Published online May 28, 2017. doi: 10.3748/wjg.v23.i20.3607
Peer-review started: January 6, 2017
First decision: January 19, 2017
Revised: February 6, 2017
Accepted: March 30, 2017
Article in press: March 30, 2017
Published online: May 28, 2017
Processing time: 141 Days and 11.8 Hours
Crohn’s disease (CD) is a chronic, non-specific granulomatous inflammatory disorder that commonly affects the small intestine and is a phenotype of inflammatory bowel disease (IBD). CD is prone to relapse, and its incidence displays a persistent increase in developing countries. However, the pathogenesis of CD is poorly understood, with some studies emphasizing the link between CD and the intestinal microbiota. Specifically, studies point to the brain-gut-enteric microbiota axis as a key player in the occurrence and development of CD. Furthermore, investigations have shown white-matter lesions and neurologic deficits in patients with IBD. Based on these findings, brain activity changes in CD patients have been detected by blood oxygenation level dependent functional magnetic resonance imaging (BOLD-fMRI). BOLD-fMRI functions by detecting a local increase in relative blood oxygenation that results from neurotransmitter activity and thus reflects local neuronal firing rates. Therefore, biochemical concentrations of neurotransmitters or metabolites may change in corresponding brain regions of CD patients. To further study this phenomenon, brain changes of CD patients can be detected non-invasively, effectively and accurately by BOLD-fMRI combined with magnetic resonance spectroscopy (MRS). This approach can further shed light on the mechanisms of the occurrence and development of neurological CD. Overall, this paper reviews the current status and prospects on fMRI and MRS for evaluation of patients with CD based on the brain-gut-enteric microbiota axis.
Core tip: The occurrence and development of Crohn’s disease (CD) have strong links to the brain-gut-enteric microbiota axis and are associated with psychological factors such as stress, anxiety and depression. In patients with inflammatory bowel disease, studies have revealed white-matter lesions and neurologic disorders. Brain activity and biochemical changes in brain regions can be detected accurately by blood oxygenation level dependent functional magnetic resonance imaging combined with magnetic resonance spectroscopy in patients with CD. This approach can further shed light on the mechanism of occurrence of neurologic CD.
- Citation: Lv K, Fan YH, Xu L, Xu MS. Brain changes detected by functional magnetic resonance imaging and spectroscopy in patients with Crohn's disease. World J Gastroenterol 2017; 23(20): 3607-3614
- URL: https://www.wjgnet.com/1007-9327/full/v23/i20/3607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i20.3607
Crohn’s disease (CD) is a chronic, non-specific granulomatous inflammatory disorder which can affect any part of the digestive tract. Most commonly, CD affects the small intestine. CD is a phenotype of inflammatory bowel disease (IBD). Geographically, CD is most prevalent in developed Western countries. However, recent epidemiologic studies definitely support a rapid increase in the incidence of CD in developing countries. Factors such as urbanization, improved sanitation, increased use of antibiotics and modern Western diets have all contributed to the rise of CD[1,2]. Therefore; it should be highly valued that the prevalence of CD will increase in the near future.
To date, the pathogenesis of CD is not fully understood. The current dogma attributes CD to biological factors such as immune response, genetic susceptibility, intestinal dysbiosis, and external environmental factors[3]. Many studies have demonstrated that the onset and development of CD are closely related to the intestinal microbiome, which has a strong relationship with the brain-gut-enteric microbiota axis in particular. In recent years, the mechanism of the brain-gut-enteric microbiota axis in CD patients has gained more attention from researchers. In particular, the advent of blood oxygenation level dependent functional magnetic resonance imaging (BOLD-fMRI) and hydrogen proton magnetic resonance spectroscopy (1H-MRS) has allowed non-invasive detection of brain activity and biochemical changes. Using fMRI, abnormal functional activity has been detected in the cerebral cortex of patients diagnosed with CD. This paper therefore serves as a comprehensive review of the current status and prospects on MRS and fMRI for evaluation of patients with CD based on the brain-gut-enteric microbiota axis.
The microbiome refers to the microorganisms, their genomes and the local environment in the human intestine[4]. A healthy gut microbiome is traditionally comprised of around 100 trillion species of microbes, most of which are bacteria[5]. Interruption of the symbiotic relationship between microbiota and the gastrointestinal tract is referred to as dysbiosis, which perturbs host functions and is a precursor to disorders, such as IBD[6,7]. As Prosberg et al[8] demonstrated through a systematic review, patients with active IBD had lower abundance of intestinal flora compared to patients in remission. Furthermore, the phenotypes of CD and ulcerative colitis (UC) are distinct. Therefore, the pathogenesis of IBD involves complex interactions between the immune system, the microbiome and environmental factors in genetically susceptible individuals. Specifically, an imbalance in intestinal microflora for genetically susceptible individuals can lead to abnormal immune responses within the gut and damage in the intestinal mucosal barrier[9]. This imbalance plays a key role in the progression of CD inflammation. In a study conducted by Erickson et al[10], the ileum of CD patients was found to exhibit altered carbohydrate metabolism, bacterial-host interactions and the presence of human host-secreted enzymes. It is hypothesized that the aforementioned changes in intestinal function are directly induced by an imbalanced microbiota. This result further highlights potential targets for the treatment of IBD patients[11].
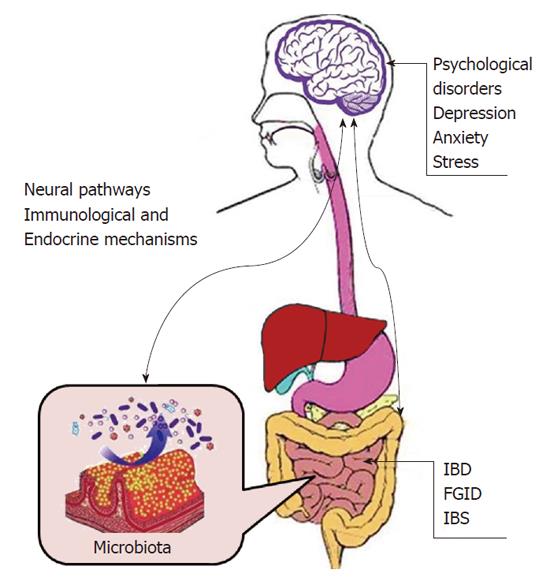
The bidirectional signaling between the gastrointestinal tract and the brain is vital for sustaining homeostasis and is regulated at the neural level by both the central and enteric nervous systems. Hormonal and immunological regulations are also known to play a part. Studies[12,13] have indicated that bacteria such as commensal, probiotic, and pathogenic bacteria in the gastrointestinal tract can activate peripheral neural pathways and central nervous system (CNS) signaling pathways. This result is hardly surprising as the gut microbiome plays an important role in basic neuroregenerative processes such as the formation of the blood-brain barrier, myelination, neurogenesis, and microglia maturation. Therefore, neural pathways in the enteric, autonomic and limbic systems along with the intestinal microbiota, immunological and endocrine systems are all regulated by the enteric microbiome. Meanwhile, the enteric nervous system is composed of small nerve cells, enteric ganglia, the nerve connectors between these ganglia, and nerve fibers that supply effector tissues. Effector tissues include intestinal smooth muscle, mucosal epithelium, intrinsic vascular and gastroenteropancreatic endocrine cells[14]. This relationship between the brain and enteric system involving the nervous system and microbiome is known as the brain-gut-enteric microbiota axis (Figure 1). A malfunction in just one of these pathways can influence the progression of CD[15-17].
Evidently, the enteric microbiome strongly impacts brain-gut communication in the brain-gut-enteric microbiota axis. Not surprisingly, intestinal bacterial colonization plays a major role in the development and maturation of immune and endocrine systems, which are the key factors underlying CNS signaling[18]. Under control of the CNS, cells from the intrinsic layer of the lumen release chemokines into the intestinal lumen, which can lead to gastrointestinal motility, secretions, and changes in the intestinal permeability. All these factors perturb the gastrointestinal bacterial environment[19,20].
Animal studies[21,22] have confirmed that behavioral disorders (such as stress) can change the composition of the intestinal flora. One proposed mechanism regards mucus and norepinephrine secretion by epithelial cells under stress, which results in gastrointestinal motility changes and specific strain growth. A large number of studies[23-27] have confirmed that factors such as stress, anxiety and depression can affect the activity and recurrence of CD. Recent data suggest that gastrointestinal inflammation caused by stress may be induced upon the dysfunction of the hypothalamic-pituitary-adrenal axis. This inflammation is known to alter the interaction between bacterial and mucosal mast cells through corticotrophin releasing factor.
Current research[28] has also confirmed that intestinal microbiota can directly alter neural biochemistry and that dysbiosis may directly contribute to mental illnesses in patients with intestinal disorders. The study compared parameters of anxiety-like behavior and motor activity between specific pathogen free mice with a normal gut microbiota and germ free (GF) mice. The GF mice exhibited decreased anxiety and increased motor activity. Further studies[29-31] also revealed that animals with non-invasive infections with pathogens in the cecum showed rapid activation of brainstem nuclei and exhibited anxiety like behavior. This reaction is believed to be mediated by signals from the vagal afferents to the nuclei of the solitary tract and the lateral parabrachial nuclei.
These studies have proved that the occurrence and development of CD have strong links to the brain-gut-enteric microbiota axis and involve psychological factors such as stress, anxiety and depression.
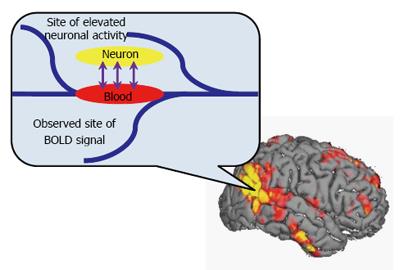
BOLD-fMRI[32,33] was first reported by Ogawa et al[33] in 1990, and since then, it has become a powerful method for detecting brain activity. BOLD-fMRI functions by detecting a local increase in relative blood oxygenation that results from neurotransmitter activity and thus reflects local neuronal firing rates (Figure 2). The activity of nervous system activity is therefore detected indirectly by assaying the proportion between deoxyhemoglobin and oxyhemoglobin in blood. Inspection methods are divided into the task state and resting state (rs-fMRI). The task state is further subdivided into block design and event related methods.
The resting state is characterized by behaviors such as slow breathing and minimal physical or mental activity. In the absence of design tasks, subjects are known to cooperate willingly with high consistency. Replicative measures are therefore more easily obtained in large numbers in the resting state. During the resting state, Rs-fMRI detects low frequency fluctuations in functional brain regions based on blood oxygen level. Rs-fMRI analytical methods include regional homogeneity (ReHo)[34], functional connectivity (FC)[35] and amplitude of low-frequency fluctuation (ALFF)[36]. Using fMRI, Agostini et al[37] and Bao et al[38] found abnormal functional activity in the cerebral cortex of patients with CD (Table 1).
| Ref. | Destination | Inspection | Location | Main metabolites | Results |
| Agostini et al[37], 2013 | Habituation to stress in CD | BOLD-fMRI (Task-state) | Brain | NA | Different neural activities in the amygdala, hippocampus, insula, putamen and cerebellar between CD patients and controls |
| Bao et al[38], 2016 | Brain activity in paracmastic CD patients | BOLD-fMRI (Resting-state) | Brain | NA | ReHo values: Abdominal pain: insula, MCC, SMA↑, temporal pole↓; Without abdominal pain: hoppocampal/parahippocampial cortex↑, dorsomedial prefrontal cortex↓ |
| Bezabeh et al[41], 2001 | Diagnosis in CD and UC | MRS | Colonic mucosal | Taurine, lysine, lipid, choline, creatine | The diagnostic spectral regions include taurine, lysine, and lipids |
| Varma et al[42], 2007 | Early screening of IBD | MRS | Colonic mucosal | Creatinine and phosphatidylcholine | Triglycerides, creatine, phosphocholine and glycerol backbone of lipids are the most discriminatory metabolites |
| Fathi et al[43], 2014 | Biomarkers of CD | MRS | Serum | Alanine, glutamine, leucine/isoleucine, lysine and valine | Two chemical shifts of isoleucine (0.99 ppm) and valine (1.03 ppm) have considerable impact for discriminating patient and normal samples |
Agostini et al[37] hypothesized inadequate habituation to stress as a characteristic for CD patients, and their study sought to compare neural habituation between CD patients and healthy subjects. During a high-stress task, different neural regions were activated between the two groups. Particular differences arose in the activation of the amygdala, hippocampus, insula, putamen and cerebellar regions. These contrasts revealed a stark difference in the habituation to stress between CD affected individuals and controls. Particularly, CD patients demonstrated inadequate habituation to stress as previously hypothesized, which contribute a link between stress and exacerbated inflammation. These results suggested that the self-regulation of stress levels in CD patients is decreased, which can be an important factor in exacerbating intestinal inflammation.
Bao et al[38] investigated changes in resting-state brain activity in paracmastic CD patients with and without abdominal pain. Regional homogeneity (ReHo) was used to assess resting-state brain activity. They found that patients with abdominal pain exhibited lower ReHo values in the insula, middle cingulate cortex (MCC) and supplementary motor area (SMA), with higher ReHo values in the temporal pole. In contrast, patients without abdominal pain exhibited lower ReHo values in the hippocampal/parahippocampal cortex and higher ReHo values in the dorsomedial prefrontal cortex. These results showed a significant negative correlation between the ReHo values of the insular and MCC activities with the daily pain scores of patients with abdominal pain. The results of this study confirmed a difference in resting state brain activity between CD patients with and without abdominal pain. Furthermore, the abnormal activity of insular and MCC regions was closely related to the severity of abdominal pain.
MRS[39] utilizes nuclear magnetic resonance phenomena and chemical shifts to quantitatively analyze specific atomic nuclei and their compounds. As a non-invasive, quantitative measurement for physiological and biochemical changes of internal organs and tissue metabolism, MRS offers unparalleled versatility and safety. Not only does MRS characterize functional groups, but describes the relationships between appropriate nuclei different constitutional isomers and stereoisomers. To date, in vivo MRS of the brain in CD patients has yet to be implemented. Studies of MRS frequently use ex vivo samples of CD patients, such as serum/plasma, urine, stool and colonic mucosal samples (Table 1).
MRS proves useful in the study of metabolomics, which involves the high throughput analysis, characterization and quantification of small molecular metabolites. By assaying different fluids, such as serum/plasma, urine and stool samples, the presence or absence of different metabolites can be used to distinguish between IBD and healthy volunteers. MRS can even be used to discern the different subtypes (CD and UC) of IBD as metabolite changes are directly associated with changes in intestinal bacteria[40]. These findings demonstrate that IBD is a disorder of the intestinal flora.
The differential diagnosis between CD and UC is often difficult. However, Bezabeh[41] utilized 1H-MRS combined with spectral data to delineate UC and CD. Tissue samples from the colon of affected patients were assayed by spectral analysis, with a 98.6% accuracy rate for discerning UC and CD. The diagnostic spectral regions include taurine, lysine, and lipids.
In another study using MRS for metabolomics, Varma et al[42] performed 1H-MRS on ex vivo colonic mucosal samples for the early screening of IBD. Their results revealed differing levels of creatinine and phosphatidylcholine over time between IBD affected and healthy groups, and suggest the existence of biochemical changes in IBD.
Separately, Fathi et al[43] explored the biomarkers of metabolism in patients with CD. Using 1H-MRS metabolic profiling of the serum samples, it was shown that valine and isoleucine levels are also useful in the differential diagnosis of CD metabolites, and these metabolites can be used for high risk screening for early diagnosis of CD patients.
MRS has been most widely used in the assessment of neurologic disorders. Previous studies[44,45] have shown that in vivo quantitative or semi-quantitative detection of brain tissue metabolites including glutamine (Glu), glutamate (Gln), γ-aminobutyric acid (GABA), N-acetylaspartate, myo-inositol, choline, creatine, glycerophosphorylcholine and phosphorylcholine can indicate neurological cell density, metabolism, permeability and other factors. The functions of clinically detectable neurochemical metabolites are listed in Table 2. Therefore, in vivo MRS is instrumental in the diagnosis of brain diseases such as tumors, ischemia, infection, epilepsy, metabolic disorders, dementia, mental diseases and so on[46,47]. MRS combined with clinical evaluations and conventional MRI is essential for diagnosing certain entities. Further studies[48,49] have shown that white-matter lesions and neurologic deficits in IBD patients may be an additional extra-intestinal manifestation of this disease. Despite the versatility of MRS, however, current studies more frequently employed CD spectrum analysis of urine and fecal samples of CD patients. Meanwhile, in vivo MRS in the brain of CD patients has yet to be implemented.
| Metabolite | Chemical shift (ppm) | Concentration range (mmol/kgww) | Functions |
| NAA | 2.02 | 7.9-16.6 (average 10.3) | An osmolite, a storage form of aspartate, a precursor of NAAG, a marker of neuronal density |
| GABA | 3.01 | 1.3-1.9 | A primary inhibitory neurotransmitter |
| tCho | 3.20 | 0.9-2.5 | An essential nutrient that is required for synthesis of the neurotransmitter acetylcholine, and of phosphatidylcholine, a major constituent of membranes |
| Cr | 3.05 | 5.1-10.6 | A concentration reference |
| Glu | 2.04-2.35 | 6.0-12.5 | An excitatory neurotransmitter |
| Gln | 2.12-2.46 | 3.0-5.8 | A precursor and storage form of glutamate |
| mI | 3.56 | 3.8-8.1 | An essential requirement for cell growth, and a storage form for glucose |
| Lac | 1.33-1.35 | 0.4 | The end product of anaerobic glycolysis |
Neurotransmitter mediated signal transduction plays an important role in the regulation of cerebral blood flow, which is mainly controlled by astrocytes[50]. BOLD-fMRI studies have confirmed changes in local cerebral blood oxygen concentrations in patients with CD. Based on previous observations[37,38], it is hypothesized that metabolites of functional brain areas will be changed accordingly in patients with CD. Specifically, the excitatory and inhibitory neurotransmitters associated with mental and psychological factors such as Glu and GABA[51,52], respectively, are thought to vary. The coordination between excitatory and inhibitory neurotransmitters is the basis of regulated neuronal activity, and is correlated with the amplitude of the BOLD-fMRI signal. GABA baseline levels were negatively correlated with BOLD-fMRI signals of brain activity, and this result indicates the correlation between the BOLD-fMRI signals and GABA levels[53].
In addition, Glu and Gln serve as major excitatory neurotransmitters. Due to their similar molecular structures, the pair is often referred to as Glx. Studies of MRS in chronic pain[54-56] suggested that altered levels of Glx and GABA are present in patients with chronic pain, suggesting the role of neurotransmitters in pain management. Mullins et al[57] further used 1H-MRS to investigate changes of brain metabolites in patients scoring high on the self-assessed pain scale. Results indicated that the onset of pain can induce a dynamic increase in Glu concentration in the anterior cingulate cortex. It was also found that an increased Glu concentration was significantly related to the pain level of participants’ subjective experience.
While increases in Glx are corresponded to increased pain perception, GABA[44,58,59] is an inhibitory neurotransmitter which plays an important role with Glx in neurotransmission and pain. GABA concentration in brain tissue in vivo is relatively low (< 2 mmol/L), and the MRS spectrum shows strong overlap with other metabolites. Therefore, traditional MRS is not optimal for detecting GABA. In light of this limitation, the spectral editing technique MEGA-PRESS (MEscher-GArwood Point RESolved Spectroscopy)[60] was designed to allow accurate detection of GABA. Results are encouraging and offer potential applications in the screening of neurodegenerative diseases, mental disorders, acute and chronic pain.
Functional magnetic resonance spectroscopy (fMRS) has been proposed for various applications[61-65], especially in conditions involving psychological factors. MRS combined with fMRI is known as fMRS and can be used to detect changes in brain metabolites with high accuracy.
Current BOLD-fMRI studies have confirmed changes in local cerebral blood oxygen concentrations in patients with CD. Therefore, it is hypothesized that the level of metabolites in functional brain areas will be changed accordingly. The correlation of these changes can be related with the pathogenesis of CD. Meanwhile, the progression of CD with respect to psychological factors and symptom of abdominal pain requires further investigation. Based on the brain-gut-enteric microbiota axis, fMRS can be used to study brain activity and biochemical concentrations of key neurotransmitters, particularly Glx and GABA, in patients with CD. fMRS studies therefore offer unpatrolled versatility for evaluation of patients with CD and serve to prevent further disease progression and relieve symptoms of abdominal pain for patients suffering from CD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Ozen H, Tantau A S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3517] [Article Influence: 270.5] [Reference Citation Analysis (5)] |
| 2. | Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, O’Morain C, Moum B, Colombel JF. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 440] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 3. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1272] [Article Influence: 66.9] [Reference Citation Analysis (2)] |
| 4. | Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3709] [Cited by in RCA: 3187] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 5. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7817] [Article Influence: 521.1] [Reference Citation Analysis (4)] |
| 6. | Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 408] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 7. | Lepage P, Colombet J, Marteau P, Sime-Ngando T, Doré J, Leclerc M. Dysbiosis in inflammatory bowel disease: a role for bacteriophages? Gut. 2008;57:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Prosberg M, Bendtsen F, Vind I, Petersen AM, Gluud LL. The association between the gut microbiota and the inflammatory bowel disease activity: a systematic review and meta-analysis. Scand J Gastroenterol. 2016;51:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Boyapati R, Satsangi J, Ho GT. Pathogenesis of Crohn’s disease. F1000Prime Rep. 2015;7:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Erickson AR, Cantarel BL, Lamendella R, Darzi Y, Mongodin EF, Pan C, Shah M, Halfvarson J, Tysk C, Henrissat B. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS One. 2012;7:e49138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 11. | Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 12. | Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1513] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 13. | Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 996] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 14. | Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 1059] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 15. | Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 639] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 16. | Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 17. | Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1121] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 18. | Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 622] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 19. | Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 914] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 20. | Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861-869. [PubMed] |
| 21. | Chen C, Brown DR, Xie Y, Green BT, Lyte M. Catecholamines modulate Escherichia coli O157: H7 adherence to murine cecal mucosa. Shock. 2003;20:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 23. | Ananthakrishnan AN, Khalili H, Pan A, Higuchi LM, de Silva P, Richter JM, Fuchs CS, Chan AT. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the Nurses’ Health Study. Clin Gastroenterol Hepatol. 2013;11:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Cámara RJ, Schoepfer AM, Pittet V, Begré S, von Känel R. Mood and nonmood components of perceived stress and exacerbation of Crohn’s disease. Inflamm Bowel Dis. 2011;17:2358-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 408] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 26. | Mardini HE, Kip KE, Wilson JW. Crohn’s disease: a two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci. 2004;49:492-497. [PubMed] |
| 27. | Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 28. | Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1983] [Cited by in RCA: 2281] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 29. | Wang X, Wang BR, Zhang XJ, Xu Z, Ding YQ, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol. 2002;8:540-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Goehler LE, Lyte M, Gaykema RP. Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun. 2007;21:721-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Matthews PM, Jezzard P. Functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2004;75:6-12. [PubMed] |
| 33. | Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68-78. [PubMed] |
| 34. | Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1520] [Cited by in RCA: 1982] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 35. | Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 650] [Cited by in RCA: 626] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 36. | Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1781] [Cited by in RCA: 1603] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 37. | Agostini A, Filippini N, Benuzzi F, Bertani A, Scarcelli A, Leoni C, Farinelli V, Riso D, Tambasco R, Calabrese C. Functional magnetic resonance imaging study reveals differences in the habituation to psychological stress in patients with Crohn’s disease versus healthy controls. J Behav Med. 2013;36:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Bao CH, Liu P, Liu HR, Wu LY, Jin XM, Wang SY, Shi Y, Zhang JY, Zeng XQ, Ma LL. Differences in regional homogeneity between patients with Crohn’s disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain. 2016;157:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Jackman LM, Sternhell S. Application of Nuclear Magnetic Resonance Spectroscopy in Organic Chemistry: International Series in Organic Chemistry. 2013;. |
| 40. | De Preter V. Metabolomics in the Clinical Diagnosis of Inflammatory Bowel Disease. Dig Dis. 2015;33 Suppl 1:2-10. |
| 41. | Bezabeh T, Somorjai RL, Smith IC, Nikulin AE, Dolenko B, Bernstein CN. The use of 1H magnetic resonance spectroscopy in inflammatory bowel diseases: distinguishing ulcerative colitis from Crohn’s disease. Am J Gastroenterol. 2001;96:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Varma S, Bird R, Eskin M, Dolenko B, Raju J, Bezabeh T. Detection of inflammatory bowel disease by proton magnetic resonance spectroscopy (1H MRS) using an animal model. J Inflamm (Lond). 2007;4:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Fathi F, Majari-Kasmaee L, Mani-Varnosfaderani A, Kyani A, Rostami-Nejad M, Sohrabzadeh K, Naderi N, Zali MR, Rezaei-Tavirani M, Tafazzoli M. 1H NMR based metabolic profiling in Crohn’s disease by random forest methodology. Magn Reson Chem. 2014;52:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RA, Mohamed MA, Welsh RC, Carlos RC, Barker PB, Feldman EL. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol. 2013;70:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Xu M, Ye J, Yang D, Xu X, Yeo TT, Ng WH, Lim CC. Ex-vivo NMR of unprocessed tissue in water: a simplified procedure for studying intracranial neoplasms. Anal Bioanal Chem. 2007;389:2153-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dinçer A. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 468] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 47. | Xu M, See SJ, Ng WH, Arul E, Back MF, Yeo TT, Lim CC. Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery. 2005;56:919-926; discussion 919-926. [PubMed] |
| 48. | Geissler A, Andus T, Roth M, Kullmann F, Caesar I, Held P, Gross V, Feuerbach S, Schölmerich J. Focal white-matter lesions in brain of patients with inflammatory bowel disease. Lancet. 1995;345:897-898. [PubMed] |
| 49. | Morís G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol. 2014;20:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2007] [Cited by in RCA: 1755] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 51. | Schür RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joëls M, Klomp DW, Kahn RS, Vinkers CH. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37:3337-3352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 52. | Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 53. | Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 54. | Hansen TM, Olesen AE, Simonsen CW, Drewes AM, Frøkjær JB. Cingulate metabolites during pain and morphine treatment as assessed by magnetic resonance spectroscopy. J Pain Res. 2014;7:269-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci Lett. 2012;520:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Watson CJ. Insular balance of glutamatergic and GABAergic signaling modulates pain processing. Pain. 2016;157:2194-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Mullins PG, Rowland LM, Jung RE, Sibbitt WL. A novel technique to study the brain’s response to pain: proton magnetic resonance spectroscopy. Neuroimage. 2005;26:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 58. | Aguila ME, Lagopoulos J, Leaver AM, Rebbeck T, Hübscher M, Brennan PC, Refshauge KM. Elevated levels of GABA+ in migraine detected using (1) H-MRS. NMR Biomed. 2015;28:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. Neuroimage. 2015;105:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266-272. [PubMed] |
| 61. | Apšvalka D, Gadie A, Clemence M, Mullins PG. Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. Neuroimage. 2015;118:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Jahng GH, Oh J, Lee DW, Kim HG, Rhee HY, Shin W, Paik JW, Lee KM, Park S, Choe BY. Glutamine and Glutamate Complex, as Measured by Functional Magnetic Resonance Spectroscopy, Alters During Face-Name Association Task in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis. 2016;52:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Huang Z, Davis HH, Yue Q, Wiebking C, Duncan NW, Zhang J, Wagner NF, Wolff A, Northoff G. Increase in glutamate/glutamine concentration in the medial prefrontal cortex during mental imagery: A combined functional mrs and fMRI study. Hum Brain Mapp. 2015;36:3204-3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Taylor R, Neufeld RW, Schaefer B, Densmore M, Rajakumar N, Osuch EA, Williamson PC, Théberge J. Functional magnetic resonance spectroscopy of glutamate in schizophrenia and major depressive disorder: anterior cingulate activity during a color-word Stroop task. NPJ Schizophr. 2015;1:15028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Bednařík P, Tkáč I, Giove F, DiNuzzo M, Deelchand DK, Emir UE, Eberly LE, Mangia S. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab. 2015;35:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 66. | Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129-153. [PubMed] |