Published online May 28, 2017. doi: 10.3748/wjg.v23.i20.3702
Peer-review started: September 29, 2016
First decision: November 21, 2016
Revised: January 15, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: May 28, 2017
Processing time: 240 Days and 10.5 Hours
to investigate the short-term outcomes and risk factors indicating postoperative death of patients with lesions adjacent to the hepatocaval confluence.
We retrospectively analyzed 54 consecutive patients who underwent hepatectomy combined with inferior vena cava (IVC) and/or hepatic vein reconstruction (HVR) from January 2012 to January 2016 at our liver surgery center. The patients were divided into 5 groups according to the range of IVC and hepatic vein involvement. The patient details, indications for surgery, operative techniques, intra- and postoperative outcomes were compared among the 5 groups. Univariate and multivariate analyses were performed to explore factors predictive of overall operative death.
IVC replacement was carried out in 37 (68.5%) patients and HVR in 17 (31.5%) patients. Type I2H2 had the longest operative blood loss, operative duration and overall liver ischemic time (all, P < 0.05). Three patients of Type I3H1 with totally occluded IVC did not need IVC reconstruction. Total postoperative morbidity rate was 40.7% (22 patients) and the operative mortality rate was 16.7% (9 patients). Factors predictive of operative death included IVC replacement (P = 0.048), duration of liver ischemia (P = 0.005) and preoperative liver function being Child-Pugh B (P = 0.025).
IVC replacement, duration of liver ischemia and preoperative poor liver function were risk factors predictive of postoperative death. We should be cautious about IVC replacement, especially in Type I2H2. For Type I3H1, it was unnecessary to replace IVC when the collateral circulation was established.
Core tip: The proposed IH classification, which divided the patients into 5 groups according to the range of vascular invasion, may be meaningful in selecting procedures for patients with hepatocaval confluence infiltration. inferior vena cava replacement, duration of liver ischemia and preoperative poor liver function were risk factors predictive of postoperative death for patients with lesions adjacent to the hepatocaval confluence.
- Citation: Li W, Han J, Wu ZP, Wu H. Surgical management of liver diseases invading the hepatocaval confluence based on IH classification: The surgical guideline in our center. World J Gastroenterol 2017; 23(20): 3702-3712
- URL: https://www.wjgnet.com/1007-9327/full/v23/i20/3702.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i20.3702
Liver malignancies including hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) and colorectal liver metastases, combined with liver parasitic diseases like alveolar echinococcosis (AE), often show an infiltrative growth pattern. If major vessels such as the inferior vena cava (IVC) and hepatic vein adjacent to its caval confluence are invaded by these lesions, combined liver and IVC resection followed by IVC and/or hepatic outflow reconstruction with other materials is necessary to achieve R0 resection[1-3]. As a consequence of recent advances in perioperative management and surgical technique, liver and IVC resection combined with major vascular reconstruction has become a reasonably safe treatment option with acceptable short- and long-term survival.
Preoperative portal vein embolism, associating liver partition with portal vein ligation for staged hepatectomy (commonly referred to as ALPPS), systemic chemotherapy (mainly for colorectal liver metastases) and other innovative treatments increase the tumor resectability[4-6]. Total vascular exclusion (TVE) and other vascular exclusion techniques offer chances of resection for tumor with major vascular involvement. In situ perfusion technique can be applied in patients with TVE longer than 60 min. Moreover, the utilization of anti-situm and ex vivo technique makes it easier to acquire a better operative filed and obtain tumor-free surgical margins[7-9]. Venovenous bypass (VVB) is necessary in some patients under TVE with drastic hemodynamic fluctuations[7].
Though technically challenging, hepatectomy combined with major vascular resection and reconstruction has been performed in many centers[7-10]. However, due to the lack of surgical protocols, different standards have been used in different centers. Here, we present our surgical guideline and outcomes for the combined liver and IVC resection in 54 patients with different kinds of liver lesions invading the hepatocaval confluence. The “IH classification” outlined herein was established based on our experience, and was the surgical guideline in our center.
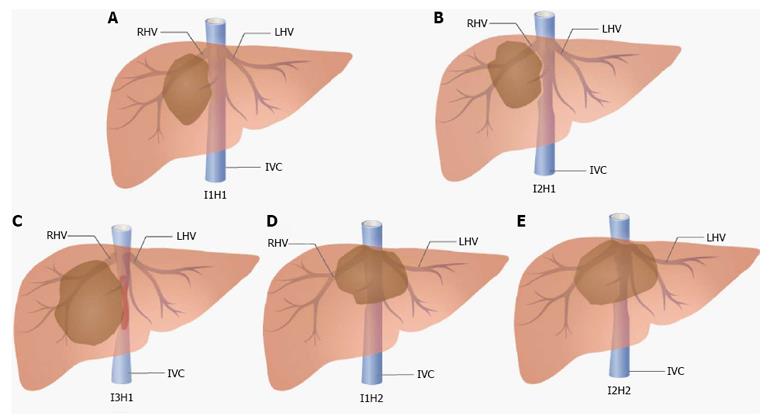
We retrospectively analyzed 54 consecutive patients who underwent liver resection combined with IVC resection and reconstruction from January 2012 to January 2016 at our liver surgery center at the West China Hospital, Sichuan University. Cases with IVC involvement that could be detached primarily without reconstruction were not included in this study. Patients with tumor thrombus in the IVC or hepatic veins were also excluded. The final diagnoses were confirmed by histopathological examinations after surgery. We have established classifications for this challenging situation based on our experience and the patients were divided into 5 groups according to the classifications (Figure 1). The indications for surgery of these patients are summarized in Table 1. All procedures described in this study were approved by the Ethics Committee of West China Hospital, Sichuan University.
| Indications for surgery | n | Sex (M:F), n | Classifications | ||||
| I1H1 | I2H1 | I1H2 | I2H2 | I3H1 | |||
| Hepatocellular carcinoma | 11 | 6:05 | 2 | 5 | 1 | 3 | 0 |
| Cholangiocarcinoma | 26 | 18:08 | 3 | 10 | 4 | 6 | 3 |
| Colorectal metastases | 8 | 5:03 | 1 | 5 | 0 | 1 | 1 |
| Alveolar echinococcosis | 9 | 5:04 | 3 | 3 | 0 | 2 | 1 |
Classification based on varying degrees of IVC infiltration: I1: Less than 50% of IVC circumference is involved and the IVC is not totally occluded; I2: More than 50% of IVC circumference is involved and the IVC is not totally occluded; and I3: The encroached IVC is totally occluded.
Classification based on hepatic outflow conditions: H1: The hepatic outflow of the residual liver is not involved; and H2: The hepatic outflow of the residual liver is involved (3 hepatic veins are all infiltrated).
The ultrasonography and contrast computed tomography (CT) scan or magnetic resonance imaging of the abdomen were performed to evaluate the number and extent of lesions, gross type, liver volume, presence of major vascular infiltration, and regional or distant metastasis. Our standard indication for hepatectomy was Child-Pugh grade A or B, or indocyanine green retention rate at 15 min < 10%. Some patients with metastatic colorectal cancer received systemic chemotherapy after evaluation in the cancer center of our hospital, and all of them underwent colonoscopy before surgery. Our policy for indication of portal vein embolism is when the predicted future liver remnant is less than 40% of the total non-tumorous functional liver volume[11].
The procedures for hepatectomy have been reported elsewhere[11,12]. Our preferred abdominal incision was J-shaped thoracoabdominal incision. After mobilization, the intraoperative ultrasound was performed routinely to confirm the number and location of lesions as well as to evaluate the relation of tumor to major vessels. The other major procedures before hepatectomy included: portal pedicle division and ligation, exposing and encircling the infra- and supra-hepatic IVC (the supradiaphragmatic IVC was encircled if the diaphragm was invaded), and dividing and ligating the short hepatic veins if possible. Liver parenchyma transaction (Pringle’s maneuver was used if necessary) was carried out with the Kelly crush technique or other instruments including CUSA (Valleylab Corp., Somerville, NJ, United States) or Harmonic scalpel (Johnson & Johnson Corp., Princeton, NJ, United States). The anterior approach was used if bulky lesions resided in the right lobe of the liver.
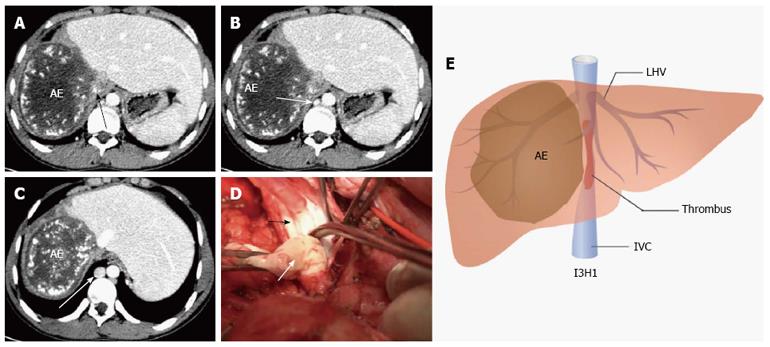
When the critical remaining parenchyma and vascular structures were exposed, various vascular control techniques were applied. For type I1H1, we clamped the IVC tangentially without IVC exclusion or clamped IVC below the hepatic vein (CIBH) of the remnant liver without hepatic outflow exclusion. In our experience, if IVC involvement was less than 30% of the IVC circumference and 2 cm of the length, the defect was usually sutured transversely after removing the invaded IVC wall. If IVC involvement was 30% to 50% of IVC circumference and longer than 2 cm, we used autogenous veins such as great saphenous vein patches or expanded polytetrafluoroethylene (ePTFE; Gore-Tex, Flagstaff, AZ, United States) patches for IVC repair. As for type I1H2, IVC reconstruction was similar to type I1H1. TVE (clamping the infra-hepatic IVC, portal triad and supra-hepatic IVC sequentially) was utilized for IVC and hepatic vein reconstruction. In this type, 3 hepatic veins were involved, and the remaining stump of hepatic vein was reimplanted directly into the vena cava, or an interposed reinforced ePTFE graft. With respect to type I2H1, we used TVE or CIBH (if there was enough room below the hepatic vein) for blood control. And, if longitudinal infiltration was longer than 3 cm, IVC replacement was performed. Regarding type I2H2, TVE was necessary to complete tumor resection and vascular reconstruction. In this type, vascular reconstruction included hepatic outflow and IVC reconstruction. IVC was replaced with ePTFE tube graft and the hepatic vein was reimplanted into the ePTFE tube graft if it was totally invaded. Otherwise, if hepatic vein of the residual liver was partially involved, we used autogenous vein patches or ePTFE patches for hepatic vein plasty. With respect to I3H1, if collateral circulation including ascending lumbar veins, hemiazygos vein, and azygos vein were dilated and compensated portal hypertension and caval flow effectively, we only performed liver and IVC resection without IVC replacement (Figure 2).
Ex vivo, in situ perfusion and anti-situm technique were predominately used in type I2H2. In situ hypothermic perfusion as described by DuBay et al[13] can be performed when TVE lasts longer than 60 min. When TVE was utilized, the patient’s hemodynamic condition was carefully monitored and VVB (installed from the inferior mesenteric vein, and the right femoral vein to the left internal jugular vein) was applied when the patient could not tolerate the hemodynamic fluctuation. Ex vivo technique, which we have reported elsewhere[14], is easier to obtain tumor-free surgical margins and reconstruct the vessels. However, given the higher complications (including bile leakage, bile duct stricture and prosthetic graft infection caused by bile leakage) associated with biliary tract anastomosis[15,16], ex vivo was performed only on patients with the IVC, hepatic vein confluence, and/or portal structures infiltrated extensively. VVB was needed for most of the patients who underwent ex vivo. Anti-situm technique, first introduced by Pichlmayr et al[17] 20 years ago, did not need to divide the portal structures. After cutting off the supra-hepatic IVC, the liver together with the IVC was rotated to the anterior position, away from their anatomic location. Then, hepatectomy could be achieved rather easily with infra-hepatic IVC and the portal triad exclusion, hypothermic hepatic perfusion and percutaneous VVB.
All patients were treated with low-molecular weight heparin sodium anticoagulation solution (1 mg per kg bodyweight) from 2 d after surgery, with close monitoring. After discharge from hospital, the patients were given warfarin (2.5 mg, qd, po) for 3 mo. Enhanced abdominal CT or ultrasonography was performed every 7 d in the first mo postoperatively to detect the patency of reconstructed vessels. For hepatitis B virus-infected patients, anti-viral drugs were applied.
Postoperative mortality was defined as death within 90 d of operation. Clavien-Dindo classification was used to classify all general complications occurring at any time during the hospital stay[18]. Liver failure was defined as peak bilirubin concentration > 7 mg/dl, peak international normalized ratio > 2.0, refractory ascites, or encephalopathy[19]. Bile leakage was defined as a drain fluid-to-serum total bilirubin concentration ratio ≥ 3.0[20]. Renal insufficiency was defined as increase of serum urea and/or creatinine level (50% above the baseline). Clinically significant ascites was defined when abdominal drainage was more than 500 ml/d for longer than 3 d.
The clinicopathologic characteristics and short-term surgical outcomes of these patients were compared among the 5 groups. Categorical variables were expressed as number and tested by chi-square test or Fisher’s exact test. Continuous variables were summarized as mean (range) and tested by one-way ANOVA (Student-Newman-Keuls test was used when ANOVA was significant) or Kruskal-Wallis H rank test when necessary. The prognostic significance of the variables in predicting operative death was performed by univariate and multivariate binary logistic regression analysis. All statistical analyses were 2-tailed and P values < 0.05 were regarded as statistically significant. All analyses were performed by SPSS 19.0 statistical software (IBM Corp., Armonk, NY, United States).
Fifty-four patients (34 males, 20 females) underwent hepatectomy combined with vascular resection and reconstruction, with a median (range) age of 49.7 (39-72) years. The indications for surgery were: ICC (n = 26), HCC (n = 11), colorectal metastases (n = 8) and AE (n = 9) (Table 1). The intra- and postoperative data for the different types of liver lesions treated by hepatectomy combined with IVC and/or HVR are summarized in Table 2. The resection concerned 4.7 liver segments medially (range, 1-6 segments). IVC replacement was performed in 37 (68.5%) patients and HVR in 17 (31.5%) patients.
| Variables | I1H1 (n = 9) | I2H1 (n = 23) | I1H2 (n = 5) | I2H2 (n = 12) | I3H1 (n = 5) | |
| IVC resection and replacement (n = 2) | Only IVC resection (n = 3) | |||||
| RL:REL:RT:LEL:LT | 3:2:2:1:1 | 4:6:8:2:3 | 0:1:2:2:0 | 2:4:3:2:1 | 1:0:1:0:0 | 1:2:0:0:0 |
| Tumor size (cm) | 7.2 (2.9-14.3) | 8.7 (7.1-15.4) | 9.2 (3.9-9.9) | 9.6 (7.2-16.1) | 9.4 (6.6-12.2) | 8.3 (7.1-10.2) |
| Operative blood loss (mL) | 460 (310-950) | 740 (450-1250) | 570 (450-1050) | 1020 (550-1700)a | 680 (550-810) | 450 (350-560) |
| Need for blood transfusion | 1 | 8 | 0 | 7 | 1 | 0 |
| Transfusion volume (mL) | 400 | 550 (200-950) | 0 | 600 (300-850)c | 400 | 0 |
| Operation duration (min) | 290 (210-420) | 592 (480-800) | 520 (250-860) | 750 (310-1150)a | 580 (490-670) | 320 (240-440) |
| No. of patients using TVE | 2 | 23 | 3 | 12 | 2 | 0 |
| No. of patients using PM | 5 | 17 | 2 | 8 | 2 | 2 |
| Duration of TVE (min) | 50 (40,60) | 62 (46-90) | 48 (35-68) | 73 (37-89)a | 49 (39-57) | 0 |
| Duration of PM (min) | 25 (10-35) | 36 (15-45) | 22.5(20-25) | 38 (10-50) | 30 (15-15) | 30 (15-15) |
| Reconstruction detail | IVCR: direct suture in 4, with a patch in 5; HVR: no | IVCR: replacement; HVR: no | IVCR: with a patch in 5; HVR: reimplant to ePTFE in 1, to residual IVC in 4 | IVCR: replacement; HVR: with a patch in 3, reimplant to ePTFE in 5, to residual IVC in 4 | IVCR: replacement; HVR: no | IVCR: no; HVR: no |
| Surgical technique | ||||||
| Anti-situm | 0 | 0 | 0 | 2 | 1 | 0 |
| Ex vivo | 0 | 0 | 0 | 6 | 0 | 0 |
| In situ perfusion | 0 | 2 | 1 | 5 | 0 | 0 |
| Postoperative liver function | ||||||
| Serum maximum AST (IU/L) | 460 (220-870) | 557 (240-1240) | 490 (230-590) | 630 (330-1350) | 520 (370,670) | 665 (265-768) |
| Serum maximum ALT (IU/L) | 565 (345-1350) | 695 (230-1510) | 520 (280-1020) | 710 (340-1405) | 610 (410-810) | 685 (210-830) |
| Serum maximum PT (s) | 14.1 (12.2-16.3) | 15.4 (13.3-16.9) | 14.8 (12.8-15.9) | 15.4 (13.4-17.5) | 16.4 (15.5-17.3) | 15.5 (13.7-16.7) |
| Serum maximum TB (mmol/L) | 33.4 (28.5-44.7) | 36.8 (29.4-56.9) | 33.7 (31.2-47.7) | 45.0 (34.1-55.6) | 35.0 (27.0-43.0) | 33.1 (28.0-56.1) |
| Hospital stay (d) | 11 (7-17) | 15 (9-24) | 12 (8-22) | 19 (13-28) | 14 (11-17) | 16 (13-19) |
Type I2H2 had the longest operative blood loss, operation duration and overall liver ischemic time than the other 4 types (all, P < 0.05). The other clinical characteristics of the 5 types including tumor size, postoperative liver function, and hospital stay were listed in Table 2 in detail. Type I2H2 had the most complex procedure, which needed IVC replacement and hepatic vein plasty (n = 3; with autogenous vein patches in 2 and ePTFE patches in 1) or reimplantation (n = 9; reimplant to ePTFE graft in 5 and to residual IVC in 4). Anti-situm (n = 2), ex vivo (n = 6) and in situ perfusion (n = 5) were mainly utilized in I2H2. Three patients of type I3H1 with totally occluded IVC did not need IVC reconstruction. The other 2 patients underwent IVC resection and replacement due to the uncompensated collateral circulation. The surgical procedures for the other 3 types were described in Table 2.
Total postoperative morbidity rate was 40.7% (22 patients) and the operative mortality rate was 16.7% (9 patients) (Table 3). Total morbidity and mortality rates of type I2H2 were higher than for type I1H1 (both, P < 0.05). Artificial graft infection (n = 4; 2 in type I2H2 and 2 in type I2H1), liver failure (n = 4; 2 in type I2H1 and 2 in type I2H2) and thrombosis of reconstructed vessels (n = 1; 1 in type I2H2) were the main reasons leading to postoperative death. Univariate analysis of factors predictive of death were Child-Pugh B (P = 0.004), IVC replacement (P = 0.044), duration of ischemia (P < 0.001) and duration of operation (P < 0.001) (Table 4). Factors predictive of operative death in multivariate analysis included IVC replacement (P = 0.048), duration of liver ischemia (P = 0.005) and preoperative liver function being Child-Pugh B (P = 0.025) (Table 5).
| Variable | I1H1 (n = 9) | I2H1 (n = 23) | I1H2 (n = 5) | I2H2 (n = 12) | I3H1 (n = 5) | |
| IVC resection and replacement (n = 2) | Only IVC resection (n = 3) | |||||
| Total number | 1 | 8 | 2 | 8a | 2 | 1 |
| Biliary leak | 1 | 1 | 1 | |||
| Liver failure | 2 | 2 | ||||
| Ascites | 1 | 4 | 1 | 2 | 1 | 1 |
| Jaundice | 1 | |||||
| Hemorrhage requiring reoperation | 1 | |||||
| Thrombosis of reconstructed vessels | ||||||
| Hepatic vein | 1 | |||||
| Inferior vena cava | 1 | |||||
| Intraabdominal abscess | 1 | |||||
| Reconstructed vessel infection | 2 | 2 | 1 | |||
| Wound infection | ||||||
| Respiratory complication | 1 | 1 | ||||
| Clavien-Dindo classification | ||||||
| Grade I-II | 1 | 7 | 2 | 6 | 1 | 1 |
| Grade III-V | 1 | 4 | 0 | 5 | 1 | 0 |
| 90-d mortality | 0 | 4 | 0 | 5a | 0 | 0 |
| All patients (n = 54) | Operative death | P value | ||
| Yes (n = 9) | No (n = 45) | |||
| Age (yr) | 49.7 (39-72) | 53(45-72) | 49 (39-67) | 0.249 |
| Sex ratio (M:F) | 34:20 | 6:3 | 28:17 | 0.801 |
| Preoperative chemotherapy | 4 | 1 | 3 | 1.000 |
| Preoperative PVE | 9 | 2 | 7 | 1.000 |
| Tumor type | ||||
| Colorectal metastases | 8 | 2 | 6 | 0.864 |
| Hepatocellular carcinoma | 11 | 2 | 9 | 1.000 |
| Cholangiocarcinoma | 26 | 4 | 22 | 1.000 |
| Alveolar echinococcosis | 9 | 1 | 8 | 1.000 |
| Preoperative TB > 34 μmol/L | 6 | 1 | 5 | 1.000 |
| ICG-R15 over 10% | 9 | 3 | 6 | 0.327 |
| Child-Pugh B | 6 | 4 | 2 | 0.004 |
| No. of segments resected | 4.7 (3-6) | 5.1 (4-6) | 4.6 (4-6) | 0.189 |
| Classifications | 0.157 | |||
| I1H1 | 9 | 0 | 9 | 0.328 |
| I2H1 | 23 | 4 | 19 | 1.000 |
| I1H2 | 5 | 0 | 5 | 0.576 |
| I2H2 | 12 | 5 | 7 | 0.028 |
| I3H1 | 5 | 0 | 5 | 0.576 |
| IVC replacement | ||||
| Yes (I2 + 2 cases in I3) | 37 | 9 | 28 | 0.044 |
| No (I1 + 3 cases in I3) | 17 | 0 | 17 | |
| Hepatic vein reconstruction | ||||
| Yes (H2) | 17 | 4 | 13 | 0.600 |
| No (H1) | 37 | 5 | 32 | |
| Duration of ischemia (min) | 68.7 (0-112) | 87.7 (62-112) | 64.9 (0-106) | < 0.001 |
| Operative blood loss (mL) | 721.5 (310-1250) | 769.0 (550-1250) | 712.3 (310-780) | 0.389 |
| Blood transfused amount (mL) | 174.1 (0-950) | 219.4 (0-950) | 165.8 (0-850) | 0.501 |
| Duration of operation (min) | 554.8 (210-1150) | 709.6 (310-1150) | 523.7 (210-860) | < 0.001 |
| R0 resection | 49 | 7 | 42 | 0.401 |
| Tumor size | 8.7 (2.9-16.1) | 9.5 (8.8-16.1) | 8.6 (2.9-15.4) | 0.062 |
| OR (95%CI) | P value | |
| IVC replacement | 37.56 (1.46-945.32) | 0.048 |
| Duration of ischemia | 1.65 (1.02-2.58) | 0.005 |
| Child B or C | 1.82 (1.14-2.89) | 0.025 |
The median follow-up time was 20 mo (range, 2-48 mo). No patient was lost during follow-up. A total of 8 patients (3 in type I2H2, 2 in type I2H1, 2 in type I1H2, and 1 in type I1H1) died from tumor recurrence within 6 mo after the operations. Overall 1- and 3-year actuarial survival rates for HCC were 60% and 45% and for ICC were 55% and 38%. Twenty-five patients developed recurrence. Local recurrence in the liver occurred in 16 patients, in brain in 3, and in lung in 4, and abdominal cavity metastasis was detected in 2. Disease-free 1- and 3-year survival rates for patients with HCC were 18% and 8% respectively, and for patients with ICC were 16% and 9%. All AE patients were alive without recurrence and metastasis at the last follow-up.
In the present study, 54 patients who underwent liver resection combined with IVC and/or HVR were included. ICC, HCC, AE and colorectal metastasis were the main causes leading to IVC encroach. Undoubtedly, when liver diseases have involved the hepatocaval confluence, resection and reconstruction of the vascular structures remain technically difficult. A variety of vascular exclusion techniques, IVC reconstruction strategies, and other innovative surgical methods have brought hope for patients in this late stage[21,22].
Due to the high postoperative morbidity and mortality rates, though technically feasible, it remains controversial as to whether or not we should perform radical resection with vascular reconstruction for lesions invading IVC and other major vessels. However, prognosis of malignant tumor involved IVC is unfavorable when performing hepatectomy without IVC reconstruction[10]. R0 resection combined with IVC reconstruction may have a better short- and long-term prognosis than cases which only underwent hepatectomy or conservative treatment, but further prospective studies are needed to investigate it.
In Table 6, we summarized the morbidity and mortality rates of the patients who underwent liver resection and IVC reconstruction in previous reports. In the present study, total postoperative morbidity rate was 40.7% (22 patients) and the operative mortality rate was 16.7% (9 patients; Table 3). Artificial vascular graft was the most commonly used material due to the shortage of xenogenous vessels and a larger surgery injury when utilizing autogenous vein[23]. Though graft infection was a life-threatening complication of artificial tube graft, many studies including ours showed that graft infection rate after artificial graft replacement was < 10%[15,24,25]. For type I2H2, postoperative mortality rate was higher than the other types, which may be related to the longer operation time, longer ischemic time, more blood loss and higher postoperative morbidity rate. Consequently, for patients in type I2H2, it was still controversial about whether we should perform such an extensive operation.
| Ref. | Hospital mortality | Hospital morbidity | No. alive/total (follow-up time) |
| DuBay et al[13] | 11.1% (1 of gastrointestinal bleeding and multiple organ failure) | 22.2% | 6/9 (2-33 mo) |
| Malde et al[15] | 11.4% (4 of multiple organ failure) | 40.0% | 16/35 (1-140 mo) |
| Azoulay et al[31] | 4.5% (1 of sepsis and multiple organ failure) | 64.0% | 11/22 (7-84 mo) |
| Madariaga et al[21] | 11.0% (1 of liver failure) | 22.2% | 6/9 (3-156 mo) |
| Giordano et al[25] | 4.0% (1 of liver failure) | 39.1% | 16/23 (1-33 mo) |
| Hemming et al[24] | 8.3% (liver failure and multiple organ failure) | 43.0% | 46/60 (median 31 mo) |
| Yamamoto[29] | 28.6% (1 of sepsis, 1 of liver failure) | 28.6% | 2/7 (2-72 mo) |
| Lodge et al[10] | 25% (1 of sepsis and multiple organ failure, 1 of respiratory and renal failure) | 87.5% | 7/8 (0.5-30 mo) |
Most of the previous studies demonstrated that it was difficult to assess IVC involvement preoperatively relying on imaging technique[15,24,25]. Though intraoperative ultrasonography and cavography were performed to help confirm the IVC invasion, the true IVC invasion rate confirmed by pathological examinations after surgery was only 60% in our study (data not shown). For malignant infiltrative-growth diseases, including ICC and AE (characterized by tumor-like growth), R0 resection was a primary goal of treatment. IVC resection was necessary when it was infiltrated or embraced by the lesions which cannot be divided totally. For HCC and colorectal liver metastases (the tumor usually compress rather than encroaches the vessels), sometimes we could not achieve R0 resection when the IVC was surrounded by the tumor; thus, IVC replacement was performed in some of these patients. Multi-organ infiltration was not a surgical contraindication for AE. Given the lack of alternative curative approaches, a radical operation with complete removal of the parasitic lesions was the best beneficial way to achieve radical treatment[26-28]. However, IVC resection combined with reconstruction in AE patients was still controversial considering the severe complications related to the IVC replacement.
Moreover, multivariate analysis in the present study showed that IVC replacement was a prognostic factor predictive of operative death (P = 0.048); thus, indications of IVC replacement should be controlled strictly. In our experience, we have established the IH classification according to the range of tumor invasion. According to the extent of caval involvement, the IVC was reconstructed using a tube graft (I2), direct suture or with patches (I1). For I3 (IVC was totally occluded), if there were no symptoms and life-threatening complications associated with caval obstruction and portal hypertension (Figure 2), the IVC was removed without replacement (empirically, when renal vein pressure was < 40 mmHg, the kidney function was not affected). Once the collateral circulation could not compensate the IVC stricture or occlusion, IVC replacement was necessary. In our study, 3 patients with AE were given IVC resection without reconstruction and had good short- and long-term survival. As for H1, we protected the hepatic vein of the residual liver during the operation and HVR was unnecessary. If 3 hepatic veins were involved (H2), hepatic vein plasty (with autogenous vein graft or ePTFE patches) or reimplantation (to the tube graft or residual IVC) was carried out to recover hepatic outflow. However, the criteria of IVC reconstruction in different centers are not identical due to the small sample size and patient heterogeneity (Table 7).
| Ref. | Year | No. of cases | Indication | IVC repair type | Hepatic vein reconstruction | VVB | Perfusion | Technique | IVC reconstruction criteria | ||
| Tube | Patch | Suture | |||||||||
| DuBay et al[13] | 2009 | 9 | IVC leiomyosarcoma = 4; ICC = 2; PCC = 1; Metastases = 1; Malignant schwannoma = 1 | 7 | 0 | 0 | Into the native IVC = 1; Into the graft = 5; Primary repair = 1 | Not described | 9 | In situ perfusion | Not described |
| Malde et al[15] | 2011 | 35 | metastasis = 21; HCC = 6; ICC = 3; Other conditions = 5 | 11 | 2 | 22 | Not described | Not described | 12 | In situ perfusion = 13; Anti situm = 3; Ex vivo = 6 | < 2 cm: direct suture; > 2 cm: with patches; > 50% of the circumference and longitudinally infiltration: replacement |
| Azoulay et al[31] | 2006 | 22 | Metastasis = 9; ICC =8; HCC = 2; Other cancers = 3 | 10 | 4 | 8 | Into the native IVC = 4; Into the graft =2 | 12 | 9 | In situ perfusion = 9; Anti situm and ex vivo = 0; TVE only = 12; Others = 1 | < 30% circumference: longitudinally suture; 30%-50% circumference: transversely suture; > 50% circumference: replacement |
| Madariaga et al[21] | 2000 | 9 | Metastasis = 1 IVC leiomyosarcoma = 3; ICC = 3; other cancers = 2 | 8 | 0 | 1 | Into the graft = 1; Primary repair = 1 | 1 | 0 | In situ perfusion, Anti situm and ex vivo = 0; TVE only = 3 | Not described |
| Giordano et al[25] | 2011 | 23 | Metastases = 13; ICC = 3; HCC = 4; Others = 3 | 7 | 0 | 16 | Into the graft = 1 | 4 | 4 | In situ perfusion = 4; Anti situm = 0; Ex vivo = 0 | < 30% of the circumference: suture; > 50% of the circumference: replacement |
| Hemming et al[24] | 2012 | 60 | ICC = 26; HCC = 16; Metastases = 13; Others = 5 | 38 | 14 | 8 | Into the graft = 4 | 6 (ex vivo) | 8 | In situ perfusion = 8; Ex vivo = 6; Anti situm = 0 | < 3 cm longitudinally: end-to-end anastomosis; > 5 cm sections of the anterolateral wall: with patches; 3-8 cm longitudinally : replacement |
| Yamamoto[29] | 2012 | 7 | ICC = 2; HCC = 5 | 4 | 1 | 2 | Into the graft = 4 | 0 | 7 | Anti-situm = 7 | > 50% of the circumference: replacement |
| Lodge et al[10] | 1999 | 8 | Metastasis = 8 | 3 | 4 | 1 | Into the native IVC = 1; Into the graft = 3 | 6 (4 ex vivo and 2 TVE) | Not described | Ex vivo = 4; TVE only = 4; Anti situm = 0 | < 60° circumferentially and < 2 cm longitudinally: clamp tangentially |
Vascular exclusion methods, including intermittent Pringle maneuver, TVE and CIBH, are all widely utilized in different centers[24,25,29-31]. In our study, multivariate analysis showed that duration of liver ischemia was a factor predictive of operative death (P = 0.005). When the duration of anticipated TVE was longer than 60 min, hypothermic hepatic perfusion (University of Wisconsin solution, chilled to 4 °C) was applied to acquire an extended period of time (the longest was 102 min in our study) and protect the remnant liver. Kim et al[32] used a new technique of extracorporeal hepatic venous bypass to avoid hypothermic perfusion successfully. They sutured a part of cryopreserved iliac vein to the hepatic vein stump of the remnant liver and a cannula for hepatic venous bypass was placed in it to drain the blood to the internal jugular vein. When we carried out ex vivo and anti-situm, consistent with some of the previous reports[10,21,29], we used VVB if hemodynamic intolerance and splanchnic congestion occurred. Our criterion was: a decrease in mean arterial pressure > 30% and/or a decrease in cardiac index > 50%. However, Zhang et al[33] have performed ex vivo liver resection and liver autotransplantation without VVB in order to shorten anhepatic time. After removing en bloc liver and IVC, they replaced the IVC transiently with a tube graft before reconstructing the IVC with autogenous veins. In one of our patients, we also utilized synthetic caval graft to replace the resected part of IVC combined with transient portacaval shunt reconstruction. A vena cava vessel made by autogenous veins was applied to replace the IVC eventually. This technique is feasible and it could take place of VVB in selected patients.
If the lesions involved 3 hepatic veins at the hepatic vein confluence (H2), then ex vivo, in situ perfusion and anti-situm technique were applied. In these cases, hepatic vein reconstruction of the remnant liver should be done[17]. In situ perfusion and anti-situm technique were preferable for protection of the portal structures. However, if the portal triads were also involved, ex vivo technique had to be used. We have performed ex vivo liver resection followed by autotransplantation on several patients with advanced AE. The IVC were replaced using autogenous vein graft or artificial graft. We propose that AE may be a specific indication for ex vivo technique, with better prognosis than in malignant cancers.
In conclusion, liver resection combined with IVC and/or HVR is technically feasible with acceptable short-term survival. However, IVC replacement should be prudent as it was a risk factor related to postoperative death. In addition, preoperative liver function should be given special attention and intraoperative liver ischemia time should be shortened to reduce postoperative mortality. The proposed IH classification, which divided the patients into 5 groups according to the range of vascular invasion, may be meaningful in selecting procedures for patients with hepatocaval confluence infiltration. However, due to the small sample size and patient heterogeneity in the present study, this classification still needs to be investigated in more studies. For example, IVC replacement and HVR must be applied in type I2H2 patients to achieve R0 resection. Nevertheless, such an aggressive treatment is controversial for colorectal liver metastasis and HCC because alternative treatment approaches with lower morbidity and mortality could be applied. Consequently, the proposed IH classification describes anatomic issues but may not have identical significance in guiding surgical approach and indicating postoperative prognosis in different liver diseases.
We are grateful to Jean T for revising the language of the manuscript.
Though technically challenging, hepatectomy combined with major vascular resection and reconstruction has been performed in many centers because it was the only way to achieve R0 resection. However, the surgical indications and protocols were different and controversial in different centers.
The authors investigated the short-term outcomes and risk factors predictive of postoperative death for patients with lesions adjacent to the hepatocaval confluence.
The authors established the “IH classification” dividing the patients into 5 groups according to the range of vascular invasion, which was meaningful in selecting procedures for patients with hepatocaval confluence infiltration.
In this study, the authors present their surgical guideline and outcomes about the combined liver and inferior vena cava (IVC) resection in 54 patients with different kinds of liver lesions invading the hepatocaval confluence. The IH classification was established based on our experience, which can be a reference for other surgeons.
This is an interesting paper reviewing the center’s experience with a very challenging group of patients with liver malignancies and IVC or hepatic vein involvement.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Augustin G, Smith RC, Tsoulfas G S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Machado MA, Herman P, Bacchella T, Machado MC. Resection and reconstruction of retrohepatic vena cava without venous graft during major hepatectomies. J Surg Oncol. 2007;96:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Varma D, Ogata S, Belghiti J. Isolated total caval clamping with “preserved remnant liver perfusion” for combined hepatic and venacaval resection in tumors involving venacava. Surgery. 2007;141:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Lechaux D, Megevand JM, Raoul JL, Boudjema K. Ex vivo right trisegmentectomy with reconstruction of inferior vena cava and “flop” reimplantation. J Am Coll Surg. 2002;194:842-845. [PubMed] |
| 4. | Croome KP, Hernandez-Alejandro R, Parker M, Heimbach J, Rosen C, Nagorney DM. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford). 2015;17:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Malinowski M, Geisel D, Stary V, Denecke T, Seehofer D, Jara M, Baron A, Pratschke J, Gebauer B, Stockmann M. Portal vein embolization with plug/coils improves hepatectomy outcome. J Surg Res. 2015;194:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Chung KY, Kemeny N. Regional and systemic chemotherapy for primary hepatobiliary cancers and for colorectal cancer metastatic to the liver. Semin Radiat Oncol. 2005;15:284-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Azoulay D, Lim C, Salloum C, Andreani P, Maggi U, Bartelmaos T, Castaing D, Pascal G, Fesuy F. Complex Liver Resection Using Standard Total Vascular Exclusion, Venovenous Bypass, and In Situ Hypothermic Portal Perfusion: An Audit of 77 Consecutive Cases. Ann Surg. 2015;262:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Hoti E, Salloum C, Azoulay D. Hepatic resection with in situ hypothermic perfusion is superior to other resection techniques. Dig Surg. 2011;28:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Mehrabi A, Fonouni H, Golriz M, Hofer S, Hafezi M, Rahbari NN, Weitz J, Büchler MW, Schmidt J. Hypothermic ante situm resection in tumors of the hepatocaval confluence. Dig Surg. 2011;28:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Lodge JP, Ammori BJ, Prasad KR, Bellamy MC. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471-479. [PubMed] |
| 11. | Qiu J, Wu H, Bai Y, Xu Y, Zhou J, Yuan H, Chen S, He Z, Zeng Y. Mesohepatectomy for centrally located liver tumours. Br J Surg. 2013;100:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Huang J, Tang W, Hernandez-Alejandro R, Bertens KA, Wu H, Liao M, Li J, Zeng Y. Intermittent hepatic inflow occlusion during partial hepatectomy for hepatocellular carcinoma does not shorten overall survival or increase the likelihood of tumor recurrence. Medicine (Baltimore). 2014;93:e288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Dubay D, Gallinger S, Hawryluck L, Swallow C, McCluskey S, McGilvray I. In situ hypothermic liver preservation during radical liver resection with major vascular reconstruction. Br J Surg. 2009;96:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Jianyong L, Jingcheng H, Wentao W, Lunan Y, Jichun Z, Bing H, Ding Y. Ex vivo liver resection followed by autotransplantation to a patient with advanced alveolar echinococcosis with a replacement of the retrohepatic inferior vena cava using autogenous vein grafting: a case report and literature review. Medicine (Baltimore). 2015;94:e514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Malde DJ, Khan A, Prasad KR, Toogood GJ, Lodge JP. Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford). 2011;13:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Pichlmayr R, Weimann A, Oldhafer KJ, Schlitt HJ, Klempnauer J, Bornscheuer A, Chavan A, Schmoll E, Lang H, Tusch G. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg. 1995;19:807-813. [PubMed] |
| 18. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] |
| 19. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1724] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 20. | Taguchi Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Kokuryo T, Wakai K, Nagino M. The determination of bile leakage in complex hepatectomy based on the guidelines of the International Study Group of Liver Surgery. World J Surg. 2014;38:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Madariaga JR, Fung J, Gutierrez J, Bueno J, Iwatsuki S. Liver resection combined with excision of vena cava. J Am Coll Surg. 2000;191:244-250. [PubMed] |
| 22. | Fu SY, Lau WY, Li AJ, Yang Y, Pan ZY, Sun YM, Lai EC, Zhou WP, Wu MC. Liver resection under total vascular exclusion with or without preceding Pringle manoeuvre. Br J Surg. 2010;97:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Orimo T, Kamiyama T, Yokoo H, Kakisaka T, Wakayama K, Tsuruga Y, Kamachi H, Taketomi A. Usefulness of artificial vascular graft for venous reconstruction in liver surgery. World J Surg Oncol. 2014;12:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Hemming AW, Mekeel KL, Zendejas I, Kim RD, Sicklick JK, Reed AI. Resection of the liver and inferior vena cava for hepatic malignancy. J Am Coll Surg. 2013;217:115-24; discussion 124-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Nuzzo G, Giordano M, Giuliante F, Lopez-Ben S, Albiol M, Figueras J. Complex liver resection for hepatic tumours involving the inferior vena cava. Eur J Surg Oncol. 2011;37:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1333] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 27. | Graeter T, Ehing F, Oeztuerk S, Mason RA, Haenle MM, Kratzer W, Seufferlein T, Gruener B. Hepatobiliary complications of alveolar echinococcosis: A long-term follow-up study. World J Gastroenterol. 2015;21:4925-4932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Qu B, Liu C, Yang Y, Li J, Jin C, Wang B, Yu L, Lv Y. Giant hepatic alveolar echinococcosis in an adult. Am J Surg. 2009;198:e23-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Yamamoto Y. Ante-situm hepatic resection for tumors involving the confluence of hepatic veins and IVC. J Hepatobiliary Pancreat Sci. 2013;20:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Gallagher TK, Udupa KV, Geoghegan JG, Maguire D, Traynor OJ, Hoti E. Techniques and outcomes of combined inferior vena cava and visceral resection for benign and malignant disease. Int J Surg. 2014;12:864-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Azoulay D, Andreani P, Maggi U, Salloum C, Perdigao F, Sebagh M, Lemoine A, Adam R, Castaing D. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Kim DS, Yu YD, Jung SW, Ji W, Suh SO. Extracorporeal hepatic venous bypass during en bloc resection of right trisection, caudate lobe, and inferior vena cava: a novel technique to avoid hypothermic perfusion. J Am Coll Surg. 2013;216:e47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Zhang KM, Hu XW, Dong JH, Hong ZX, Wang ZH, Li GH, Qi RZ, Duan WD, Zhang SG. Ex-situ liver surgery without veno-venous bypass. World J Gastroenterol. 2012;18:7290-7295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |