy GA, Sibaii H. Comparison of Serum IgG Antibody Test with Gastric Biopsy for the Detection of Helicobacter Pylori Infection among Egyptian Children. Open Access Maced J Med Sci. 2015;3:303-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Guo T, Yang J, Yao J, Zhang Y, Da M, Duan Y. Expression of MACC1 and c-Met in human gastric cancer and its clinical significance. Cancer Cell Int. 2013;13:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Guo CG, Chen YJ, Ren H, Zhou H, Shi JF, Yuan XH, Zhao P, Zhao DB, Wang GQ. A nomogram for predicting the likelihood of lymph node metastasis in early gastric signet ring cell carcinoma: A single center retrospective analysis with external validation. Medicine (Baltimore). 2016;95:e5393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Herreros-Villanueva M, Bujanda L, Gil I, Caballero MC, Cosme A. Triple synchronous gastric tumors: A rare combination diffuse adenocarcinoma, B-cell MALT lymphoma and large cell neuroendocrine carcinoma. Gastroenterol Hepatol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Huang Z, Li Y, Zhao H, Zhao JJ, Cai JQ. Prognositic factors and clinicopathologic characteristics of small gastrointestinal stromal tumor of the stomach: a retrospective analysis of 31 cases in one center. Cancer Biol Med. 2013;10:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Markar SR, Hanna GB. Surgical resection of gastric cancer hepatic metastases: expanding the indications for curative treatment. Transl Gastroenterol Hepatol. 2016;1:80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Liu K, Zhang W, Chen X, Chen X, Yang K, Zhang B, Chen Z, Zhou Z, Hu J. Comparison on Clinicopathological Features and Prognosis Between Esophagogastric Junctional Adenocarcinoma (Siewert II/III Types) and Distal Gastric Adenocarcinoma: Retrospective Cohort Study, a Single Institution, High Volume Experience in China. Medicine (Baltimore). 2015;94:e1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Lim HK, Park JM, Chi KC, Lee EJ, Jeong EM. Disappearance of Serum Methylated p16 Indicates Longer Survival in Patients with Gastric Cancer. J Gastric Cancer. 2013;13:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Wang H, Chen MM, Zhu GS, Ma MG, Du HS, Long YP. Lymph node mapping with carbon nanoparticles and the risk factors of lymph node metastasis in gastric cancer. J Huazhong Univ Sci Technolog Med Sci. 2016;36:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Cong X, Lu C, Huang X, Yang D, Cui X, Cai J, Lv L, He S, Zhang Y, Ni R. Increased expression of glycinamide ribonucleotide transformylase is associated with a poor prognosis in hepatocellular carcinoma, and it promotes liver cancer cell proliferation. Hum Pathol. 2014;45:1370-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Aristatile B, Al-Numair KS, Al-Assaf AH, Veeramani C, Pugalendi KV. Protective Effect of Carvacrol on Oxidative Stress and Cellular DNA Damage Induced by UVB Irradiation in Human Peripheral Lymphocytes. J Biochem Mol Toxicol. 2015;29:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Balta S, Unlu M, Arslan Z, Demırkol S. Neutrophil-to-Lymphocyte Ratio in Prognosis of Gastric Cancer. J Gastric Cancer. 2013;13:196-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, Xu D. Preoperative C-Reactive Protein/Albumin Ratio Predicts Prognosis of Patients after Curative Resection for Gastric Cancer. Transl Oncol. 2015;8:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Çalik M, Demirci E, Altun E, Çalik İ, Gündoğdu ÖB, Gürsan N, Gündoğdu B, Albayrak M. Clinicopathological importance of Ki-67, p27, and p53 expression in gastric cancer. Turk J Med Sci. 2015;45:118-128. [PubMed] |
| 33. | Cidon EU, Ellis SG, Inam Y, Adeleke S, Zarif S, Geldart T. Molecular targeted agents for gastric cancer: a step forward towards personalized therapy. Cancers (Basel). 2013;5:64-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Polotskaia A, Xiao G, Reynoso K, Martin C, Qiu WG, Hendrickson RC, Bargonetti J. Proteome-wide analysis of mutant p53 targets in breast cancer identifies new levels of gain-of-function that influence PARP, PCNA, and MCM4. Proc Natl Acad Sci USA. 2015;112:E1220-E1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Lv Q, Zhang J, Yi Y, Huang Y, Wang Y, Wang Y, Zhang W. Proliferating Cell Nuclear Antigen Has an Association with Prognosis and Risks Factors of Cancer Patients: a Systematic Review. Mol Neurobiol. 2016;53:6209-6217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Kleiner S, Faisal A, Nagamine Y. Induction of uPA gene expression by the blockage of E-cadherin via Src- and Shc-dependent Erk signaling. FEBS J. 2007;274:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Calaf GM, Roy D, Narayan G, Balajee AS. Differential expression of cell adhesion molecules in an ionizing radiation-induced breast cancer model system. Oncol Rep. 2013;30:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Kang BW, Kim JG, Park H, Park BE, Jeon SW, Bae HI, Kwon OK, Chung HY, Yu W. Clinical Significance of MET Gene Copy Number in Patients with Curatively Resected Gastric Cancer. Chonnam Med J. 2015;51:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Coelho E, Magalhães A, Dinis-Ribeiro M, Reis CA. [Molecular Mechanisms for Adhesion and Colonization of Human Gastric Mucosa by Helicobacter pylori and its Clinical Implications]. Acta Med Port. 2016;29:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Guo YL, Zhu TN, Guo W, Dong ZM, Zhou Z, Cui YJ, Zhao RJ. Aberrant CpG Island Shore Region Methylation of CAV1 Is Associated with Tumor Progression and Poor Prognosis in Gastric Cardia Adenocarcinoma. Arch Med Res. 2016;47:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Jin EH, Lee DH, Jung SA, Shim KN, Seo JY, Kim N, Shin CM, Yoon H, Jung HC. Clinicopathologic factors and molecular markers related to lymph node metastasis in early gastric cancer. World J Gastroenterol. 2015;21:571-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Lv H, Liu R, Fu J, Yang Q, Shi J, Chen P, Ji M, Shi B, Hou P. Epithelial cell-derived periostin functions as a tumor suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway. Cell Cycle. 2014;13:2962-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Park KK, Yang SI, Seo KW, Yoon KY, Lee SH, Jang HK, Shin YM. Correlations of Human Epithelial Growth Factor Receptor 2 Overexpression with MUC2, MUC5AC, MUC6, p53, and Clinicopathological Characteristics in Gastric Cancer Patients with Curative Resection. Gastroenterol Res Pract. 2015;2015:946359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Kinami S. Perspective of new techniques overcoming laparoscopic sentinel node biopsy for early gastric cancer. Transl Gastroenterol Hepatol. 2016;1:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Bayrak M, Olmez OF, Kurt E, Cubukcu E, Evrensel T, Kanat O, Manavoglu O. Prognostic significance of c-erbB2 overexpression in patients with metastatic gastric cancer. Clin Transl Oncol. 2013;15:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Gatenby PA, Shaw C, Hine C, Scholtes S, Koutra M, Andrew H, Hacking M, Allum WH. Retrospective cohort study of an enhanced recovery programme in oesophageal and gastric cancer surgery. Ann R Coll Surg Engl. 2015;97:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Xing GL, Tian SH, Xie XL, Fu J. HS-4, a highly potent inhibitor of cell proliferation of human cancer cell. Asian Pac J Trop Med. 2015;8:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Martowicz A, Rainer J, Lelong J, Spizzo G, Gastl G, Untergasser G. EpCAM overexpression prolongs proliferative capacity of primary human breast epithelial cells and supports hyperplastic growth. Mol Cancer. 2013;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Turanli S, Bozdogan N, Mersin H, Berberoglu U. The Effect of Helicobacter pylori on Gastric Cancer Treated with Adjuvant Chemotherapy After Curative Resection. Indian J Surg. 2015;77:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Ray D, Morimoto M. Malrotation of the Intestine in Adult and Colorectal Cancer. Indian J Surg. 2015;77:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Dong LL, Liu L, Ma CH, Li JS, Du C, Xu S, Han LH, Li L, Wang XW. E-cadherin promotes proliferation of human ovarian cancer cells in vitro via activating MEK/ERK pathway. Acta Pharmacol Sin. 2012;33:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |