Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2556
Peer-review started: February 8, 2017
First decision: March 3, 2017
Revised: March 13, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: April 14, 2017
Processing time: 67 Days and 13.9 Hours
To evaluate a laparoscopic approach to gallbladder lesions including polyps, wall-thickening lesions, and suspected T1 and T2 gallbladder cancer (GBC).
We performed 50 cases of laparoscopic whole-layer cholecystectomy (LCWL) and 13 cases of laparoscopic gallbladder bed resection (LCGB) for those gallbladder lesions from April 2010 to November 2016. We analyzed the short-term and long-term results of our laparoscopic approach.
The median operation time was 108 min for LCWL and 211 min for LCGB. The median blood loss was minimal for LCWL and 28 ml for LCGB. No severe morbidity occurred in either procedure. Nine patients who underwent LCWL and 7 who underwent LCGB were postoperatively diagnosed with GBC. One of these patients had undergone LCGB for pathologically diagnosed T2 GBC after LCWL. All of the final surgical margins were negative. Three of these 15 patients underwent additional open surgery. The mean follow-up period was 26 mo, and only one patient developed recurrence.
LCWL and LCGB are safe and useful procedures that allow complete resection of highly suspected or early-stage cancer and achieve good short-term and long-term results.
Core tip: Laparoscopic cholecystectomy is commonly performed for the treatment of benign diseases. Gallbladder carcinoma (GBC) is typically managed by open surgery because of various concerns associated with potential dissemination, recurrence, and technical difficulties. However, many benign lesions are difficult to differentiate from GBC, including polyps and lesions that cause wall thickening. We use a laparoscopic approach for many types of gallbladder lesions including gallbladder carcinoma. This study demonstrated that our laparoscopic approach is safe, useful, and allows for the complete resection of highly suspected or early-stage gallbladder cancer.
- Citation: Ome Y, Hashida K, Yokota M, Nagahisa Y, Okabe M, Kawamoto K. Laparoscopic approach to suspected T1 and T2 gallbladder carcinoma. World J Gastroenterol 2017; 23(14): 2556-2565
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2556.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2556
Laparoscopic cholecystectomy (LC) is a basic approach for benign diseases such as cholecystolithiasis. However, laparoscopic surgery for gallbladder carcinoma (GBC) has not been widely employed. This is because of the highly malignant potential of GBC, higher rates of port-site recurrence (PSR) and peritoneal dissemination caused by intraoperative perforation of the gallbladder in LC than in open cholecystectomy, and the technical difficulties involved in the laparoscopic performance of standard GBC procedures[1]. Meanwhile, many benign lesions are difficult to differentiate from GBC, including polyps and wall thickening lesions such as chronic cholecystitis and adenomyomatosis[2-12]. Such lesions are sometimes diagnosed as GBC postoperatively by pathological examination. Achieving the correct preoperative diagnosis and stage of GBC is very difficult[13]. Tumor exposure as well as intraoperative gallbladder perforation can increase the risk of cancer relapse; therefore, the above-mentioned lesions, which are associated with suspected GBC, should be carefully managed. We use a laparoscopic approach depending on the type of gallbladder lesion, including suspected T1 and T2 GBC, in our institution. In this study, we evaluated the short-term and long-term outcomes of our strategy.
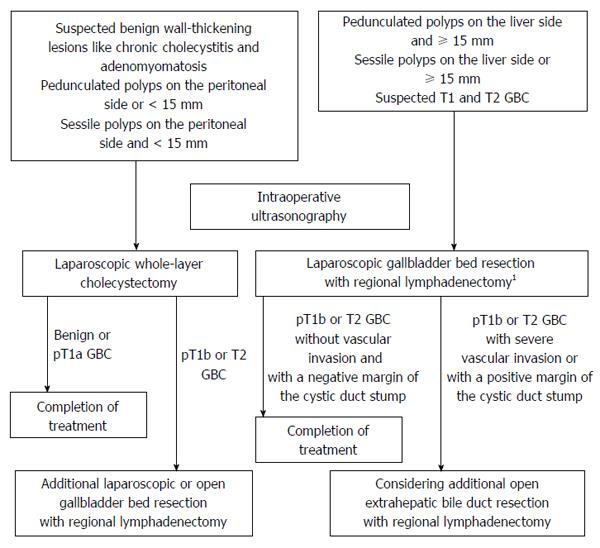
Since April 2010, we have used a laparoscopic approach depending on the type of gallbladder lesion being treated. A laparoscopic approach is indicated for polyps larger than 10 mm, growing polyps, wall-thickening lesions including chronic cholecystitis and adenomyomatosis, and suspected T1 or T2 GBC. Computed tomography, magnetic resonance imaging, and abdominal ultrasonography are routinely carried out as preoperative examinations. When GBC is highly suspected and a more exact differential diagnosis is required, clinicians may consider the use of endoscopic ultrasonography, positron emission tomography, and multidetector computed tomography. An algorithm of our laparoscopic approach to gallbladder lesions is shown in Figure 1. Intraoperative ultrasonography (IOUS) is usually performed first during the operation. We perform laparoscopic whole-layer cholecystectomy (LCWL) for suspected benign lesions rather than for GBC. In conventional cholecystectomy, the gallbladder is dissected along the inner layer of the subserosal layer[14]. On the other hand, the gallbladder is removed including the cystic plate by dissecting along the outer layer of the subserosal layer in whole-layer cholecystectomy. These benign lesions include wall-thickening lesions such as adenomyomatosis and chronic cholecystitis, pedunculated polyps on the peritoneal side or smaller than 15 mm, and sessile polyps on the peritoneal side and smaller than 15 mm. When intraoperative or postoperative pathological examination unexpectedly reveals the presence of GBC invading beyond the muscular layer, additional gallbladder bed resection and regional lymphadenectomy are considered. These additional procedures were previously performed by open surgery but can now be performed laparoscopically because of technical improvements. However, we perform laparoscopic gallbladder bed resection (LCGB) for pedunculated polyps on the liver side and larger than 15 mm, sessile polyps on the liver side or larger than 15 mm, and suspected T1 and T2 GBC. In Japan, D1 lymphadenectomy is defined as removal of the lymph nodes around the cystic duct and common bile duct, and D2 lymphadenectomy is defined as removal of the lymph nodes in the hepatoduodenal ligament, around the common hepatic artery, and around the posterosuperior region of the pancreas head. We perform LCGB with D2 lymphadenectomy for strongly suspected or definite T2 GBC, but with D1 lymphadenectomy for other lesions.
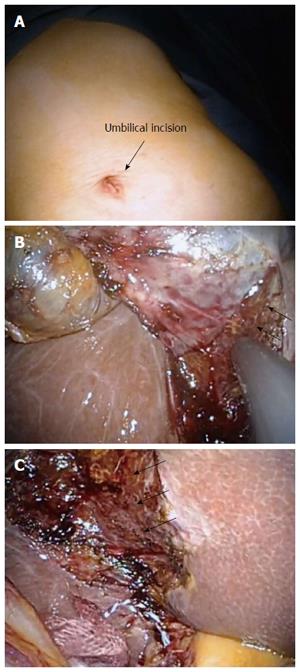
The trocars in LCWL are positioned as in conventional LC. A 12-mm trocar for the laparoscope is placed on the umbilicus, a 5-mm trocar in the epigastric region, and two 5-mm trocars in the right subcostal area. Alternatively, LCWL can be performed by a single-incision approach (Figure 2A). First, IOUS is performed to examine the lesion and investigate the extent of the tumor. The cystic duct and the cystic artery are separated and cut, and the sentinel lymph nodes (around the cystic duct) are removed to check for lymph node metastasis. When the tumor extension approaches the cystic duct, the cystic artery and duct are cut at their origin. We take special care to avoid grasping the tumor site and causing perforation during the cholecystectomy. The adipose tissues and cystic plate of Calot’s triangle are resected. The surface of the liver parenchyma, which is covered by a glossy membrane called Laennec’s capsule, is then exposed. The whole-layer gallbladder wall, which includes the cystic plate, is easily detached from the liver bed by blunt dissection without bleeding, leaving Laennec’s capsule on the liver surface (Figure 2B and C). A drain is usually unnecessary. The resected specimen is inserted into a retrieval bag and extracted though the umbilical port site.
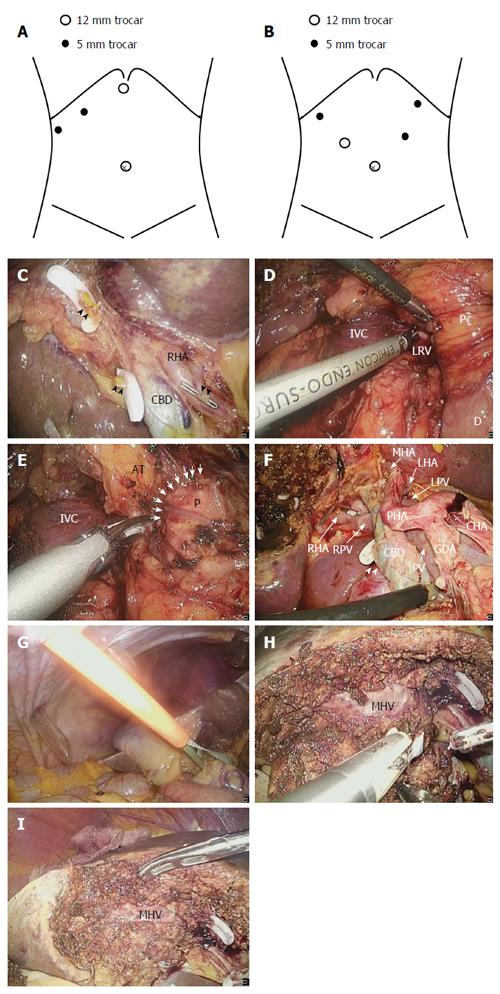
A 12-mm trocar for the laparoscope is usually placed on the umbilicus, a 12-mm trocar is placed in the epigastric region, and two 5-mm trocars are placed in the right subcostal area. When we plan to perform D2 regional lymphadenectomy, a 12-mm trocar for the laparoscope is also inserted through the umbilicus, a 12-mm trocar is inserted into the right flank region, a 5-mm trocar is inserted into the left flank region, and two 5-mm trocars are inserted into the right and left subcostal areas, respectively (Figure 3A and B). First, IOUS is performed as described above. We sometimes cut and retract the round ligament to maintain a good operative field. In D1 lymphadenectomy, the hepatoduodenal ligament above the upper margin of the pancreas is dissected using laparoscopic coagulating shears (LCS). The right hepatic artery and cystic artery are identified, and the cystic artery is cut at its origin. The cystic duct is clamped and cut at its origin, and the lymph nodes are removed from the common bile duct and right hepatic artery (Figure 3C). We do not completely remove the lymph nodes in the hepatoduodenal ligament during the first operation when the presence of GBC and the depth of its invasion are uncertain; however, we intend to resect the lymph nodes around the cystic artery, cystic duct, and common bile duct, including the sentinel lymph nodes, and to achieve a negative surgical margin. In D2 lymphadenectomy for suspected or definite T2 GBC, Kocher’s mobilization is fully performed and the inferior vena cava and left renal vein are then identified (Figure 3D). Next, the lymph nodes around the posterosuperior region of the pancreas head are removed (Figure 3E). The magnified laparoscopic view enables us to accurately identify the boundary between the pancreatic parenchyma and surrounding adipose tissues to allow for safe dissection of the lymph nodes from the pancreas. The vessels around the pancreas, such as the posterior superior pancreaticoduodenal artery and vein, are effective guides for the dissection, and the dissection proceeds along those vessels. Lymphadenectomy is continued along the superior border of the pancreas and common hepatic artery. The lymph nodes are then dissected along the portal vein, proper hepatic artery, left and right hepatic arteries, and common bile duct. We are careful to avoid excessive exposure of the common bile duct and to preserve the pericholedochal vessels, thus avoiding delayed biliary stenosis. The cystic artery and duct are clamped and cut at their origin, and regional lymphadenectomy is completed (Figure 3F). The Pringle maneuver is usually performed with an extracorporeal tourniquet during liver parenchymal transection (Figure 3G). The resection line of the liver is determined about 1 to 2 cm away from the gallbladder bed margin. The superficial layer of the resection area is dissected using LCS. The liver parenchyma is transected by the clamp crushing method using LCS or a bipolar device, and the remaining fibrous tissues and small vessels are cut using LCS (Figure 3H). A comparatively large vein should be carefully separated, clamped, and cut. After resection of the gallbladder bed has been completed, careful hemostasis is finally confirmed (Figure 3I). A drain is placed through the foramen of Winslow. The resected specimen is inserted into a retrieval bag and extracted though the enlarged umbilical port site.
From April 2010 to November 2016, 52 patients underwent LCWL and 13 underwent LCGB for gallbladder lesions suspected to be GBC. Two of the 52 patients who underwent LCWL with simultaneous resection of another cancer site were excluded from this study. The patient characteristics, perioperative findings, pathological findings, and postoperative outcomes of the patients who underwent LCWL or LCGB were retrospectively reviewed, and the short-term and long-term outcomes of our laparoscopic approach were analyzed.
The patients’ characteristics are expressed as median with range for continuous data and as number with percentage for categorical data. The RFS rates were estimated using the Kaplan-Meier method. These analyses were performed using SPSS, version 20.0 (IBM Corp., Armonk, NY, United States).
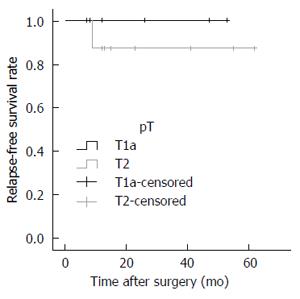
The patients’ perioperative characteristics, including their clinicopathological and surgical data, are summarized in Table 1. There were no conversions from either LCWL or LCGB to open surgery. The median operation time was 108 (61-221) min for LCWL and 211 (111-269) min for LCGB. The median blood loss was minimal (> 0-150) mL for LCWL and 28 (> 0-150) mL for LCGB. Intraoperative perforation was seen in only one patient (2.0%) who underwent LCWL. There were no instances of severe postoperative complications (Clavien-Dindo grade ≥ 3) and no mortality in either procedure. The length of the postoperative hospital stay was 3 (1-6) d for LCWL and 6 (4-11) d for LCGB. The data of the patients pathologically diagnosed with GBC are shown in Table 2. Among patients who underwent LCWL, nine were postoperatively diagnosed with GBC. The depth of invasion was pT1a in four patients, pT2 in four patients, and pT3 in one patient. Only the one patient with pT3 invasion had lymph node metastases. Two patients with pT2 GBC and one patient with pT3 GBC underwent additional resection. We performed open S4a and S5 segmentectomy with extrahepatic bile duct resection (EBR) and regional lymphadenectomy in one patient with pT2 and one patient with pT3 GBC. One patient (Case No. 9) with pT2 GBC underwent LCGB with D2 lymphadenectomy without EBR as additional resection. The other two patients with pT2 GBC did not undergo additional resection because of their old age. On the other hand, among the patients who underwent LCGB, seven were diagnosed with GBC. One of them was the above-mentioned patient (Case No. 9) who had previously undergone LCWL. The depth of invasion in patients who underwent LCGB was pT1a in two patients and pT2 in five patients. Two of the five patients with pT2 GBC had lymph node metastases. We performed LCGB with D2 lymphadenectomy for three patients with pT2 GBC, including the above-mentioned patient (Case No. 9). One patient with pT2 GBC underwent additional open lymphadenectomy with EBR. In both LCWL and LCGB, all of the final surgical margins were pathologically negative. The mean follow-up period after the operation for GBC was 26 mo, and only one patient (6.7%) who had undergone LCGB with D2 lymphadenectomy for pT2 GBC with multiple lymph node metastases (Case No. 7) developed recurrence 9 mo after the operation and died 14 mo postoperatively. One patient (Case No. 3) died 2 mo after LCWL and 1 mo after the additional resection because of another disease that was not associated with the surgical procedure or the GBC. No port site recurrence or peritoneal dissemination was found. The postoperative RFS rate in patients who underwent the laparoscopic approach is shown in Figure 4.
| LCWL(n = 50) | LCGB(n = 13) | |
| Clinical findings | ||
| Sex | ||
| Male | 30 (60) | 6 (46.2) |
| Female | 20 (40) | 7 (53.8) |
| Age, median (range, yr) | 58.5 (30-92) | 67 (50-85) |
| Surgical findings | ||
| Operation time, median (range, min) | 108 (61-221) | 211 (111-293) |
| Intraoperative blood loss, median (range, mL) | Minimal (> 0-150) | 28 (> 0-150) |
| Intraoperative perforation | 1 (2.0) | 0 (0) |
| Conversions to the open approach | 0 (0) | 0 (0) |
| Perioperative outcomes | ||
| Severe postoperative complications1 | 0 (0) | 0 (0) |
| Mortality | 0 (0) | 0 (0) |
| Postoperative hospital stay, days -median (range) | 3 (1-6) | 6 (4-11) |
| Pathological findings | ||
| Gallbladder carcinoma | 9 (18) | 7 (53.8) |
| Depth of invasion2 | ||
| pT1a | 3 | 2 |
| pT1b | 0 | 0 |
| pT2 | 5 | 53 |
| pT3 | 1 | 0 |
| pT4 | 0 | 0 |
| Lymph node metastasis | ||
| pN0 | 9 | 5 |
| pN1 | 0 | 2 |
| Surgical margin | ||
| positive | 0 | 0 |
| negative | 9 | 7 |
| Postoperative outcomes | ||
| Additional operation performed | 3 | 1 |
| Recurrence | ||
| Yes | 0 | 1 |
| No | 9 | 6 |
| Case | Sex | Age | Preoperative diagnosis | Type of operation | pT | pN | pSM | Additional surgery | Adjuvant therapy | Recurrence | RFS (mo) | Outcome |
| 1 | F | 71 | polyp | LCWL | T2 | N0 | Negative | S4a and S5 segmentectomy with extrahepatic bile duct resection | No | No | 62 | Alive |
| 2 | F | 80 | polyp | LCWL | T1a | N0 | Negative | - | No | No | 53 | Alive |
| 3 | M | 79 | chronic cholecystitic | LCWL | T3 | N1 | Negative | S4a and S5 segmentectomy with extrahepatic bile duct resection | No | No | 2 | Dead |
| 4 | F | 80 | GBC | LCGB | T2 | N0 | Negative | Extrahepatic bile duct resection | No | No | 55 | Alive |
| 5 | F | 80 | GBC | LCGB | T1a | N0 | Negative | - | No | No | 47 | Alive |
| 6 | F | 83 | polyp | LCWL | T2 | N0 | Negative | - | No | No | 41 | Alive |
| 7 | F | 61 | GBC | LCGB | T2 | N1 | Negative | - | No | Liver and bone metastases | 9 | Dead |
| 8 | F | 85 | GBC | LCGB | T1a | N0 | Negative | - | No | No | 26 | Alive |
| 9 | F | 66 | polyp | LCWL | T2 | N0 | Negative | LCGB with lymphadenectomy | No | No | 23 | Alive |
| 9 | F | 66 | definite GBC | LCGB | T2 | N0 | Negative | - | No | No | - | Alive |
| 10 | M | 83 | GBC | LCGB | T2 | N0 | Negative | - | No | No | 15 | Alive |
| 11 | M | 84 | polyp | LCWL | T2 | N0 | Negative | - | No | No | 13 | Alive |
| 12 | F | 78 | GBC | LCGB | T2 | N1 | Negative | - | No | No | 12 | Alive |
| 13 | M | 50 | polyp | LCWL | T1a | N0 | Negative | - | No | No | 12 | Alive |
| 14 | F | 86 | GBC | LCWL | T1a | N0 | Negative | - | No | No | 8 | Alive |
| 15 | F | 92 | polyp | LCWL | T1a | N0 | Negative | - | No | No | 7 | Alive |
LC is a common procedure for treatment of benign disease. Several studies on laparoscopic radical resection for GBC have also been reported[15-18]. However, the performance of laparoscopic surgery for GBC has not become widespread. The reasons for this are associated with the highly malignant potential of GBC and technical difficulties in performing regional lymphadenectomy and gallbladder bed resection.
The dissection during conventional LC on the liver side is performed along the inner layer of the subserosal layer. When the depth of invasion of the GBC extends to the subserosal layer, residual GBC may exist after conventional LC. Even a mucosal carcinoma in the Rokitansky-Aschoff sinus may result in a positive surgical margin. Moreover, LC for GBC has serious problems with respect to PSR and peritoneal dissemination because of the bile leakage caused by intraoperative perforation. The incidence of PSR after LC for GBC has been reported to range from 11% to 16%[19-22]. Ouchi et al[23] reported that gallbladder perforation occurred in 20% of patients with GBC who underwent LC. Wakai et al[19] reported that gallbladder injury occurred in 25% of patients, of whom PSR or local recurrence developed in 43% who underwent LC among 28 patients with GBC. Both research groups found that patients with gallbladder perforation had a significantly poorer prognosis than did those without perforation. Lee et al[24] described that the incidence of PSR and peritoneal dissemination in patients who underwent LC was higher than that in patients who underwent open cholecystectomy. As just described, laparoscopic surgery for GBC is associated with several difficulties. However, laparoscopic surgery has been widely employed for other various malignancies, and it provides patients with a minimally invasive treatment and early recovery from the surgery. If we can overcome the defects of laparoscopic surgery for GBC, such patients will benefit from this procedure.
GBC is sometimes encountered incidentally. Accurate preoperative diagnosis of GBC and its depth of invasion is difficult. In particular, some lesions, such as chronic cholecystitis (the prime example is xanthogranulomatous cholecystitis) and adenomyomatosis, are important differential diagnoses of GBC that may be difficult to confirm[3-7]. Several studies have reported that polypoid lesions are highly suspected to be GBC when they are larger than 10 mm, solitary, sessile, or rapidly growing[8-12]. Yeh et al[2] reported that polypoid gallbladder lesions larger than 15 mm are more strongly suspected to be malignancies. The above-mentioned lesions should be more carefully treated to avoid causing bile spillage and tumor exposure.
We now use the laparoscopic approach on the types of gallbladder lesions shown in Figure 1. The present study has shown that the risk of gallbladder perforation in both LCWL and LCGB was much lower (2.0% and 0.0%, respectively) than that in conventional LC because the dissection layer is more outside than usual. The entire subserosal layer is removed by LCWL; thus, T1 or T2 GBC can be completely resected in theory. However, suspected GBC is now indicated for LCGB so that a safety margin from the tumor is secured. Proper indications for LCWL and LCGB help to avoid tumor exposure and achieve complete resection of GBC. Intraoperative pathological examination is not always performed because of our institutional system. The specimen is macroscopically evaluated immediately after its extraction to confirm the existence of GBC and negative surgical margins. Further investigation by pathological examination is performed postoperatively. We believe that it is most important to achieve complete resection of the tumor and an accurate diagnosis, including staging, without increasing the risk of recurrence in the first-stage operation. Therefore, we take extreme care to avoid tumor exposure and bile spillage during the first-stage operation; if necessary, we plan an additional second-stage surgery.
When the diagnosis of GBC is made by intraoperative or postoperative pathological examination, an additional resection including regional lymphadenectomy should be considered depending on the depth of GBC invasion. In patients diagnosed with T1a carcinoma, an additional resection is not necessary if the surgical margin of the cystic duct stump is negative. However, an additional extended radical resection including regional lymphadenectomy is recommended in patients with T1b or more advanced GBC because vascular and perineural invasion and positive lymph node metastasis are observed at high rates[25-29]. Such an additional resection was previously performed by open surgery. However, now it can be carried out laparoscopically because of technical improvements. The laparoscopic magnified view allows for more accurate identification of the dissection plane and performance of finer procedures than in open surgery. These are great advantages of laparoscopic surgery, and can lead to reduced bleeding volume and accurate lymph node dissection.
The necessity of routine EBR is controversial[25,30-33]. There is currently no obvious evidence that recommends the routine EBR[1]. We usually perform regional lymphadenectomy without EBR when ductal involvement is not present. The advantages of EBR are facilitation of regional lymphadenectomy, removal of the possible presence of microscopic periductal involvement, and avoidance of postoperative ischemic biliary stenosis. The laparoscopic magnified view allows for sufficient lymph node dissection around the common bile duct and preservation of pericholedochal small vessels to prevent biliary ischemia. However, the lymphatic infiltration around the bile duct is a main pathway for tumor spread[25,34]. Therefore, when positive lymph node metastasis or advanced microscopic neurovascular invasion is detected, we now perform thorough regional lymphadenectomy with EBR by laparotomy, not by laparoscopic surgery.
Several studies have revealed that the long-term survival of patients with T1 or T2 GBC treated by laparoscopic radical cholecystectomy was comparable with that of patients treated by open surgery[17,18]. In the present study, our laparoscopic approach achieved good short-term outcomes and provided acceptable long-term outcomes although there was a limitation that the follow-up period was comparatively short. Until now, there have been no reports on effective laparoscopic approaches to the lesions suspected of GBC as well as definite GBC. Our laparoscopic approach to suspected T1 and T2 GBC is feasible and valid. It is necessary to accumulate the experience of the laparoscopic surgery for GBC and to compare the long-term results with open surgery.
Our laparoscopic approach to suspected T1 and T2 GBC is a safe and useful procedure that overcomes the risk of recurrence caused by conventional LC.
Laparoscopic surgery for gallbladder carcinoma (GBC) has not been widely employed yet. GBC is highly malignant, and laparoscopic surgery for GBC may increase the risk of port-site recurrence and peritoneal dissemination caused by intraoperative gallbladder injury. However, achieving the correct preoperative diagnosis and stage of GBC is very difficult. Many benign lesions including polyps and wall thickening lesions such as chronic cholecystitis and adenomyomatosis are difficult to differentiate from GBC. Such lesions are sometimes diagnosed as GBC postoperatively by pathological examination. Therefore, the above-mentioned lesions, which are associated with suspected GBC, should be carefully managed because tumor exposure as well as intraoperative gallbladder perforation can increase the risk of cancer relapse. We use a laparoscopic approach depending on the type of gallbladder lesion, including suspected T1 and T2 GBC, in this institution. In this study, the authors evaluated the usefulness of our laparoscopic approach.
Laparoscopic cholecystectomy is a common procedure for treatment of benign disease. However, laparoscopic surgery for patients with suspected GBC is not recommended now due to the risk of port-site recurrence and peritoneal dissemination caused by intraoperative gallbladder perforation. This study suggested that the laparoscopic approach is feasible and useful for the management of suspected T1 and T2 GBC.
There have been no reports on effective laparoscopic approaches to the lesions suspected of GBC. The laparoscopic approach could overcome the risk of gallbladder injury which was reported to increase by laparoscopic surgery, and good results were obtained. This new laparoscopic approach to suspected T1 and T2 GBC is a safe and useful procedure.
This study suggests that our laparoscopic approach with an appropriate algorithm is useful for suspected T1 and T2 GBC. Laparoscopic surgery can be employed even in well-selected patients with T2 GBC.
Laparoscopic whole-layer cholecystectomy (LCWL): In conventional laparoscopic cholecystectomy, the gallbladder is dissected along the inner layer of the subserosal layer, whereas in LCWL, the gallbladder is removed by dissecting along the outer layer of the subserosal layer. Laparoscopic gallbladder bed resection (LCGB): LCGB is a procedure to resect the gallbladder including 1 to 2 cm of adherent liver parenchyma laparoscopically.
This is a retrospective but interesting study aiming to evaluate laparoscopic surgery for “suspected” T1 and T2 gallbladder cancer. Wide spread of the laparoscopic approach has been hampered by the risk of tumor dissemination as well as by the difficulties in preoperative (and operative) diagnosis for malignancy and staging, as described by the authors. Their operative outcomes shown in the manuscript, with a precise algorithm for surgical management, are likely to be acceptable.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Komatsu S, Lee SC, Liu XF S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Miyazaki M, Yoshitomi H, Miyakawa S, Uesaka K, Unno M, Endo I, Ota T, Ohtsuka M, Kinoshita H, Shimada K. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Yeh CN, Jan YY, Chao TC, Chen MF. Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surg Laparosc Endosc Percutan Tech. 2001;11:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Deng YL, Cheng NS, Zhang SJ, Ma WJ, Shrestha A, Li FY, Xu FL, Zhao LS. Xanthogranulomatous cholecystitis mimicking gallbladder carcinoma: An analysis of 42 cases. World J Gastroenterol. 2015;21:12653-12659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Singh VP, Rajesh S, Bihari C, Desai SN, Pargewar SS, Arora A. Xanthogranulomatous cholecystitis: What every radiologist should know. World J Radiol. 2016;8:183-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Suzuki H, Wada S, Araki K, Kubo N, Watanabe A, Tsukagoshi M, Kuwano H. Xanthogranulomatous cholecystitis: Difficulty in differentiating from gallbladder cancer. World J Gastroenterol. 2015;21:10166-10173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Ootani T, Shirai Y, Tsukada K, Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Nishimura A, Shirai Y, Hatakeyama K. Segmental adenomyomatosis of the gallbladder predisposes to cholecystolithiasis. J Hepatobiliary Pancreat Surg. 2004;11:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Chijiiwa K, Tanaka M. Polypoid lesion of the gallbladder: indications of carcinoma and outcome after surgery for malignant polypoid lesion. Int Surg. 1994;79:106-109. [PubMed] |
| 9. | Kubota K, Bandai Y, Noie T, Ishizaki Y, Teruya M, Makuuchi M. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery. 1995;117:481-487. [PubMed] |
| 10. | Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH, Yu SJ, Kang HY, Lee JY, Park MJ. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008;2:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Cha BH, Hwang JH, Lee SH, Kim JE, Cho JY, Kim H, Kim SY. Pre-operative factors that can predict neoplastic polypoid lesions of the gallbladder. World J Gastroenterol. 2011;17:2216-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000;87:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Kokudo N, Makuuchi M, Natori T, Sakamoto Y, Yamamoto J, Seki M, Noie T, Sugawara Y, Imamura H, Asahara S. Strategies for surgical treatment of gallbladder carcinoma based on information available before resection. Arch Surg. 2003;138:741-50; discussion 750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Honda G, Hasegawa H, Umezawa A. Universal safe procedure of laparoscopic cholecystectomy standardized by exposing the inner layer of the subserosal layer (with video). J Hepatobiliary Pancreat Sci. 2016;23:E14-E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Gumbs AA, Hoffman JP. Laparoscopic radical cholecystectomy and Roux-en-Y choledochojejunostomy for gallbladder cancer. Surg Endosc. 2010;24:1766-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Gumbs AA, Hoffman JP. Laparoscopic completion radical cholecystectomy for T2 gallbladder cancer. Surg Endosc. 2010;24:3221-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Shirobe T, Maruyama S. Laparoscopic radical cholecystectomy with lymph node dissection for gallbladder carcinoma. Surg Endosc. 2015;29:2244-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Itano O, Oshima G, Minagawa T, Shinoda M, Kitago M, Abe Y, Hibi T, Yagi H, Ikoma N, Aiko S. Novel strategy for laparoscopic treatment of pT2 gallbladder carcinoma. Surg Endosc. 2015;29:3600-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Wakai T, Shirai Y, Yokoyama N, Nagakura S, Watanabe H, Hatakeyama K. Early gallbladder carcinoma does not warrant radical resection. Br J Surg. 2001;88:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 20. | Paolucci V, Schaeff B, Schneider M, Gutt C. Tumor seeding following laparoscopy: international survey. World J Surg. 1999;23:989-95; discussion 996-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 203] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Lundberg O, Kristoffersson A. Port site metastases from gallbladder cancer after laparoscopic cholecystectomy. Results of a Swedish survey and review of published reports. Eur J Surg. 1999;165:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Z’graggen K, Birrer S, Maurer CA, Wehrli H, Klaiber C, Baer HU. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery. 1998;124:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Ouchi K, Mikuni J, Kakugawa Y; Organizing Committee, The 30th Annual Congress of the Japanese Society of Biliary Surgery. Laparoscopic cholecystectomy for gallbladder carcinoma: results of a Japanese survey of 498 patients. J Hepatobiliary Pancreat Surg. 2002;9:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Lee JM, Kim BW, Kim WH, Wang HJ, Kim MW. Clinical implication of bile spillage in patients undergoing laparoscopic cholecystectomy for gallbladder cancer. Am Surg. 2011;77:697-701. [PubMed] |
| 25. | Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Kato A, Miyazaki M. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012-107; discussion 1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Wakai T, Shirai Y, Yokoyama N, Ajioka Y, Watanabe H, Hatakeyama K. Depth of subserosal invasion predicts long-term survival after resection in patients with T2 gallbladder carcinoma. Ann Surg Oncol. 2003;10:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Schauer RJ, Meyer G, Baretton G, Schildberg FW, Rau HG. Prognostic factors and long-term results after surgery for gallbladder carcinoma: a retrospective study of 127 patients. Langenbecks Arch Surg. 2001;386:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Chijiiwa K, Nakano K, Ueda J, Noshiro H, Nagai E, Yamaguchi K, Tanaka M. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg. 2001;192:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, Shirai Y, Muto T, Yoshida K. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery. 1996;120:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Choi SB, Han HJ, Kim WB, Song TJ, Suh SO, Choi SY. Surgical strategy for T2 and T3 gallbladder cancer: is extrahepatic bile duct resection always necessary? Langenbecks Arch Surg. 2013;398:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012;18:2775-2783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Pitt HA, Nakeeb A. Operative approach to gallbladder cancer. Curr Gastroenterol Rep. 2006;8:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Wiggers JK, Groot Koerkamp B, Ovadia Z, Busch OR, Gouma DJ, van Gulik TM. Patterns of recurrence after resection of gallbladder cancer without routine extrahepatic bile duct resection. HPB (Oxford). 2014;16:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Shirai Y, Yoshida K, Tsukada K, Ohtani T, Muto T. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg. 1992;79:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (1)] |