Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2566
Peer-review started: December 20, 2016
First decision: January 10, 2017
Revised: January 17, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 14, 2017
Processing time: 116 Days and 23.7 Hours
To elucidate the epidemiological characteristics and associated risk factors of perforated peptic ulcer (PPU).
We retrospectively reviewed medical records of patients who were diagnosed with benign PPU from 2010 through 2015 at 6 Hallym university-affiliated hospitals.
A total of 396 patients were identified with postoperative complication rate of 9.1% and mortality rate of 0.8%. Among 174 (43.9%) patients who were examined for Helicobacter pylori (H. pylori) infection, 78 (44.8%) patients were positive for H. pylori infection, 21 (12.1%) were on non-steroidal anti-inflammatory drugs (NSAIDs) therapy, and 80 (46%) patients were neither infected of H. pylori nor treated by any kinds of NSAIDs. Multivariate analysis indicated that older age (OR = 1.09, 95%CI: 1.04-1.16) and comorbidity (OR = 4.11, 95%CI: 1.03-16.48) were risk factors for NSAID-associated PPU compared with non-H. pylori, non-NSAID associated PPU and older age (OR = 1.04, 95%CI: 1.02-1.07) and alcohol consumption (OR = 2.08, 95%CI: 1.05-4.13) were risk factors for non-H. pylori, non-NSAID associated PPU compared with solely H. pylori positive PPU.
Elderly patients with comorbidities are associated with NSAIDs-associated PPU. Non-H. pylori, non-NSAID peptic ulcer is important etiology of PPU and alcohol consumption is associated risk factor.
Core tip: The incidence of complications of peptic ulcer has not been decreasing and only a few data is available about epidemiological characteristics and associated risk factors of perforated peptic ulcer. In a retrospective review of medical records from multicenter in Korea revealed that elderly patients with comorbidities were associated with non-steroidal anti-inflammatory drugs (NSAIDs)-associated peptic ulcer perforation and non-Helicobacter pylori (H. pylori), non-NSAID peptic ulcer is important etiology in the development of peptic ulcer perforation. In a multivariate logistic regression analysis, alcohol consumption was suspected to be associated risk factors for the development of non-H. pylori, non-NSAID peptic perforation.
- Citation: Yang YJ, Bang CS, Shin SP, Park TY, Suk KT, Baik GH, Kim DJ. Clinical characteristics of peptic ulcer perforation in Korea. World J Gastroenterol 2017; 23(14): 2566-2574
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2566
The decreasing prevalence of Helicobacter pylori (H. pylori) infection and improvement of peptic ulcer treatment such as proton pump inhibitors (PPIs) or eradication therapies for H. pylori resulted in reduction of the incidence of uncomplicated peptic ulcer disease in recent decades[1-3]. However, several studies have shown controversial results showing constant incidence of complicated peptic ulcer disease[4-7], which may be due to multifactorial risk factors including the increased consumption of non-steroidal anti-inflammatory drugs (NSAIDs) or acetylsalicylic acid (ASA), especially in elderly patients with multiple comorbidities, smoking habits, or unknown etiologies[8,9].
Previous studies evaluated the epidemiologic characteristics and associated risk factors of perforated peptic ulcer (PPU) and demonstrated increasing incidence of PPU by age[7-15]. However, these studies used national registry database rather than those from hospitals, which have potential for underestimation of true incidence or misinterpretation of characteristics of PPU. Also, H. pylori infection status in patients with PPU was rarely evaluated except 1 study, which included suboptimal number of subjects at early 2000s[12]. Also, the effect of NSAIDs or ASA on PPU was inconsistent according to the studies[5,13,16]. Therefore, this study aimed to investigate the epidemiological characteristics and associated risk factors of benign PPU using multicenter clinical data.
We retrospectively reviewed the medical records of 402 patients who were diagnosed with PPU (either gastric or duodenal ulcer) from January 2010 through December 2015 at Hallym university-affiliated hospitals, including the Chuncheon, Kangdong, Dongtan, Hangang, Kangnam and Hallym University Sacred Heart Hospital. Except 6 patients with unknown histology of PPU, remaining 396 ulcers were verified as benign ulcers by histology after surgical resection or endoscopic biopsy. This study was approved by the institutional review board of Chuncheon Sacred Heart Hospital (2016-86).
We retrospectively collected the clinical data including age, sex, body mass index (BMI), smoking status and alcohol consumption for the last 3 mo, presence of any comorbidities, and current medications, such as NSAIDs or ASA, steroid, H2-blockers, or PPIs. BMI was calculated as weight in kilograms divided by the square of height in meters. Positive alcohol consumption was defined as those who drink more than 20 g of alcohol amount in a week.
Chief complaints and laboratory data including white blood count (WBC), hemoglobin (Hb), serum creatinine (SCr), C-reactive protein (CRP) at admission period were obtained. Also, sites of perforation, treatment methods, the development of postoperative complication if surgical management was done, the length of hospital stay, and mortality rate were identified. The sites of perforation were divided into 3 areas in stomach (from cardiac to body area, proximal antrum, and from prepyloric to pyloric area) and 2 areas in duodenum (bulb, and 2nd portion). The size of perforated peptic ulcer was categorized on the basis of centimeter. The methods of operation were classified into 3 groups: (1) simple closure with or without omentopexy; (2) pyloroplasty with or without vagotomy; and (3) any other form of gastrectomy (total, subtotal, or antrectomy). If patients were assessed H. pylori infection status, diagnostic methods such as rapid urease test, 13C-urea breath test, or the serological test and the infection status were identified. All patients who were examined for H. pylori were discontinued PPIs or H2-blockers at least 4 wk before H. pylori test. Treatment regimen and whether the treatment was successful or not were also identified.
Continuous variables were expressed as mean ± SD. Categorical variables were expressed as number and percentage. We compared the differences in the clinical characteristics and therapeutic outcomes of the study population using the Student’s t-test for the continuous variables and the Fisher’s exact test for the categorical variables. To identify the risk of non-H. pylori, non-NSAIDs associate PPU, we performed univariate and subsequent multivariate logistic regression analysis. In this study, a P value < 0.05 (2-tailed) was adopted as the threshold of statistical significance for all tests. All of the analyses were performed using SPSS version 20.0. (SPSS Inc., Chicago, IL, United States).
The baseline characteristics of the study population and site specific characteristics classified according to the site of perforation are shown in Table 1. We identified a total of 396 benign PPU patients, consisting of 173 (43.7%) in gastric ulcer group and 223 (56.3%) in duodenal ulcer group. Men predominance was observed (85.1%). The mean age and BMI of the subjects were 50.6 ± 18.3 years and 21.7 ± 2.9 kg/m2, respectively. And about half of patients had alcohol consumption (47.2%) and smoking habit (55.8%). Of all, 54 (13.6%) patients had been diagnosed with peptic ulcer at median 12 mo before the time of perforation (interquartile range: 2-36 mo). In terms of the comorbidities, 123 (31.1%) patients had at least one comorbidities, which were cardiovascular disease (67.5%), diabetes mellitus (33.3%), chronic liver disease (10.6%) and cerebrovascular disease (8.9%) in the order. The proportion of taking medication was as follows; 44 (11.2%) patients on NSAIDs including ASA (n = 23), 8 (2%) patients on steroid, and 31 (7.8%) patents on anti-ulcer medication such as PPIs (n = 19) or H2-blocker (n = 12).
| Variables | Total | Gastric ulcer | Duodenal ulcer | P value |
| (n = 396) | (n = 173) | (n = 223) | ||
| Sex (men) | 337 (85.1) | 145 (83.8) | 192 (86.1) | 0.57 |
| Age | 50.6 ± 18.3 | 51.4 ± 19.0 | 50.1 ± 17.8 | 0.49 |
| BMI (kg/m2) | 21.7 ± 2.9 | 21.5 ± 2.9 | 21.9 ± 3.0 | 0.14 |
| Alcohol consumption | 187 (47.2) | 76 (43.9) | 111 (49.8) | 0.27 |
| Current smoking | 221 (55.8) | 98 (56.6) | 123 (55.2) | 0.84 |
| Previous ulcer history | 54 (13.6) | 27 (15.6) | 27 (12.1) | 0.38 |
| Comorbidity | 123 (31.1) | 61 (35.3) | 62 (27.8) | 0.13 |
| Cardiovascular disease | 83 (67.5) | 38 (62.3) | 45 (72.6) | 0.22 |
| DM | 41 (33.3) | 18 (29.5) | 23 (37.1) | 0.45 |
| Chronic liver disease | 13 (10.6) | 8 (13.1) | 5 (8.1) | 0.40 |
| Cerebrovascular disease | 11 (8.9) | 4 (6.6) | 7 (11.3) | 0.36 |
| Malignancy | 9 (7.3) | 6 (9.8) | 3 (4.8) | 0.24 |
| Chronic kidney injury | 6 (4.9) | 4 (6.6) | 2 (3.2) | 0.33 |
| Pulmonary disease | 4 (3.3) | 3 (4.9) | 1 (1.6) | 0.30 |
| Infectious disease | 3 (2.4) | 2 (3.3) | 1 (1.6) | 0.49 |
| Current medication | ||||
| NSAIDs | 44 (11.2) | 20 (11.7) | 24 (10.8) | 0.87 |
| Steroid | 8 (2.0) | 5 (2.9) | 3 (1.3) | 0.23 |
| Proton pump inhibitor | 19 (4.8) | 11 (6.4) | 8 (3.6) | 0.24 |
| H2-blocker | 12 (3.0) | 5 (2.9) | 7 (3.1) | > 0.99 |
| Presentation | 0.39 | |||
| Abdominal pain | 365 (92.2) | 155 (89.6) | 210 (94.2) | |
| Melena/hematemesis | 16 (4.0) | 9 (5.2) | 7 (3.1) | |
| Shock | 5 (1.3) | 2 (1.2) | 3 (1.3) | |
| Epigastric soreness | 6 (1.5) | 4 (2.3) | 2 (0.9) | |
| Nausea/vomiting | 4 (1.0) | 3 (1.7) | 1 (0.4) | |
| Laboratory findings | ||||
| White blood count (x103/uL) | 13.4 ± 7.8 | 12.9 ± 5.3 | 13.8 ± 9.4 | 0.24 |
| Hemoglobin (g/dL) | 13.7 ± 6.0 | 13.7 ± 8.8 | 13.7 ± 2.2 | 0.95 |
| Serum Creatinine (mg/dL) | 1.02 ± 0.7 | 1.01 ± 0.7 | 1.03 ± 0.6 | 0.87 |
| C-reactive protein (mg/L) | 93.7 ± 89.0 | 88.9 ± 86.0 | 97.2 ± 91.1 | 0.39 |
| Anatomical findings | ||||
| Location | ||||
| Stomach | 173 (43.7) | |||
| Body | 11 (6.4) | |||
| Antrum | 62 (35.8) | |||
| Pylorus | 100 (57.8) | |||
| Duodenum | 223 (56.3) | |||
| Bulb | 218 (97.8) | |||
| 2nd portion | 5 (2.2) | |||
| Size | 0.82 | |||
| ≥ 1 cm | 126 (37.6) | 58 (38.4) | 68 (37.0) | |
| < 1 cm | 209 (62.4) | 93 (61.6) | 116 (63.0) | |
| H. pylori test | ||||
| Positivity H. pylori test | 78/174 (44.8) | 30/72 (41.7) | 48/102 (47.1) | 0.48 |
| Rapid urease test | 60 (76.9) | 26 (86.7) | 34 (70.8) | |
| Urea breath test | 5 (6.4) | 2 (6.7) | 3 (6.2) | |
| Serology test | 13 (16.7) | 2 (6.7) | 11 (22.9) | |
| Operation | 388 (98.0) | 170 (98.3) | 218 (97.8) | 0.51 |
| Primary closure and/or omentopexy | 307 (79.5) | 139 (81.7) | 168 (77.8) | |
| Pyloroplasty and/or vagotomy | 43 (11.1) | 15 (8.8) | 28 (13.0) | |
| Total/subtotal gastrectomy or antrectomy | 36 (9.3) | 16 (9.5) | 20 (9.2) | |
| Others (Whipple’s operation, drainage) | 2 (0.5) | 0 (0.0) | 2 (0.9) | |
| Medical treatment | 8 (2.0) | 3 (1.7) | 5 (2.2) | 0.09 |
| Clinical course | ||||
| Hospital stay | 13.1 ± 9.4 | 13.4 ± 10.4 | 12.8 ± 8.7 | 0.54 |
| Complication | 36 (9.1) | 21 (12.1) | 15 (6.7) | 0.08 |
| In hospital mortality | 3 (0.8) | 2 (1.2) | 1 (0.4) | 0.58 |
At admission, the majority of patients (92.2%) complained abdominal pain and 16 (4.0%) patients experienced melena or hematemesis. The mean levels of WBC (13.4 ± 7.8 × 103/uL) and CRP (93.7 ± 89.0 mg/L) were above normal range. However, the mean levels of Hb (13.7 ± 6.0 g/dL) and SCr (1.02 ± 0.7 mg/dL) were within normal value. Among 174 (43.9%) patients who were tested for H. pylori infection, 78 (44.8%) patients were positive for H. pylori tests, which were rapid urease test (n = 60), urea breath test (n = 5), and serologic test (n = 13). Comparing with the 222 patients who did not perform H. pylori test, patients who tested for H. pylori infection were significantly younger (47.6 ± 16.8 vs 53.0 ± 19.1 years, P = 0.003) and none of them had malignant disease. The other baseline characteristics were comparable between the patients who were tested for H. pylori infection or not (Table 2). Except 9 patients who were lost to follow-up, 69 (88.5%) patients were prescribed with 7 or 14 d of standard triple therapy (n = 66), or 14 d of bismuth-based quadruple therapy (n = 3) as the first-line regimen. Among them, 33 patients achieved successful eradication after the first-line treatment (eradication rate of 47.8%) and 4 patients who failed to eradication after first line regimen achieved successful eradication after 2nd line treatment (overall eradication rate of 53.6%). We could not evaluate eradication status in the remaining 32 patients due to lost to follow up during or after eradication treatment.
| Variables | Total (n = 396) | Gastric ulcer (n = 173) | Duodenal ulcer (n = 223) | ||||||
| Patients who were testedfor H. pylori infection(n = 174) | Patients who were not tested for H. pylori infection(n = 222) | P value | Patients who were testedfor H. pylori infection(n = 72) | Patients who were not tested for H. pylori infection(n = 101) | P value | Patients who were testedfor H. pylori infection(n = 102) | Patients who were not tested for H. pylori infection(n = 121) | P value | |
| Sex (men) | 151 (86.8) | 186 (83.8) | 0.480 | 60 (83.3) | 85 (84.2) | > 0.99 | 91 (89.2) | 101 (83.5) | 0.25 |
| Age (yr) | 47.6 ± 16.8 | 53.0 ± 19.1 | 0.003 | 47.5 ± 18.3 | 54.1± 19.1 | 0.02 | 47.6 ± 15.7 | 52.2 ± 19.1 | 0.05 |
| < 60 | 133 (76.4) | 142 (64.0) | 0.008 | 56 (77.8) | 61 (60.4) | 0.02 | 77 (75.5) | 81 (66.9) | 0.18 |
| ≥ 60 | 41 (23.6) | 80 (36.0) | 16 (22.2) | 40 (39.6) | 25 (24.5) | 40 (33.1) | |||
| BMI (kg/m2) | 22.0 ± 3.1 | 21.5 ± 2.8 | 0.150 | 21.5 ± 3.1 | 21.4 ± 2.7 | 0.83 | 22.3 ± 3.0 | 21.6 ± 3.00 | 0.10 |
| Alcohol drinking | 87 (50.0) | 100 (45.0) | 0.360 | 33 (45.8) | 43 (42.6) | 0.76 | 54 (52.9) | 57 (47.1) | 0.42 |
| Current smoking | 97 (55.7) | 124 (55.9) | > 0.99 | 39 (54.2) | 59 (58.4) | 0.64 | 58 (56.9) | 65 (53.7) | 0.69 |
| Both alcohol consumption and smoking | 69 (39.7) | 85 (38.3) | 0.840 | 24 (33.3) | 36 (35.6) | 0.87 | 45 (44.1) | 49 (40.5) | 0.59 |
| Previous ulcer history | 30 (17.2) | 24 (10.8) | 0.08 | 14 (19.4) | 13 (12.9) | 0.29 | 16 (15.7) | 11 (9.1) | 0.15 |
| Comorbidity | 44 (25.3) | 79 (35.6) | 0.03 | 21 (29.2) | 40 (39.6) | 0.2 | 23 (22.5) | 39 (32.2) | 0.13 |
| HTN | 27 (61.4) | 55 (69.6) | 0.43 | 14 (66.7) | 24 (60.0) | 0.78 | 13 (56.5) | 31 (79.5) | 0.08 |
| DM | 13 (29.5) | 28 (35.4) | 0.55 | 5 (23.8) | 13 (32.5) | 0.56 | 8 (34.8) | 15 (38.5) | > 0.99 |
| Cardiovascular disease | 6 (13.6) | 15 (19.0) | 0.62 | 3 (14.3) | 7 (17.5) | 0.53 | 3 (13.0) | 8 (20.5) | 0.35 |
| Chronic liver disease | 5 (11.4) | 8 (10.1) | 0.53 | 1 (4.8) | 7 (17.5) | 0.24 | 4 (17.4) | 1 (2.6) | 0.06 |
| Malignancy | 0 (0.0) | 9 (11.4) | 0.02 | 0 (0.0) | 6 (15.0) | 0.07 | 0 (0.0) | 3 (7.7) | 0.24 |
| Chronic kidney injury | 3 (6.8) | 3 (3.8) | 0.17 | 2 (9.5) | 2 (5.0) | 0.43 | 1 (4.3) | 1 (2.6) | 0.61 |
| Pulmonary disease | 0 (0.0) | 4 (5.1) | 0.37 | 0 (0.0) | 3 (7.5) | 0.28 | 0 (0.0) | 1 (2.6) | 0.63 |
| Infectious disease | 1 (2.3) | 2 (2.5) | 0.71 | 1 (4.8) | 1 (2.5) | 0.57 | 0 (0.0) | 1 (2.6) | 0.63 |
| Current medication | |||||||||
| NSAIDs | 21 (12.1) | 23 (10.4) | 0.63 | 9 (12.7) | 11 (11.0) | 0.81 | 12 (11.8) | 12 (9.9) | 0.67 |
| Steroid | 2 (1.2) | 6 (2.7) | 0.24 | 1 (1.4) | 4 (4.0) | 0.31 | 1 (1.0) | 2 (1.7) | 0.56 |
| Proton pump inhibitor | 7 (4.0) | 12 (5.4) | 0.64 | 3 (4.2) | 8 (7.9) | 0.26 | 4 (3.9) | 4 (3.3) | 0.54 |
| H2-blocker | 4 (2.3) | 8 (3.6) | 0.56 | 3 (4.2) | 2 (2.0) | 0.34 | 1 (1.0) | 6 (5.0) | 0.09 |
In terms of the site of perforation, bulb of duodenum (55.1%) was the most common site, followed by pylorus (25.3%), and antrum (15.7%). The proportion of duodenal ulcer perforation was 56.3% and the gastric ulcer perforation was 43.7%, respectively. Except 8 (2.0%) patients who were treated by medical management, remaining 388 patients (98.0%) underwent surgical management. The operative methods were primary closure with or without omentopexy (n = 307), pyloroplasty with or without vagotomy (n = 43), and any other form of gastrectomy (total, subtotal or antrectomy, n = 36). The mean duration of hospital stay was 13.1 ± 9.4 d. Though 36 (9.1%) patients experienced postoperative complication, only 3 (0.8%) patients died during hospitalization because of acute respiratory distress syndrome or uncontrolled sepsis. All of the baseline characteristics and clinical manifestations were comparable between perforated gastric ulcer and duodenal ulcer group. The detailed characteristics of all of the enrolled population are described in Tables 1 and 2.
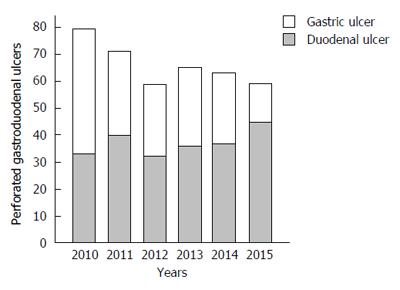
The annual incidences of PPU showed decreasing trend for study periods, especially in gastric ulcer (Figure 1). The incidence of gastric ulcer perforation was 49.8% in the first 3 years and 36.9% in the last 3 years, which was statistically significant (P = 0.01). The decreasing incidence of perforated gastric ulcer was mainly observed in male under the age of 60. In these patients, the proportions of H. pylori infection, NSAIDs use, alcohol consumption, and any comorbidities were increased during study period, whereas the proportion of smoking habit was decreased from 65.9% in the first 3 years to 57.9% in the last 3 years, although the statistical significance was not reached.
Among 396 patients, 121 (30.6%) patients were older than 60 years and the proportion of women was significantly higher in patients older than 60 years (old age group) compared with patients younger than 60 years (young age group) (5.5% vs 36.4%, P < 0.001). The proportion of alcohol consumption (56.0% vs 27.3%) and smoking habit (62.5% vs 40.5%) was higher in young age group than those of patients in old age group (P < 0.001). The proportions of patients with comorbidities (14.9% vs 67.8%) and taking NSAIDs (2.9% vs 30.0%) were significantly higher in old age group (P < 0.001), whereas the proportion of patients with H. pylori infection was significantly higher in young age group (50.4% vs 26.8%, P = 0.008). Although the site of perforation was comparable between two groups, the higher proportion of patients in old age group had PPU over 1 cm (31.1% vs 53.0%, P < 0.001). Moreover, the length of hospitalization (11.3 ± 7.7 vs 17.0 ± 11.7 d) and postoperative complication rate (4.0% vs 20.7%) were significantly higher in old age group (P < 0.001). All of the in-hospital mortality cases were also occurred in old age group (Table 3).
| Variables | < 60 yr | ≥ 60 yr | P value |
| (n = 275) | (n = 121) | ||
| Sex (men) | 260 (94.5) | 77 (63.6) | < 0.001 |
| BMI (kg/m2) | 21.8 ± 2.7 | 21.5 ± 3.4 | 0.33 |
| Alcohol consumption | 154 (56.0) | 33 (27.3) | < 0.001 |
| Current smoking | 172 (62.5) | 49 (40.5) | < 0.001 |
| Previous ulcer history | 31 (11.3) | 23 (19.0) | 0.04 |
| Comorbidity | 41 (14.9) | 82 (67.8) | < 0.001 |
| Current medication | |||
| NSAIDs | 8 (2.9) | 36 (30.0) | < 0.001 |
| Steroid | 4 (1.5) | 4 (3.3) | 0.26 |
| Proton pump inhibitor | 9 (3.3) | 10 (8.3) | 0.04 |
| H2-blocker | 5 (1.8) | 7 (5.8) | 0.05 |
| H. pylori test | |||
| Positivity H. pylori test | 67/133 (50.4) | 11/41 (26.8) | 0.008 |
| Rapid urease test | 52 (77.6) | 8 (72.7) | |
| Urea breath test | 4 (6.0) | 1 (9.1) | |
| Serology test | 11 (16.8) | 2 (18.2) | |
| Anatomical findings | |||
| Location | 0.490 | ||
| Gastric ulcer | 117 (42.5) | 56 (46.3) | |
| Duodenal ulcer | 158 (57.5) | 65 (53.7) | |
| Size | < 0.001 | ||
| ≥ 1 cm | 73 (31.1) | 53 (53.0) | |
| < 1 cm | 162 (68.9) | 47 (47.0) | |
| Clinical course | |||
| Hospital stay | 11.3 ± 7.7 | 17.0 ± 11.7 | < 0.001 |
| Complication | 11 (4.0) | 25 (20.7) | < 0.001 |
| In hospital mortality | 0 (0.0) | 3 (2.5) | 0.009 |
A total of 174 patients who were tested for H. pylori infection status were categorized into 4 groups in terms of the etiology of peptic ulcer (both H. pylori positive and NSAIDs use, either H. pylori positive or NSAID use, and Non-H. pylori, non-NSAIDs group). The patients with solely H. pylori positive were 73 and the patients taking NSAIDs without H. pylori infection were 16. Five patients were infected H. pylori and also taking NSAIDs (Both H. pylori positive and NAIDs user group). The remaining 80 patients who were negative for H. pylori test and not taking any kinds of NSAIDs or ASA were categorized into Non-H. pylori, non-NSAIDs group. Men predominance was observed consistently in all of the 4 groups. The mean age (69.5 ± 12.2 years) and the proportion of patients with any comorbidities (75.0%) were significantly higher in NSAIDs user group (P < 0.001). The mean BMI level and the proportion of patients with alcohol consumption, current smoking, and peptic ulcer history were similar among the 4 groups. More than half of patients in each group experienced duodenal ulcer perforation, which were most commonly in bulb area. Also, the proportion of patients with perforation more than 1 cm in diameter was significantly higher in NSAIDs group (66.7%) than the other groups (P = 0.002). The lengths of hospital stay and postoperative complication rates were comparable among 4 group. There was no mortality during hospitalization in 4 groups. The detailed clinical characteristics of PPU according to the etiology are described in Table 4.
| Variables | Both H. pylori positive and NSAIDs user group (n = 5) | H. pylori positive group (n = 73) | NSAIDs user group (n = 16) | Non-H. pylori, Non-NSAIDs group (n = 80) | P value |
| Sex (men) | 4 (80.0) | 67 (91.8) | 9 (56.2) | 71 (88.8) | 0.005 |
| Age | 57.6 ± 16.0 | 40.3 ± 15.2 | 69.5 ± 12.2 | 49.1 ± 14.6 | < 0.001 |
| BMI (kg/m2) | 24.7 ± 4.1 | 21.6 ± 3.0 | 21.2 ± 4.3 | 22.2 ± 2.8 | 0.090 |
| Alcohol consumption | 3 (60.0) | 33 (45.2) | 4 (25.0) | 47 (58.8) | 0.060 |
| Current smoking | 3 (60.0) | 36 (49.3) | 6 (37.5) | 52 (65.0) | 0.090 |
| Previous ulcer history | 0 (0.0) | 9 (12.3) | 4 (25.0) | 17 (21.2) | 0.290 |
| Comorbidity | 2 (40.0) | 11 (15.1) | 12 (75.0) | 19 (23.8) | < 0.001 |
| Anatomical findings | |||||
| Location | 0.710 | ||||
| Gastric ulcer | 1 (20.0) | 29 (39.7) | 8 (50.0) | 34 (42.5) | |
| Duodenal ulcer | 4 (80.0) | 44 (60.3) | 8 (50.0) | 46 (57.5) | |
| Size | 0.002 | ||||
| ≥ 1 cm | 3 (60.0) | 15 (23.8) | 8 (66.7) | 33 (48.5) | |
| < 1 cm | 2 (40.0) | 48 (76.2) | 4 (33.3) | 35 (51.5) | |
| Clinical course | |||||
| Hospital stay | 10.6 ± 4.2 | 10.3 ± 4.5 | 12.8 ± 4.8 | 13.2 ± 9.9 | 0.120 |
| Complication | 0 (0.0) | 1 (1.4) | 1 (6.2) | 6 (7.5) | 0.240 |
| In hospital mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
To identify the associated risk factors according to the etiology, we performed univariate and subsequent multivariate regression analysis. Older age [odds ratio (OR) = 1.09, 95% confidence interval (CI): 1.04-1.16] and comorbidity (OR = 4.11, 95%CI: 1.03-16.48) were associated with NSAID-associated PPU compared with non-H. pylori, non-NSAID associated PPU (Table 5). Older age (OR = 1.04, 95%CI: 1.02-1.07) and alcohol consumption (OR = 2.08, 95%CI: 1.05-4.13) were associated with non-H. pylori, non-NSAID associated PPU compared with solely H. pylori positive PPU (Table 6).
| Variables | Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
| Sex (men) | 0.16 (0.05-0.55) | 0.003 | ||
| Age | 1.12 (1.06-1.17) | < 0.001 | 1.09 (1.04-1.16) | 0.001 |
| BMI (kg/m2) | 0.89 (0.74-1.07) | 0.210 | ||
| Alcohol consumption | 0.23 (0.07-0.79) | 0.020 | ||
| Current smoking | 0.32 (0.11-0.98) | 0.050 | ||
| Previous ulcer history | 1.24 (0.35-4.32) | 0.740 | ||
| Comorbidity | 9.63 (2.78-33.39) | < 0.001 | 4.11 (1.03-16.48) | 0.050 |
| Variables | Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
| Sex (men) | 0.71 (0.24-2.09) | 0.530 | ||
| Age | 1.04 (1.02-1.07) | 0.001 | 1.04 (1.02-1.07) | < 0.001 |
| BMI (kg/m2) | 1.09 (0.97-1.22) | 0.160 | ||
| Alcohol consumption | 1.73 (0.91-3.28) | 0.100 | 2.08 (1.05-4.13) | 0.040 |
| Current smoking | 1.91 (1.00-3.66) | 0.050 | ||
| Previous ulcer history | 1.92 (0.80-4.63) | 0.150 | ||
| Comorbidity | 1.76 (0.77-4.00) | 0.180 |
This study evaluated the clinical characteristics of PPU and assessed the associated risk factors of PPU in terms of the common etiology. Previous western studies which evaluated the incidence and changing pattern of PPU consistently revealed that most patients with PPU were aged over 60 years without gender difference and the incidence of PPU showed increasing trend by age[3,9,11]. On the other hand, a retrospective study from Middle Eastern showed that the mean age of the patients with PPU was 35.5 years and 98.3% of patients were men[13]. Also, Korean population based study using national Health Insurance claims database reported that most patients with PPU were younger than 60 years with men predominance, and increasing incidence of PPU with age, especially in women[8], which was in agreement with our study. Due to the inherent limitation of retrospective manner of this study, selection bias could be the reason of different epidemiologic characteristics. Also, there are different pattern of risk factors (H. pylori infection rate, NSAIDs consumption) according to the geographical area of each study. However, our study clearly categorized 4 patterns of PPU according to the etiology of peptic ulcer and each of these groups showed distinguishing characteristics of PPU.
H. pylori infected group was younger than the other groups. However, this was not due to the increased prevalence of H. pylori infection in younger patients. As a result of the decreasing pattern of H. pylori infection rate and increasing pattern of NSAIDs consumption due to the elderly society in the world as well as in Korea, NSAIDs user group was relatively older than the other groups. Korean epidemiologic studies also demonstrated the increasing age of peptic ulcer occurrence in the recent decades[17-19].
In NSAIDs users, the size of the PPU was larger than other groups and hospital stay was relatively longer than H. pylori infected group, although statistically insignificant. The reason of relatively larger size PPU in the NSAIDs users could not be elucidated in this study. However, considering the direct cytotoxicity in the gastric mucosa of NSAIDs other than inhibition of prostaglandin synthesis or inflammatory responses in the development of peptic ulcer, there could be a possibility of more serious injury from NSAID in the development of PPU[20].
NSAIDs users were relatively older and the proportion of women was higher than the other groups, just like the characteristics of peptic ulcer in Korea[17]. They had also more comorbidities than other groups. However, in contrast to the higher mortality rate and silent ulcer rate without significant symptoms reported in patients with NSAID induced peptic ulcer, there was no statistically significant mortality difference in NSAID induced PPU and initial symptomatic presentation was not different from those of the others[17,21].
H. pylori infection and NSAIDs consumption are still important risk factors for the development of PPU. Several studies have investigated the risk factors for the development of PPU. Gisbert et al[12] who compared the prevalence of H. pylori infection and NSAIDs treatment between PPU and uncomplicated peptic ulcer disease identified that H. pylori prevalence were significantly lower in PPU and NSAIDs treatment was associated with PPU in a multivariate analysis. Another study from Swedish population[5] suggested that NSAIDs had little influence on peptic ulcer complications reflecting declining incidences of peptic ulcer complication despite rising NSAIDs prescription after PPI introduction. However, these studies used suboptimal number of subjects with PPU or evaluated the effect of only NSAIDs on ulcer complication without consideration for H. pylori infection. Therefore, to the best of our knowledge, this is the first study which investigated the prevalence of not only patients with H. pylori infection and NSAIDs treatment but also with non-H. pylori, non-NSAIDs and included largest number of PPU patents using clinical data from hospitals.
Previous epidemiology study of peptic ulcer disease in Korea showed that there was substantial proportion of patients (40.6%) in non-H. pylori, non-NSAIDs peptic ulcer disease among peptic ulcers developed in a single tertiary center for a year[19], which was closely correlated with our study. In our study, almost half of the subjects (46%) were not associated with H. pylori infection and NSAIDs treatment and these patients had intermediate demographic characteristics between H. pylori infected group and NSAIDs treated group in terms of age and gender. Although the reported prevalence of non-H. pylori, non-NSAIDs is variable according to the geographical area due to the difference of H. pylori infection prevalence, previous Korean studies reported 16.2% to 22.2% of prevalence[22,23]. This rate was intensified in the development of PPU in our study, reflecting decreasing prevalence of H. pylori infection in Korea.
Older age and alcohol consumption were significant risk factors of non-H. pylori, non-NSAIDs associated PPU compared with solely H. pylori positive PPU, which suggested the possible effect of aging or alcohol consumption on the development of non-H. pylori, non-NSAIDs associated PPU. There has been few studies about the association between aging or alcohol consumption and complicated peptic ulcer disease. Andersen et al[15] reported that alcohol consumption was correlated with peptic ulcer bleeding, and Charpignon et al[24] and Xia et al[25] commonly showed that aging was significant risk factor for idiopathic peptic ulcers, which might be due to the association with increased comorbidities according to aging. Also, animal study suggested that decreased defense mechanism of aging such as decreased secretion of mucus, bicarbonate or prostaglandin could be the reason of peptic ulcer in elderly patients[26]. To confirm the effect of aging or alcohol consumption on PPU, further studies with large population are needed.
This study had several limitations that should be addressed. First, retrospective study design had inherently hidden bias from imperfect recall and undetectable variables. Because most patients performed only one diagnostic method to evaluate H. pylori status and took H. pylori test after the management of PPU such as antibiotics use and surgical treatment, there was possibility of false-negative results of H. pylori test. Also, surreptitious NSAIDs/ASA use might be underestimated the proportion of NSAIDs user group. Second, we could not verify the independent risk factors of perforated peptic ulcers by comparison between the patients with PPU and patients with uncomplicated peptic ulcer disease due to rare incidence of PPU compared with uncomplicated peptic ulcers. Third, although baseline characteristics between the patients who were tested or not for the H. pylori infection were comparable except age, half of the patients with PPU were not evaluated for H. pylori infection status because of lost to follow-up after discharge, which could affect as a selection bias. Fourth, because we initially identified the patients with PPU using ICD code and then review the medical records, there was a possibility of underestimation of mortality of PPU. Although the pitfalls stated above, this study included largest population of PPU not only patients with H. pylori infection and NSAIDs treatment but also with non-H. pylori, non-NSAIDs.
In conclusion, Elderly patients with comorbidities are associated with NSAIDs-associated PPU. Non-H. pylori, non-NSAID peptic ulcer is important etiology in the development of PPU and alcohol consumption is associated risk factor.
The incidence of complications of peptic ulcer has not decreased, and limited data are available regarding the epidemiological characteristics and associated risk factors of perforated peptic ulcers.
In a retrospective review of medical records from multicenter in Korea revealed that elderly patients with comorbidities are associated with non-steroidal anti-inflammatory drugs (NSAIDs)-associated peptic ulcer perforation and non- Helicobacter pylori (H. pylori), non-NSAID peptic ulcer is important etiology in the development of peptic ulcer perforation.
Thorsen et al, Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J gastroenterol 2013; 19(3): 347-354.
In the analysis for the risk factors of non-H. pylori, non-NSAID peptic ulcer perforation, alcohol consumption is suspected to be associated risk factor.
Risky patients for the development of peptic ulcer perforation should be educated and managed separately according to the different etiology to prevent the serious complications of peptic ulcer.
Non-H. pylori, non-NSAID peptic ulcer refers to etiologic terminology diagnosed by exclusion of common causes of peptic ulcer such as H. pylori, ulcerogenic drugs, and malignancy. Although clinical course of this disease entity is more serious compared with solely H. pylori or NSAID associated peptic ulcer, there has been no clinical recommendation in the management according to the etiology of peptic ulcer.
The authors elucidated the epidemiological characteristics and associated risk factors of perforated peptic ulcer in Korea. The present study was well organized and well investigated.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Harmanci O, Naito Y, Tosetti C S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007;76:1005-1012. [PubMed] |
| 3. | Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993-2002: a population-based cohort study. Am J Gastroenterol. 2006;101:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 4. | Manuel D, Cutler A, Goldstein J, Fennerty MB, Brown K. Decreasing prevalence combined with increasing eradication of Helicobacter pylori infection in the United States has not resulted in fewer hospital admissions for peptic ulcer disease-related complications. Aliment Pharmacol Ther. 2007;25:1423-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hermansson M, Ekedahl A, Ranstam J, Zilling T. Decreasing incidence of peptic ulcer complications after the introduction of the proton pump inhibitors, a study of the Swedish population from 1974-2002. BMC Gastroenterol. 2009;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Post PN, Kuipers EJ, Meijer GA. Declining incidence of peptic ulcer but not of its complications: a nation-wide study in The Netherlands. Aliment Pharmacol Ther. 2006;23:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Wysocki A, Budzyński P, Kulawik J, Drożdż W. Changes in the localization of perforated peptic ulcer and its relation to gender and age of the patients throughout the last 45 years. World J Surg. 2011;35:811-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Bae S, Shim KN, Kim N, Kang JM, Kim DS, Kim KM, Cho YK, Jung SW. Incidence and short-term mortality from perforated peptic ulcer in Korea: a population-based study. J Epidemiol. 2012;22:508-516. [PubMed] |
| 9. | Taha AS, Angerson WJ, Prasad R, McCloskey C, Gilmour D, Morran CG. Clinical trial: the incidence and early mortality after peptic ulcer perforation, and the use of low-dose aspirin and nonsteroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;28:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 11. | Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T, Søreide K. Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 2013;19:347-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 139] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (4)] |
| 12. | Gisbert JP, Legido J, García-Sanz I, Pajares JM. Helicobacter pylori and perforated peptic ulcer prevalence of the infection and role of non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2004;36:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Torab FC, Amer M, Abu-Zidan FM, Branicki FJ. Perforated peptic ulcer: different ethnic, climatic and fasting risk factors for morbidity in Al-ain medical district, United Arab Emirates. Asian J Surg. 2009;32:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Svanes C, Søreide JA, Skarstein A, Fevang BT, Bakke P, Vollset SE, Svanes K, Søoreide O. Smoking and ulcer perforation. Gut. 1997;41:177-180. [PubMed] |
| 15. | Andersen IB, Jørgensen T, Bonnevie O, Grønbaek M, Sørensen TI. Smoking and alcohol intake as risk factors for bleeding and perforated peptic ulcers: a population-based cohort study. Epidemiology. 2000;11:434-439. [PubMed] |
| 16. | Bobrzyński A, Beben P, Budzyński A, Bielański W, Plonka M, Konturek S. Incidence of complications of peptic ulcers in patients with Helicobacter pylori (Hp) infection and/or NSAID use in the era of Hp eradication. Med Sci Monit. 2002;8:CR554-CR557. [PubMed] |
| 17. | Kwon JH, Choi MG, Lee SW, Shu XX, Bae SH, Choi JY, Yoon SK, Cho YK, Park JM, Lee IS. Trends of Gastrointestinal Diseases at a Single Institution in Korea over the Past Two Decades. Gut Liver. 2009;3:252-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kim JJ, Kim N, Lee BH, Kang JM, Seo P, Lim MK, Kwon JH, Song BJ, Lee JW, Lee SH. [Risk factors for development and recurrence of peptic ulcer disease]. Korean J Gastroenterol. 2010;56:220-228. [PubMed] |
| 19. | Kim JJ, Kim N, Park HK, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH. [Clinical characteristics of patients diagnosed as peptic ulcer disease in the third referral center in 2007]. Korean J Gastroenterol. 2012;59:338-346. [PubMed] |
| 20. | Tomisato W, Tanaka K, Katsu T, Kakuta H, Sasaki K, Tsutsumi S, Hoshino T, Aburaya M, Li D, Tsuchiya T. Membrane permeabilization by non-steroidal anti-inflammatory drugs. Biochem Biophys Res Commun. 2004;323:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med. 1996;156:1530-1536. [PubMed] |
| 22. | Jang HJ, Choi MH, Shin WG, Kim KH, Chung YW, Kim KO, Park CH, Baek IH, Baik KH, Kae SH. Has peptic ulcer disease changed during the past ten years in Korea? A prospective multi-center study. Dig Dis Sci. 2008;53:1527-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Kang JM, Seo PJ, Kim N, Lee BH, Kwon J, Lee DH, Jung HC. Analysis of direct medical care costs of peptic ulcer disease in a Korean tertiary medical center. Scand J Gastroenterol. 2012;47:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Charpignon C, Lesgourgues B, Pariente A, Nahon S, Pelaquier A, Gatineau-Sailliant G, Roucayrol AM, Courillon-Mallet A. Peptic ulcer disease: one in five is related to neither Helicobacter pylori nor aspirin/NSAID intake. Aliment Pharmacol Ther. 2013;38:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Xia HH, Wong BC, Wong KW, Wong SY, Wong WM, Lai KC, Hu WH, Chan CK, Lam SK. Clinical and endoscopic characteristics of non-Helicobacter pylori, non-NSAID duodenal ulcers: a long-term prospective study. Aliment Pharmacol Ther. 2001;15:1875-1882. [PubMed] |
| 26. | Kang JM, Kim N, Kim JH, Oh E, Lee BY, Lee BH, Shin CM, Park JH, Lee MK, Nam RH. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1147-G1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |