Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3066
Peer-review started: September 12, 2016
First decision: October 20, 2016
Revised: December 7, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: May 7, 2017
Processing time: 236 Days and 22.8 Hours
To comparatively investigate the cellular and molecular characteristics of low-grade slightly elevated adenomas and polypoid adenomas.
Colorectal tumors were collected from 24 patients with slightly elevated adenomas and 23 patients with polypoid adenomas. Five commonly mutated genes (APC, BRAF, KRAS, NRAS, and PIK3CA) were selected for mutational analysis. Paraffin-embedded tumor sections were used to calculate the apoptotic index (AI) and Ki-67 labeling index (KLI). Two pure colorectal epithelial cell lines were created by pooling the slightly elevated and polypoid tumors. Western blots, luciferase assays for β-catenin-T-cell factor protein/β-catenin-lymphoid enhancer factor (β-catenin-TCF/LEF)-driven transcriptional activity, and caspase activity assays were conducted on the two cell lines.
Slightly elevated lesions showed a significantly lower APC mutational frequency and a significantly higher KRAS mutational frequency (both P < 0.05). Slightly elevated lesions showed a significantly lower AI (P < 0.05). β-catenin and β-catenin-TCF/LEF-driven transcriptional activity was significantly upregulated in slightly elevated lesions (both P < 0.05). In slightly elevated lesions, c-Myc was significantly downregulated, while cyclin D1 was significantly upregulated (both P < 0.05). β-catenin-TCF/LEF-driven transcriptional activity was negatively correlated with c-Myc (ρ = -0.78). Slightly elevated lesions displayed significant Bcl-2 and Bcl-xL upregulation (both P < 0.05) along with significant decreases in caspase-9 and caspase-3 activity (both P < 0.05). c-Myc was negatively correlated with Bcl-2 and Bcl-xL (ρ = -0.74 and -0.78, respectively).
The lower apoptotic activity of low-grade slightly elevated adenomas can be partly attributed to upregulated β-catenin pathway activity and downregulated c-Myc expression.
Core tip: We comparatively investigated the cellular and molecular characteristics of low-grade slightly elevated adenomas and low-grade polypoid adenomas. Slightly elevated lesions displayed a significantly lower apoptotic index as well as significantly upregulated β-catenin expression and β-catenin-TCF/LEF-driven transcriptional activity. Slightly elevated lesions also displayed c-Myc downregulation, cyclin D1 upregulation, upregulation of Bcl-2 and Bcl-xL, and downregulation of caspase-9 and caspase-3 activity. Low-grade slightly elevated adenomas displayed a lower apoptotic activity relative to low-grade polypoid adenomas, which can be partly attributed to upregulated β-catenin pathway activity and downregulated c-Myc expression.
- Citation: Yang TW, Gao YH, Ma SY, Wu Q, Li ZF. Low-grade slightly elevated and polypoid colorectal adenomas display differential β-catenin-TCF/LEF activity, c-Myc, and cyclin D1 expression. World J Gastroenterol 2017; 23(17): 3066-3076
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3066.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3066
Colorectal cancer is the third most common malignancy worldwide, with a global five-year prevalence of approximately 3.2 million cases[1]. Colorectal cancer remains the third-leading cause of cancer-related death in the United States and the second-leading cause of cancer-related death in Europe[2,3]. Moreover, more highly developed East Asian countries (e.g., China, Japan, South Korea, and Singapore) have displayed two- to four-fold increases in the incidence of colorectal carcinoma over the past few decades[4]. Therefore, improving the diagnosis and treatment of colorectal cancer remains a pressing global health issue.
Fortunately, colorectal cancer mortality can be improved through early diagnosis[5]. Therefore, colorectal cancer screening programs aim to detect aberrant colorectal adenomas at the earliest possible stage when curative resection is still possible[5]. Currently, colonoscopy is considered the gold-standard screening modality, as it enables the operator to simultaneously examine the entire large intestinal mucosa and resect abnormal growths in one therapeutic session[5]. Although colonoscopy has demonstrated a significant impact on colorectal cancer incidence and mortality[5], non-polypoid colorectal adenomas, such as slightly elevated (Paris 0-IIa), flat (Paris 0-IIb), and depressed (Paris 0-IIc) lesions[6], are more difficult to detect through colonoscopy on account of their flatter morphology, and some evidence suggests that they are more likely to contain carcinoma cells than Paris 0-I (polypoid) adenomas[4,7,8]. For these reasons, the development of alternative screening modalities and targeted therapeutics for low-grade non-polypoid adenomas remain important clinical challenges[9].
To this end, better understanding of the cellular and molecular characteristics of low-grade non-polypoid adenomas is needed to develop molecular biomarkers and targeted therapeutics for these lesions[9,10]. However, the bulk of published research comparing non-polypoid and polypoid lesions has focused on calculating cohort-based mutational frequencies through genotyping[11,12]. As a result, there has been limited research distinguishing the cellular and molecular characteristics of low-grade non-polypoid adenomas from those of low-grade polypoid adenomas.
Therefore, the objective of this study was to comparatively investigate the cellular and molecular characteristics of low-grade slightly elevated adenomas and low-grade polypoid adenomas excised from two cohorts of colorectal surgery patients. Specifically, we first comparatively assessed the mutational frequency of five of the most commonly mutated genes in colorectal malignancies and found that slightly elevated lesions showed a significantly lower adenomatous polyposis coli (APC) mutational frequency and a significantly higher v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutational frequency relative to polypoid lesions. Moreover, we found a significantly lower apoptotic index (AI) in slightly elevated lesions. Based on these initial findings, we next comparatively assessed differences in the anti-apoptotic β-catenin pathway and downstream apoptosis markers. This study should shed light on the molecular phenotypic differences between low-grade slightly elevated colorectal adenomas and low-grade polypoid colorectal adenomas.
This study was approved by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University (Chongqing, China). All the subjects recruited for this study provided written informed consent prior to participation.
Patients who had undergone laparoscopic or open surgery for colorectal tumor resection at the Department of Gastrointestinal Surgery at Yongchuan Hospital were consecutively screened for study eligibility. The following inclusion criteria were applied: (1) age 18 or above; (2) a diagnosis of low-grade colorectal adenoma; and (3) the presence of at least one excisable low-grade slightly elevated adenoma or at least one low-grade polypoid adenoma. The following exclusion criteria were used: (1) presentation at the emergency department (ED) or intensive care unit (ICU); (2) severely obese body mass index (BMI greater than 35 kg/m2); (3) an American Society of Anesthesiologists (ASA) classification of IV or V; (4) diagnosis of stage 0 colorectal cancer or higher; (5) the presence of other gastrointestinal disease requiring intervention; (6) hereditary colorectal cancer or a family history of colorectal cancer; (7) a history of malignancy in the preceding five years, with the exception of superficial squamous carcinoma of the skin, basal cell carcinoma of the skin, or in situ cervical malignancy; (8) pregnancy; and (9) preoperative chemotherapy or radiotherapy.
Based on all the above-mentioned criteria, a total of 47 patients were finally included in this study, with 24 patients with slightly elevated adenomas and 23 patients with polypoid adenomas. For each included patient, the following information on relevant demographic and clinical characteristics was collected for statistical comparisons between the two cohorts, including (1) age; (2) sex; (3) colorectal cancer risk factors [i.e., BMI, smoking status, excessive alcohol consumption (i.e., a male consuming more than four drinks/day or 14 drinks/week or a female consuming more than three drinks/day or seven drinks/week)[13]]; and (4) lesion characteristics (i.e., intestinal site, diameter, Paris classification, histology, and pathological grade).
After surgical removal, each specimen was immediately split into three portions: the first and second portions were fixed in 10% buffered formalin (pH 7.2) and embedded in paraffin wax for subsequent DNA extraction and staining, while the third portion was placed on ice in complete Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), amphotericin B (2.5 μg/mL), gentamicin (100 μg/mL), penicillin (200 U/mL), streptomycin (200 μg/mL), 5 mL non-essential amino acids, and HEPES (200 mmol) (all from Gibco BRL, Grand Island, NY, United States) for primary culture.
As previously described by Voorham et al[12], the paraffin-embedded sections were incubated for five days in ATL lysis buffer (QIAmp DNA Micro Kit, Qiagen, Beijing, China). Proteinase K (10 μL from a 20 ng/μL solution) was added daily. DNA isolation was performed using a column-based technique (QIAmp DNA Micro Kit, Qiagen). A Beckman UV-vis spectrophotometer (Beckman, CA, United States) was used to measure DNA concentrations and purities.
Based on Voorham et al[12]’s previously published mutational analysis of flat colorectal adenomas, five of the most commonly mutated genes (i.e., APC, BRAF, KRAS, NRAS, and PIK3CA) were selected for mutational analysis. For APC, the mutation cluster regions (MCRs) 1286-1513 and 1291-1541 were each analyzed with four flanking PCR reactions and two semi-nested PCR reactions using an ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, United States) (Supplementary Table 1). The Vector NTI Advance 11 software package (Invitrogen, Carlsbad, CA, United States) and the Mutation Surveyor version 3.30 software suite (SoftGenetics, State College, PA, United States) were used to analyze the resulting data. Validation of mutations was performed by independent PCR runs and subsequent sequencing.
As previously described[12], the Catalogue of Somatic Mutations in Cancer database was searched for the most frequent mutations in the remaining four genes, providing a coverage of 97.9% of BRAF mutations (Supplementary Table 2), 97.3% of KRAS mutations (Supplementary Table 3), 68.8% of NRAS mutations (Supplementary Table 4), and 81.4% of PIK3CA mutations (Supplementary Table 5). A Voyager DE Pro MALDI-TOF mass spectrometer (PerSeptive BioSystems, Framingham, Massachusetts) was used to genotype the specimens. All the specimens were of high quality, with more than 75% of the nucleotide positions examined showing consistent genotyping.
As previously described, random imputation was applied using the R statistical software package (version 2.12.0) to construct missing data points from unsuccessful runs in order to prevent underestimation or overestimation of the mutation frequency. Briefly, missing data points were imputed to generate probabilities equal to the proportions of the mutations for the particular nucleotide positions in question. Precise estimates were calculated by applying multiple random imputations. From these computed mutation frequencies, odds ratios were calculated for each experimental group to enable statistical comparisons.
The paraffin-embedded sections were sliced into 4-μm sections. After staining with hematoxylin and eosin (H&E), apoptotic cells were counted using light microscopy according to Kerr et al[14]’s criteria: (1) well-delineated cell margins buffered by empty spaces; (2) homogeneous, eosinophilic cytoplasm; (3) shrunken, dark basophilic nuclei; and (4) nuclear fragmentation. For the purposes of conservative counting, apoptotic cells intermingled among infiltrating inflammatory cells were ignored. The apoptotic index (AI) was calculated as the percentage of apoptotic cells counted over a total of 1000 tumor cells per field. Three random fields were counted per specimen.
Light microscopy was used to count Ki-67-positive cells in the paraffin-embedded, 4-μm-thick sections. For Ki-67 immunostaining, antigens were first unmasked with Tris-EDTA (10 mmol/L/1 mmol/L, pH 9) by 533-watt microwaving for five minutes. Then, endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide at room temperature for 15 min. Non-specific site blockade was performed with Tris-HCl (0.05 mol/L) with 1% bovine albumin (pH 7.6) at room temperature. The specimens were then incubated with a rabbit polyclonal anti-Ki-67 antibody (diluted 1:100, Dako, Carpinteria, CA, United States) at room temperature for one hour. A streptavidin-biotin-peroxidase complex detection system [labelled streptavidin-biotin (LSAB), Dako] was used with a peroxidase-linked anti-rabbit antibody (Sigma, St. Louis, MO, United States) and a DAB kit (Dako) for signal amplification and detection. For the purpose of conservative counting, only positive nuclear Ki-67 staining was considered representative of a Ki-67-positive cell. The Ki-67 labeling index (KLI) was calculated as the percentage of Ki-67-positive cells over a total of 1000 tumor cells per field. Three random fields were counted per specimen.
As previously described by Oikonomou et al[15], two pure colorectal epithelial cell lines (i.e., slightly elevated and polypoid) were cultured by pooling all slightly elevated specimens together and pooling all polypoid specimens together, respectively. By the cross-scalpel method, fresh tumor specimens were minced into small (2-3 mm) tissue pieces and pooled together in their respective groups. The small tissue pieces were then digested for 60 min at 37 °C with collagenase type IV (50 mg/mL, Gibco BRL), pronase E (10 mg/mL, Sigma), DNAase (2000 U/mL, Sigma), and 0.1 mmol EGTA (Sigma) under mild agitation. The tissue mixture was then pipetted and filtered through a 100-μm cell strainer to isolate the free cells. The resulting cells were rinsed and resuspended in Ca2+-free and Mg2+-free Hank’s Balanced Salt Solution (CMF-HBSS, Invitrogen) for centrifugation (1000 rpm for 3 min). Epithelial cells were isolated with discontinuous Percoll gradient centrifugation [25/40/50% (v/v)] for 20 min at 600 g and then collected at the 25/40% Percoll interface with a Pasteur pipette. The epithelial cells were rinsed twice in CMF-HBSS and centrifuged (1200 rpm for 3 min). The resulting pellet was resuspended in complete DMEM with 20% FBS, gentamycin (40 μg/mL, Sigma), hydrocortisone (0.1 μg/mL, Sigma), and insulin (2 μg/mL, Sigma). The cell suspension was then seeded into a T-75 cell culture flask pre-coated with fibronectin (10 μg/mL) and grown to confluence prior to initial passaging. Since all experiments were conducted prior to four passages, the cells were suspended in complete DMEM with 20% FBS and 10% dimethyl sulfoxide (Fluka/Sigma-Aldrich) and cryopreserved in liquid nitrogen immediately following the first passage. The viability of the cell lines was confirmed by seeding T-25 flasks with 1 × 104 cells/mL complete DMEM and performing cell counts after 24 h, 48 h, 72 h, and 96 h (data not shown).
Total protein was harvested from the two cell lines. Briefly, cells were lysed with lysis buffer (Beyotime, Shanghai, China) for 30 min at 4 °C. The lysate was spun down to remove cell debris, and the protein concentrations in the resulting supernatant were quantified using a Bradford protein assay kit (Bio-Rad, Hercules, CA, United States). Then, 30 μg protein per sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Bio-Rad). Prior to immunoblotting, membranes were blocked in 5% non-fat dry milk. The following primary monoclonal antibodies were diluted in buffer (1:1000) and incubated with the membranes at 4 °C overnight: anti-β-catenin, anti-c-Myc, anti-cyclin D1, anti-Bcl-2, and anti-β-actin (all from Cell Signaling Technologies, Beverly, MA, United States). After washing in TBST, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (diluted 1:10000, Cell Signaling Technologies) for one hour at room temperature. Bands were detected with enhanced chemiluminescence (ECL, Applygen Technologies, Beijing, China). Expression levels were normalized to β-actin. This experiment was repeated in triplicate.
β-catenin-T-cell factor protein/β-catenin-lymphoid enhancer factor (β-catenin-TCF/LEF)-driven transcriptional activity was detected with a luciferase assay, which was performed as previously described with minor modifications[16]. Briefly, the two cell lines were seeded with 10% FBS and 100 μL DMEM in 96-well plates and incubated for 12 h at 37 °C in a humidified incubator (5% CO2). The cell lines were then transfected with two plasmids using Lipofectamine 2000: a SuperTopFlash (STF) β-catenin-TCF/LEF reporter plasmid (80 ng) and pF9A CMV hRluc-neoFIexi (10 ng). After 24 h, the cells were lysed, and a dual-luciferase reporter assay (Promega, Madison, WI, United States) was used to measure STF luciferase activity, with transfection efficiency normalized to Renilla luciferase activity. This experiment was repeated in triplicate.
Briefly, the two cell lines were seeded into 10-cm plates 24 h prior to assaying. The two cell lines (1 × 106 cells for each cell line) were assayed for caspase-9 activity and caspase-3 activity with the fluorogenic moieties Ac-LEHD-AMC and Ac-DEVD-AMC, respectively, as described in the kit instructions (Biomol, Plymouth Meeting, PA, United States). This experiment was repeated in triplicate.
All data were analyzed with SPSS version 13.0 (SPSS, Chicago, IL, United States). Means and their associated standard error of the means (SEMs) were reported. Differences in mutational frequencies between the two lesion types were assessed with Fisher’s exact test or the χ2 test as needed. All other statistical comparisons between the two lesion types were made with a two-tailed Student’s t-test. Spearman correlation analysis was performed to assess putative correlations between key molecular characteristics. P values of less than 0.05 were deemed statistically significant. The statistical review of this study was performed by a biomedical statistician.
In this study, two colorectal patient cohorts were recruited to compare the cellular and molecular characteristics of low-grade slightly elevated and polypoid lesions. To control for confounding factors, the key demographic and clinical characteristics from the two cohorts were recorded and analyzed (Table 1). With the obvious exception of Paris classification (0-IIa for slightly elevated lesions versus 0-I for polypoid lesions) and the exception of lesion site (i.e., slightly elevated lesions were distributed more proximally, while polypoid lesions were distributed more distally), there were no significant differences in the key demographic and clinical characteristics between the two patient cohorts (P < 0.05, Table 1).
| Characteristics | Elevated cohort, n = 24 (%) | Polypoid cohort, n = 23 (%) | P value |
| Age, mean ± SD | 54.1 ± 9.4 | 53.9 ± 9.4 | > 0.05 |
| Sex (male/female) | 13/11 | 11/12 | > 0.05 |
| BMI, mean ± SD | 23.8 (3.2) | 23.9 (3.3) | > 0.05 |
| Smoker (yes/no) | 9/15 (38/62) | 9/14 (39/61) | > 0.05 |
| Excess alcohol (yes/no) | 3/24 (13) | 3/23 (13) | > 0.05 |
| Lesion site (prox/dist/rect) | 16/5/3 (67/21/12) | 6/9/8 (26/39/35) | < 0.05 |
| Diameter (mm), mean (range) | 8 (2-50) | 9 (3-32) | > 0.05 |
| Paris classification | 0-IIa, 24 (100) | 0-I, 23 (100) | < 0.05 |
| Histology, T/TV/V/S | 14/8/2/0 (58/33/8/0) | 11/9/3/0 (48/39/13/0) | > 0.05 |
| Pathological grade | Low-grade, 24 (100) | Low-grade, 23 (100) | > 0.05 |
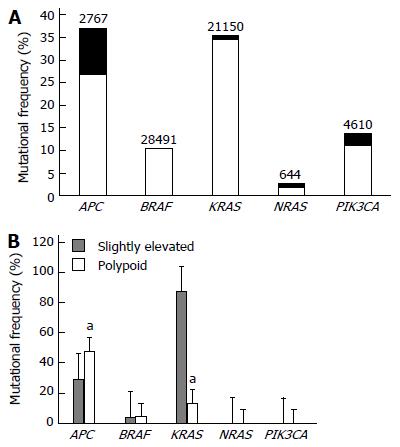
Next, we conducted a comparative mutational analysis on five of the most commonly mutated genes (APC, BRAF, KRAS, NRAS, and PIK3CA) in colorectal malignancies (Figure 1A). APC and KRAS were the most frequently mutated genes in both types of lesions (Figure 1B). Moreover, there were significant differences in the mutational frequencies of APC and KRAS between the two lesion types, with slightly elevated lesions showing a significantly lower APC mutational frequency and a significantly higher KRAS mutational frequency relative to polypoid lesions (both P < 0.05, Figure 1B). Notably, all slightly elevated lesions showed a mutation in APC, KRAS, or both genes, while less than 60% of polypoid lesions showed a mutation in either APC or KRAS (Figure 1B).
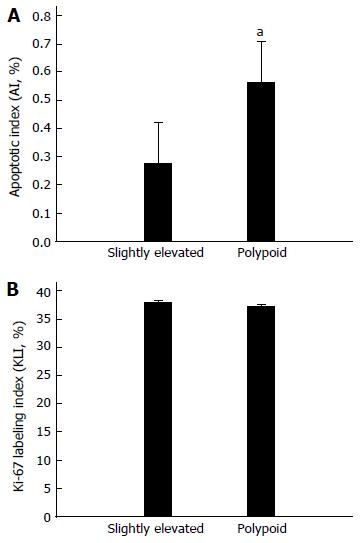
As APC and KRAS have been shown to be centrally involved in apoptotic and proliferative signaling pathways in colorectal cancer cells[17], we comparatively analyzed the apoptosis and proliferation levels of cells in both lesions. We found that slightly elevated lesions demonstrated a significantly lower AI than polypoid lesions (P < 0.05, Figure 2A), while there was no significant difference in KLI between the two lesion types (P > 0.05, Figure 2B).
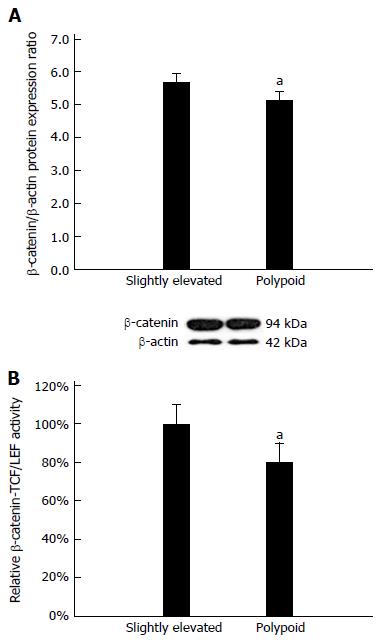
Based on the above data, we surmised that an apoptosis pathway common to both APC and KRAS must be differentially regulated in slightly elevated lesions and polypoid lesions. It is well-established that the anti-apoptotic β-catenin pathway is dysregulated by changes in either APC or KRAS activity in colorectal cancer cells[18]. Therefore, we hypothesized that the β-catenin pathway activity may be upregulated in slightly elevated lesions relative to polypoid lesions. To test this hypothesis, we constructed two primary cultures from both lesion types in order to assess β-catenin protein expression and β-catenin-TCF/LEF-driven transcriptional activity. Western blot analysis revealed that β-catenin protein expression was significantly upregulated in slightly elevated lesions relative to polypoid lesions (P < 0.05, Figure 3A). Moreover, a dual-luciferase reporter assay[19] revealed significant upregulation of β-catenin-TCF/LEF-driven transcriptional activity in slightly elevated lesions relative to polypoid lesions (P < 0.05, Figure 3B). Spearman correlation analysis showed that β-catenin protein expression was positively correlated with β-catenin-TCF/LEF-driven transcriptional activity (ρ = 0.83, P < 0.05, Table 2).
| Characteristic 1 | Characteristic 2 | Spearman correlation coefficient (ρ) | P value |
| β-catenin protein expression | β-catenin-TCF/LEF-driven transcriptional activity | 0.83 | < 0.05 |
| β-catenin-TCF/LEF-driven transcriptional activity | C-Myc protein expression | -0.78 | < 0.05 |
| β-catenin-TCF/LEF-driven transcriptional activity | Cyclin D1 protein expression | 0.79 | < 0.05 |
| Cyclin D1 protein expression | C-Myc protein expression | -0.74 | < 0.05 |
| C-Myc protein expression | Bcl-2 protein expression | -0.74 | < 0.05 |
| C-Myc protein expression | Bcl-xL protein expression | -0.78 | < 0.05 |
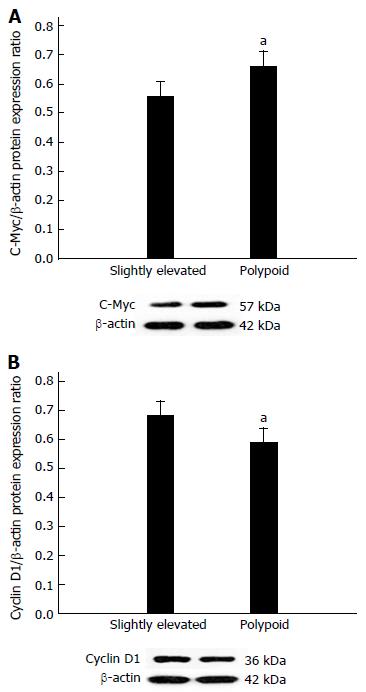
Having demonstrated that β-catenin-TCF/LEF-driven transcriptional activity is upregulated in slightly elevated lesions, we assessed the protein expression of two downstream genes that are centrally involved in regulating apoptosis in colorectal cancer cells: c-Myc and cyclin D1. We found that c-Myc expression was significantly downregulated (P < 0.05, Figure 4A), while cyclin D1 was significantly upregulated (P < 0.05, Figure 4B), in slightly elevated lesions relative to polypoid lesions. Spearman correlation analysis revealed that β-catenin-TCF/LEF-driven transcriptional activity was negatively correlated with c-Myc protein expression (ρ = -0.78, P < 0.05, Table 2) and positively correlated with cyclin D1 protein expression (ρ = 0.79, P < 0.05, Table 2).
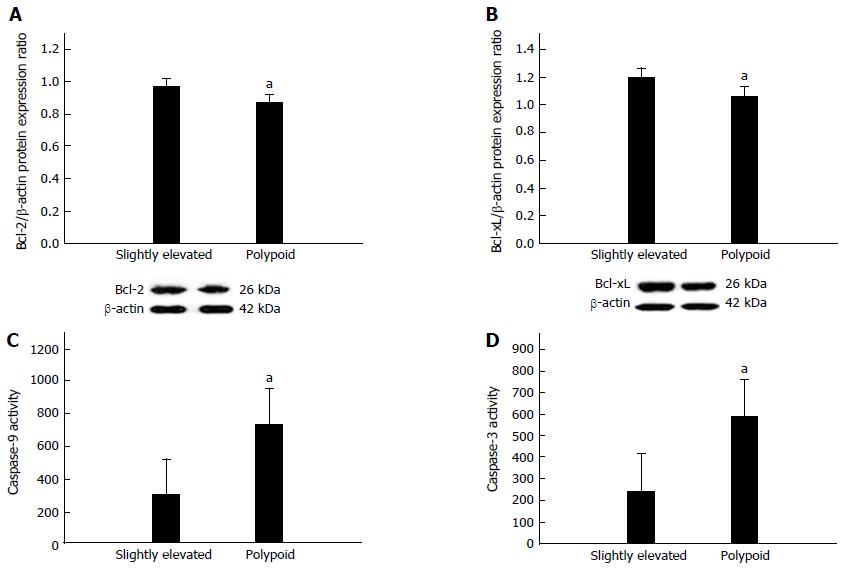
Finally, we assessed the effects of differential c-Myc and cyclin D1 regulation on the anti-apoptotic proteins Bcl-2 and Bcl-xL. We found that protein expression levels of Bcl-2 and Bcl-xL were significantly upregulated in slightly elevated lesions relative to polypoid lesions (both P < 0.05, Figure 5A and B, respectively). Spearman correlation analysis revealed that c-Myc protein expression was negatively correlated with Bcl-2 protein expression (ρ = -0.74, P < 0.05, Table 2) and Bcl-xL protein expression (ρ = -0.78, P < 0.05, Table 2). Accordingly, the activities of the pro-apoptotic enzymes caspase-9 and caspase-3 were significantly lower in slightly elevated lesions relative to polypoid lesions (both P < 0.05, Figure 5C and D, respectively).
This study comparatively investigated the cellular and molecular characteristics of low-grade slightly elevated adenomas and low-grade polypoid adenomas excised from two cohorts of colorectal surgery patients. Through comparative assessment of the mutational frequency of five commonly mutated genes, we found that the slightly elevated lesions exhibited a significantly lower APC mutational frequency and a significantly higher KRAS mutational frequency relative to polypoid lesions. Moreover, we found a significantly lower AI in slightly elevated lesions. Based on these initial findings, we next comparatively assessed differences in the anti-apoptotic β-catenin pathway and found that β-catenin protein expression and β-catenin-TCF/LEF-driven transcriptional activity were significantly upregulated in the slightly elevated lesions. We then assessed the protein expression of two downstream products of β-catenin-TCF/LEF-driven transcription - c-Myc and cyclin D1 - and found that c-Myc expression was significantly downregulated, while cyclin D1 was significantly upregulated in slightly elevated lesions. Accordingly, β-catenin-TCF/LEF-driven transcriptional activity was negatively correlated with c-Myc protein expression and positively correlated with cyclin D1 protein expression. Finally, we examined four downstream apoptosis markers and found that slightly elevated lesions displayed Bcl-2 and Bcl-xL upregulation along with decreased caspase-9 and caspase-3 activity. Notably, c-Myc protein expression was negatively correlated with Bcl-2 protein expression and Bcl-xL protein expression. These findings indicate that low-grade slightly elevated adenomas display lower apoptotic activity relative to low-grade polypoid adenomas and that this lower apoptotic activity can be partly attributed to upregulated β-catenin pathway activity and downregulated c-Myc expression.
Apoptosis is a highly conserved process for eliminating damaged cells in multi-cellular organisms[20,21]. Therefore, defects in apoptotic pathways can result in the survival and proliferation of pre-malignant cells, eventually leading to the development of human cancers[20,22]. With respect to colorectal cancer development, it is well-established that colorectal lesions undergo an “adenoma-to-carcinoma sequence”, that is, a process by which certain key mutations accumulate, thereby driving the progressive transition of pre-malignant adenomas to invasive adenocarcinomas[23]. Specifically, colorectal tumorigenesis is typically caused by initial mutations in the tumor suppressor gene APC and the oncogene KRAS, followed by mutations in other key genes, such as BRAF, CDC4, SMAD4, and TP53[23]. Although this “APC→KRAS→…” mutational sequence is typical of many colorectal tumors and thus more widely publicized[24,25], this particular sequence of mutations is not set in stone, for instance, KRAS mutations have been observed in non-malignant colorectal tissue in the absence of APC mutations[23]. Consistent with these previous findings, we found that both slightly elevated and polypoid adenomas showed the highest mutational rates in APC and KRAS relative to the other sequenced genes. Notably, slightly elevated lesions showed a significantly higher KRAS mutational frequency along with a significantly lower APC mutational frequency relative to polypoid lesions, suggesting that slightly elevated adenomas may more commonly undergo a KRAS→APC mutational sequence as opposed to the prototypical APC→KRAS mutational sequence. Similar to our current findings, several previous studies have also revealed that another type of non-polypoid colorectal lesion - colorectal lateral spreading tumors (LSTs) - display significantly greater KRAS mutational frequencies than polypoid lesions[26-29]. This is of clinical importance, as there is evidence that KRAS mutations can predict a poor response to EGFR inhibitor therapy[26]. Further mutational analysis on larger cohorts of slightly elevated adenoma cases is needed to investigate this phenomenon.
As we found that all slightly elevated lesions showed a mutation in either APC or KRAS in addition to a lower AI relative to polypoid lesions, we hypothesized that β-catenin pathway activity may be upregulated in slightly elevated lesions relative to polypoid lesions. In normal colorectal cells, β-catenin is phosphorylated by the APC/Dsh/Axin/GSK3β destruction complex - this phosphorylation signals β-catenin’s ubiquitination and subsequent proteasomic degradation, thereby maintaining low cytoplasmic levels of β-catenin[16]. However, mutations in APC or KRAS in colorectal cells can dysregulate this degradative process, leading to the accumulation of cytosolic β-catenin and its subsequent nuclear translocation[16,30]. In the nucleus, β-catenin binds with TCF/LEF to drive the transcription of key genes involved in apoptosis and cell proliferation, such as c-Myc, cyclin D1, and survivin[16]. Consistent with the above model, we found that low-grade slightly elevated lesions (which displayed a higher combined frequency of APC and KRAS mutations relative to polypoid lesions) showed significant upregulation of β-catenin protein expression and β-catenin-TCF/LEF-driven transcriptional activity.
Having demonstrated enhanced β-catenin protein expression and β-catenin-TCF/LEF-driven transcription in slightly elevated lesions, we next comparatively assessed the protein expression of two genes downstream of the β-catenin-TCF/LEF complex: c-Myc and cyclin D1. c-Myc is a transcription factor that connects upstream growth factor-driven stimulation with downstream cellular proliferation effects through enhancing the transcription of pro-proliferative genes[31]. In addition to its well-acknowledged pro-proliferative role in cancer cells[31], c-Myc overexpression has also been shown to promote apoptosis through the ARF/MDM2/p53 pathway and the p53-independent Bid/Bim/Noxa network[32]. Specifically, Topham et al[32] have postulated that c-Myc activates this pro-apoptotic BH3-only protein network that serves to suppress the activity of the anti-apoptotic proteins Bcl-2/Bcl-xL. Moreover, c-Myc acts to directly suppress Bcl-2 and Bcl-xL transcription[33]. In the case of c-Myc downregulation, the opposite effects are observed, and apoptosis is inhibited[32]. Consistent with the above model, we found that slightly elevated lesions which displayed lower apoptosis levels, exhibited c-Myc protein downregulation as well as Bcl-2 and Bcl-xL upregulation. Accordingly, we also found strong negative correlations between c-Myc protein expression and Bcl-2 protein expression as well as Bcl-xL protein expression (ρ = -0.74 and ρ = -0.78, respectively).
Cyclin D1 drives cell cycle entry by forming complexes with CDK4/6, which partially inactivates pRB to promote the downstream expression of multiple cell cycle progression genes[34]. Moreover, cyclin D1-CDK complexes phosphorylate chromatin modifiers to repress anti-proliferative gene transcription and phosphorylate the transcription factor SMAD3 to stimulate cell cycle progression[34]. Although enhanced β-catenin-TCF/LEF activity has been shown to promote cyclin D1 expression in colorectal cancer cells[35], there is evidence of c-Myc overexpression as a result of enhanced β-catenin-TCF/LEF activity which inhibits cyclin D1 expression, either directly or through c-Myc-based inhibition of NF-κB activity[36]. This situation produces conflicting signals with regard to cyclin D1 expression. Based on this “conflicting signal” model, Biliran et al[36]’s work in pancreatic tumors postulates that cancer cells can take on three distinct phenotypic alternatives with respect to c-Myc and cyclin D1 expression: (1) c-Myc-underexpressing, cyclin D1-overexpressing cells with low apoptotic potential; (2) c-Myc-overexpressing, cyclin D1-underexpressing cells with high apoptotic potential; and (3) c-Myc-overexpressing, cyclin D1-overexpressing cells with intermediate apoptotic potential. Here, we found that slightly elevated lesions which displayed lower apoptosis levels, showed c-Myc protein downregulation and cyclin D1 upregulation. Accordingly, we also found a strong negative correlation between β-catenin-TCF/LEF-driven transcriptional activity and c-Myc protein expression (ρ = -0.78) coupled with a strong positive correlation between β-catenin-TCF/LEF-driven transcriptional activity and cyclin D1 protein expression (ρ = 0.79). These combined findings align well with Biliran et al[36]’s first cancer cell phenotype described above.
There are several limitations to this study. First, the sample size of this study was small. A larger sample size would have provided more favorable statistical powering. Second, only three risk factors for colorectal carcinoma (i.e., BMI, smoking status, and excessive alcohol consumption) were assessed. Other risk factors, such as a current diagnosis of type 2 diabetes, a previous diagnosis of colorectal cancer, inflammatory bowel disease (IBD), and a family history of colorectal cancer or colorectal adenoma/polyps, should be assessed in future studies. Third, although several key genes and proteins were analyzed, other genes and proteins that have been previously found associated with colorectal malignancy, such as p53, retinoblastoma (Rb), and deleted in colon cancer (DCC), should be investigated in future studies to obtain a more exhaustive picture of the cellular and molecular changes in low-grade slightly elevated lesions[37]. Fourth, we did not analyze epigenetic factors or the mRNA expression of the proteins, which may have provided additional insight.
In conclusion, low-grade slightly elevated adenomas display lower apoptotic activity than low-grade polypoid adenomas, and this lower apoptotic activity can be partly attributed to upregulated β-catenin pathway activity and downregulated c-Myc expression.
We wish to thank Jia-Sheng Wang, Gan He, Qiang Yang, Lian Bai, Bin Jian and Qu-Gang Li at the Department of Gastrointestinal Surgery of Yongchuan Hospital (Chongqing Medical University, Chongqing, China) for their assistance and feedback.
Compared to polypoid colorectal adenomas, non-polypoid adenomas are more difficult to detect through colonoscopy on account of their flatter morphology and are more likely to contain carcinoma cells. Thus, the development of alternative screening modalities and targeted therapeutics for low-grade non-polypoid adenomas remain important clinical challenges. To address these challenges, a better understanding of the cellular and molecular characteristics of low-grade non-polypoid adenomas is needed.
The bulk of published research comparing non-polypoid and polypoid lesions has focused on calculating cohort-based mutational frequencies through genotyping. These previous studies have revealed that non-polypoid lesions display lower levels of v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) and adenomatous polyposis coli (APC) mutations, but higher levels of BRAF mutations.
This is the first study to differentiate the molecular and cellular characteristics of low-grade non-polypoid colorectal adenomas from those of low-grade polypoid adenomas. The authors found that slightly elevated lesions showed a significantly lower APC mutational frequency and significantly higher KRAS mutational frequency relative to polypoid lesions. The authors also found a significantly lower apoptotic index (AI) in slightly elevated lesions, which can be partly attributed to upregulated β-catenin pathway activity and downregulated c-Myc expression.
This study sheds light on the molecular phenotypic differences between low-grade slightly elevated colorectal adenomas and low-grade polypoid adenomas. These findings can aid the development of molecular biomarkers and targeted therapeutics for low-grade non-polypoid adenomas.
Non-polypoid colorectal adenomas refer to slightly elevated (Paris 0-IIa), flat (Paris 0-IIb), and depressed (Paris 0-IIc) lesions, which are characterized by a flatter morphology. In contrast, polypoid (Paris 0-I) colorectal adenomas refer to pedunculated or sessile polypoid lesions, which are characterized by an elevated morphology.
The article has academic and public interest. It could show some new signaling pathway to development of distinct polyps. It has clinical interest as well, because of the distinct risk of cancer into different polyps types, with or without needed of resection to colonoscopy and clinical follow up.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Priolli DG S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1226] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 2. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2073] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3659] [Article Influence: 304.9] [Reference Citation Analysis (2)] |
| 4. | Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 5. | Burt RW, Cannon JA, David DS, Early DS, Ford JM, Giardiello FM, Halverson AL, Hamilton SR, Hampel H, Ismail MK. Colorectal cancer screening. J Natl Compr Canc Netw. 2013;11:1538-1575. [PubMed] |
| 6. | Rutter MD. Importance of nonpolypoid (flat and depressed) colorectal neoplasms in screening for CRC in patients with IBD. Gastrointest Endosc Clin N Am. 2014;24:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 8. | Kim HN, Raju GS. Bowel preparation and colonoscopy technique to detect non-polypoid colorectal neoplasms. Gastrointest Endosc Clin N Am. 2010;20:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Coghlin C, Murray GI. Biomarkers of colorectal cancer: recent advances and future challenges. Proteomics Clin Appl. 2015;9:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Kuppusamy P, Govindan N, Yusoff MM, Ichwan SJ. Proteins are potent biomarkers to detect colon cancer progression. Saudi Journal of Biological Sciences 2014. . [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Voorham QJ, Rondagh EJ, Knol DL, van Engeland M, Carvalho B, Meijer GA, Sanduleanu S. Tracking the molecular features of nonpolypoid colorectal neoplasms: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:1042-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Voorham QJ, Carvalho B, Spiertz AJ, Claes B, Mongera S, van Grieken NC, Grabsch H, Kliment M, Rembacken B, van de Wiel MA. Comprehensive mutation analysis in colorectal flat adenomas. PLoS One. 2012;7:e41963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29:844-854. [PubMed] |
| 14. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [PubMed] |
| 15. | Oikonomou E, Kothonidis K, Taoufik E, Probert E, Zografos G, Nasioulas G, Andera L, Pintzas A. Newly established tumourigenic primary human colon cancer cell lines are sensitive to TRAIL-induced apoptosis in vitro and in vivo. Br J Cancer. 2007;97:73-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Li X, Bai B, Liu L, Ma P, Kong L, Yan J, Zhang J, Ye Z, Zhou H, Mao B. Novel β-carbolines against colorectal cancer cell growth via inhibition of Wnt/β-catenin signaling. Cell Death Discov. 2015;1:15033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac J Cancer Prev. 2013;14:2201-2205. [PubMed] |
| 18. | Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog. 2011;10:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Fei P, Zhang Q, Huang L, Xu Y, Zhu X, Tai Z, Gong B, Ma S, Yao Q, Li J. Identification of two novel LRP5 mutations in families with familial exudative vitreoretinopathy. Mol Vis. 2014;20:395-409. [PubMed] |
| 20. | Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 21. | Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1184] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 23. | Hadac JN, Leystra AA, Paul Olson TJ, Maher ME, Payne SN, Yueh AE, Schwartz AR, Albrecht DM, Clipson L, Pasch CA. Colon Tumors with the Simultaneous Induction of Driver Mutations in APC, KRAS, and PIK3CA Still Progress through the Adenoma-to-carcinoma Sequence. Cancer Prev Res (Phila). 2015;8:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, Garner RC, Steele RJ, Wolf CR. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci USA. 2002;99:9433-9438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Kulendran M, Stebbing JF, Marks CG, Rockall TA. Predictive and prognostic factors in colorectal cancer: a personalized approach. Cancers (Basel). 2011;3:1622-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Chang LC, Chiu HM, Shun CT, Liang JT, Lin JT, Chen CC, Lee YC, Wu MS. Mutational profiles of different macroscopic subtypes of colorectal adenoma reveal distinct pathogenetic roles for KRAS, BRAF and PIK3CA. BMC Gastroenterol. 2014;14:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Sugimoto T, Ohta M, Ikenoue T, Yamada A, Tada M, Fujishiro M, Ogura K, Yamaji Y, Okamoto M, Kanai F. Macroscopic morphologic subtypes of laterally spreading colorectal tumors showing distinct molecular alterations. Int J Cancer. 2010;127:1562-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Hiraoka S, Kato J, Tatsukawa M, Harada K, Fujita H, Morikawa T, Shiraha H, Shiratori Y. Laterally spreading type of colorectal adenoma exhibits a unique methylation phenotype and K-ras mutations. Gastroenterology. 2006;131:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Mukawa K, Fujii S, Takeda J, Kitajima K, Tominaga K, Chibana Y, Fujita M, Ichikawa K, Tomita S, Ono Y. Analysis of K-ras mutations and expression of cyclooxygenase-2 and gastrin protein in laterally spreading tumors. J Gastroenterol Hepatol. 2005;20:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Lemieux E, Cagnol S, Beaudry K, Carrier J, Rivard N. Oncogenic KRAS signalling promotes the Wnt/β-catenin pathway through LRP6 in colorectal cancer. Oncogene. 2015;34:4914-4927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 31. | Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1197] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 32. | Topham C, Tighe A, Ly P, Bennett A, Sloss O, Nelson L, Ridgway RA, Huels D, Littler S, Schandl C. MYC Is a Major Determinant of Mitotic Cell Fate. Cancer Cell. 2015;28:129-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007-9021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 34. | Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol. 2016;17:280-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 35. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2796] [Cited by in RCA: 2853] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 36. | Biliran H, Banerjee S, Thakur A, Sarkar FH, Bollig A, Ahmed F, Wu J, Sun Y, Liao JD. c-Myc-induced chemosensitization is mediated by suppression of cyclin D1 expression and nuclear factor-kappa B activity in pancreatic cancer cells. Clin Cancer Res. 2007;13:2811-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Matkowskyj KA, Chen ZE, Rao MS, Yang GY. Dysplastic lesions in inflammatory bowel disease: molecular pathogenesis to morphology. Arch Pathol Lab Med. 2013;137:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |