Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2519
Peer-review started: December 31, 2016
First decision: January 19, 2017
Revised: January 25, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: April 14, 2017
Processing time: 110 Days and 5.3 Hours
To identify simple and sensitive markers for postoperative complications after gastrectomy, the predictive values were compared among candidate preoperative factors.
Three-hundred and twelve patients with previously untreated clinical T2-4 gastric cancer who underwent a D2 standard gastrectomy (distal gastrectomy or total gastrectomy) were included in the analysis. Correlations between 21 parameters that can be determined by preoperative routine blood tests and clinically relevant postoperative complications (grade II or higher according to the Clavien-Dindo classification) were evaluated. The optimal cutoff values and clinical significance of the selected markers were further evaluated by subgroup analyses according to age, body mass index, operative procedure and clinical disease stage.
Sixty-six patients (21.1%) experienced grade II or higher postoperative complications. The platelet-lymphocyte ratio (PLR, total lymphocyte count/platelet count × 100) exhibited the highest area under the curve value (0.639) for predicting postoperative complications among the 21 parameters, and the optimal cutoff value was determined to be 0.71 (sensitivity = 70%, specificity = 56%). In the univariate analysis, the odds ratio of a low PLR for the occurrence of postoperative complications was 2.94 (95%CI: 1.66-5.35, P < 0.001), and a multivariate binomial logistic analysis involving other potential risk factors identified a low PLR as an independent risk factor for postoperative complications (OR = 3.32, 95%CI: 1.82-6.25, P < 0.001). In subgroups classified according to age, body mass index, operative procedure and clinical disease stage, the low PLR group exhibited an increased incidence of postoperative complications.
The preoperative PLR is a simple and useful predictor of complications after curative gastrectomy in patients with clinical T2-4 gastric cancer.
Core tip: The prediction of postoperative complications is important for providing appropriate perioperative management. In the present study, the predictive values for postoperative complications after gastrectomy with systemic lymphadenectomy for gastric cancer were compared among candidate preoperative factors to identify a simple and sensitive marker. Our results indicated that the preoperative platelet-lymphocyte ratio is a simple and useful predictor for complications after curative gastrectomy in patients with gastric cancer.
- Citation: Inaoka K, Kanda M, Uda H, Tanaka Y, Tanaka C, Kobayashi D, Takami H, Iwata N, Hayashi M, Niwa Y, Yamada S, Fujii T, Sugimoto H, Murotani K, Fujiwara M, Kodera Y. Clinical utility of the platelet-lymphocyte ratio as a predictor of postoperative complications after radical gastrectomy for clinical T2-4 gastric cancer. World J Gastroenterol 2017; 23(14): 2519-2526
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2519.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2519
Gastrectomy with systemic lymphadenectomy is the mainstay of treatment for resectable gastric cancer (GC)[1,2]. Despite advances in surgical techniques and devices, some patients who undergo the procedure experience clinically relevant postoperative complications, such as anastomotic leakage and intraabdominal abscess, leading to a protracted recovery period, delayed administration of adjuvant chemotherapy and impaired quality of life[3,4]. Risk management is an increasingly important healthcare issue. Developing a prediction tool based exclusively on preoperatively determined factors to identify patients most at risk of postoperative complications enables surgeons to provide appropriate informed consent information and perioperative management, ultimately minimizing the medical cost burden[5,6].
Increasing evidence indicates that multiple factors influence local infection control and the process of wound healing[7]. Accordingly, numerous reports on predictive factors for postoperative complications, including inflammatory, immunological, nutritional, coagulation and organ functional indicators, have been published[8-11]. However, limited information from cross-comparisons of these factors is available for patients who undergo a D2 gastrectomy for GC.
The aim of the present study was to compare the predictive values for postoperative complications after gastrectomy among candidate preoperative factors based on routinely obtained laboratory data. After the factor that demonstrated the highest predictive value was determined, the optimal cutoff value and its clinical significance were evaluated.
This study conforms to the ethical guidelines of the World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects, and written informed consent for surgery and the use of clinical data were obtained from all patients as required by the Institutional Review Board of Nagoya University.
A total of 1194 patients underwent a gastrectomy for GC at the Department of Gastroenterological Surgery, Nagoya University between 1999 and 2016. We retrospectively analyzed the data of 312 patients according to the following inclusion criteria: no preoperative treatment; clinical T2-4 (advanced) GC according to the TNM Classification of Malignant Tumours, 7th Edition[12]; D2 gastrectomy (distal gastrectomy or total gastrectomy) performed according to the Japanese Gastric Cancer Treatment Guidelines[13]; no combined resection of other organs except for the spleen and gallbladder; R0 gastrectomy performed and sufficient data for analysis. The choice of the reconstruction method was at the surgeon’s discretion. A first-generation cephem-based antibiotic was administered immediately before surgery and every 3 h during surgery. Oral intake was routinely started on postoperative day 1 if no obvious problems were found. Percutaneous drainage or the replacement of drainage tubes was performed when signs of inadequate drainage were noted by computed tomography or ultrasound scans. Clinically relevant postoperative complications were defined as those of grade II or higher according to the Clavien-Dindo classification[14].
Blood tests were routinely performed two days before surgery. Data were collected retrospectively from the medical records focusing on five categories: blood cell count, coagulation, nutrition, renal function and inflammation. The parameters investigated as candidate predictive factors for postoperative complications, which can be rapidly measured in every hospital, included the following: white blood cell count, neutrophil count, total lymphocyte count (TLC), hemoglobin concentration, platelet count, prothrombin time, activated partial thromboplastin time, fibrinogen, total protein, albumin, cholinesterase, total cholesterol, urea nitrogen, creatinine and C-reactive protein (CRP). In addition, some simple indices were employed as candidate indicators: neutrophil-lymphocyte ratio (NLR = neutrophil count/TLC), platelet-neutrophil ratio (PNR = neutrophil count/platelet count × 100), platelet-lymphocyte ratio (PLR = TLC/platelet count × 100), Onodera’s PNI (PNI = 10 × albumin g/dL + 0.005 × TLC/mm3), Glasgow prognostic score (GPS) and the modified GPS[7,8,15].
Subgroup analyses according to age, body mass index (BMI), operative procedure and clinical disease stage were performed to evaluate the correlations between the selected predictive factors and the incidence of clinically relevant postoperative complications.
A receiver operating characteristic (ROC) curve analysis was employed to calculate the area under the curve (AUC) and the sensitivity and specificity of the indicated variables to predict postoperative complications. The optimal cutoff value was determined using the Youden index[16]. The qualitative χ2 test and quantitative Mann-Whitney test were used to compare the two groups. Multivariable analysis was performed to identify independent risk factors for postoperative complications using binomial logistic analysis, and variables with a value of P < 0.05 were included as covariates in the final model. All statistical analyses were performed using JMP 10 software (SAS Institute Inc., NC, United States). A statistically significant difference was indicated by a P-value < 0.05.
The demographic and preoperative characteristics of the 312 patients are presented in Table 1. The median age was 66 years, and the male-to female ratio was 233:79. With respect to preoperative staging, 98, 71, 69, 55, 14 and 5 patients were classified as clinical TNM stages IB, IIA, IIB, IIIA, IIIB and IIIC, respectively.
| Variables | Number of patients |
| Age, median (range) | 66 (20-96) |
| Sex | |
| Male | 233 |
| Female | 79 |
| Diabetes mellitus | |
| Absent | 273 |
| Present | 39 |
| Cardiac comorbidities | |
| Absent | 217 |
| Present | 95 |
| Pulmonary comorbidities | |
| Absent | 292 |
| Present | 20 |
| Preoperative symptoms | |
| Absent | 172 |
| Present | 140 |
| Preoperative body mass index, mean ± SD | 21.9 ± 3.2 |
| Tumor location | |
| Entire | 8 |
| Upper third | 68 |
| Middle third | 120 |
| Lower third | 116 |
| Tumor size (mm) | |
| < 50 | 176 |
| ≥ 50 | 136 |
| UICC cT factor | |
| cT1 | 0 |
| cT2 | 150 |
| cT3 | 95 |
| cT4 | 67 |
| UICC cN factor | |
| cN0 | 157 |
| cN1 | 93 |
| cN2 | 53 |
| cN3 | 9 |
| UICC clinical stage | |
| IB | 98 |
| IIA | 71 |
| IIB | 69 |
| IIIA | 55 |
| IIIB | 14 |
| IIIC | 5 |
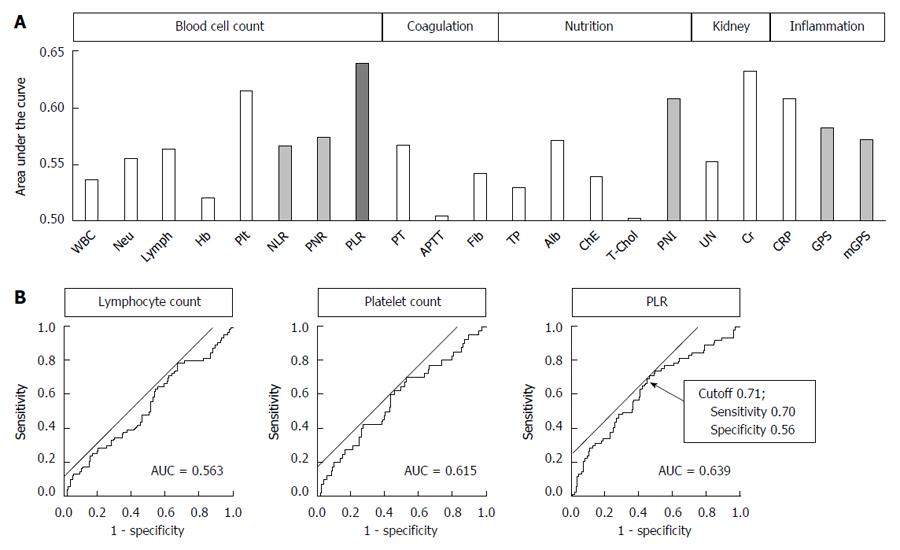
In total, 66 patients (21.1%) had grade II or higher postoperative complications. In terms of the types of complications, the cumulative numbers of patients who experienced anastomotic leakage, leakage of pancreatic fluids, intraabdominal abscess and bowel obstruction were 20 (6.4%), 12 (3.8%), 19 (6.1%) and 6 (1.9%), respectively. When the AUC value, which indicates the power to predict postoperative complications, of the 21 parameters was analyzed, the PLR demonstrated the highest AUC value (0.639) (Figure 1A). This value was greater than the values of the PLR components TLC (AUC 0.563) and platelet count (AUC 0.615) (Figure 1B). The optimal cutoff value for predicting complications using the PLR was set at 0.71 (sensitivity = 70%, specificity = 56%) (Figure 1B). The AUC values of NLR, PNR, PNI and GPS were 0.566 (cutoff = 3.06), 0.574 (cutoff = 1.36), 0.608 (cutoff = 47.0) and 0.582 (cutoff = 1), respectively (Figure 1A).
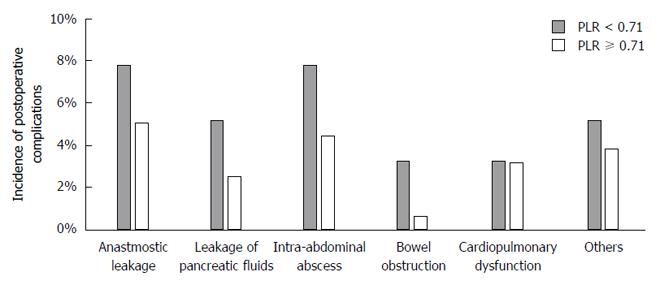
The median PLR was 0.71 (range 0.23-3.97), which is identical to the proposed cutoff. Using the cutoff indicated by the ROC curve analysis, we further evaluated the clinical significance of the PLR. Patients were categorized into two groups, the high (n = 158) and low (n = 154) PLR groups, using a PLR cutoff of 0.71. Compared to the high PLR group, patients in the low PLR group were associated with increased prevalence of pulmonary comorbidities, larger macroscopic tumor size and more frequent postoperative complications (Table 2). However, no significant differences were observed between the two groups regarding age, sex, preoperative BMI, clinical stage, type of gastrectomy, operative time, intraoperative blood loss and number of dissected lymph nodes (Table 2). In the univariate analysis, the odds ratio of a low PLR for postoperative complications was 2.94 (95%CI: 1.66-5.35, P < 0.001; Table 3). We next conducted a multivariable binomial logistic analysis involving other potential risk factors determined before surgery and found that a low PLR was an independent risk factor for postoperative complications (odds ratio 3.32, 95%CI: 1.82-6.25, P < 0.001) along with male sex and total gastrectomy (Table 3). With respect to the types of complications, the low PLR group exhibited an increased prevalence of anastomotic leakage, leakage of pancreatic fluids, intraabdominal abscess and bowel obstruction compared to the high PLR group. However, the differences were not statistically significant (Figure 2). By contrast, the incidence of cardiopulmonary dysfunction was comparable between the two groups.
| Variables | PLR < 0.71 (n = 154) | PLR ≥ 0.71 (n = 158) | P value |
| Age, median (range) | 66 (20-86) | 66 (34-96) | 0.661 |
| Sex | 0.434 | ||
| Male | 112 | 121 | |
| Female | 42 | 37 | |
| Diabetes mellitus | 0.070 | ||
| Absent | 140 | 133 | |
| Present | 14 | 25 | |
| Cardiac comorbidities | 0.785 | ||
| Absent | 106 | 111 | |
| Present | 48 | 47 | |
| Pulmonary comorbidities | 0.021 | ||
| Absent | 149 | 143 | |
| Present | 5 | 15 | |
| Preoperative symptoms | 0.838 | ||
| Absent | 84 | 88 | |
| Present | 70 | 70 | |
| Preoperative body mass index, mean ± SD | 21.6 ± 3.3 | 22.2 ± 3.1 | 0.102 |
| Tumor location | 0.673 | ||
| Entire | 3 | 5 | |
| Upper third | 32 | 36 | |
| Middle third | 57 | 63 | |
| Lower third | 62 | 54 | |
| Tumor size (mm) | 0.043 | ||
| < 50 | 78 | 98 | |
| ≥ 50 | 76 | 60 | |
| UICC cT factor | 0.502 | ||
| cT1 | 0 | 0 | |
| cT2 | 69 | 81 | |
| cT3 | 49 | 46 | |
| cT4 | 36 | 31 | |
| UICC cN factor | 0.633 | ||
| cN0 | 75 | 82 | |
| cN1 | 51 | 42 | |
| cN2 | 24 | 29 | |
| cN3 | 4 | 5 | |
| UICC clinical stage | 0.823 | ||
| IB | 45 | 53 | |
| IIA | 37 | 34 | |
| IIB | 32 | 37 | |
| IIIA | 31 | 24 | |
| IIIB | 7 | 7 | |
| IIIC | 2 | 3 | |
| Operative procedure | 0.669 | ||
| Total gastrectomy | 52 | 57 | |
| Distal gastrectomy | 102 | 101 | |
| Splenectomy | 0.191 | ||
| Absent | 128 | 122 | |
| Present | 26 | 36 | |
| Dissected lymph nodes, mean ± SD | 37.6 ± 15.8 | 38.7 ± 18.0 | 0.823 |
| Operative time (min), mean ± SD | 233 ± 56 | 238 ± 74 | 0.720 |
| Estimated blood loss (mL), median (range) | 237 (1-2350) | 245 (8-4267) | 0.491 |
| Postoperative complications | < 0.001 | ||
| Absent | 108 | 138 | |
| Present | 46 | 20 |
| Variables | Univariate | Multivariable | ||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age (≥ 65 yr) | 1.26 | 0.73-2.23 | 0.410 | |||
| Gender (male) | 4.22 | 1.88-11.3 | < 0.001 | 4.17 | 1.78-11.5 | < 0.001 |
| Preoperative symptoms | 0.95 | 0.55-1.64 | 0.864 | |||
| Diabetes mellitus | 1.14 | 0.49-2.45 | 0.755 | |||
| Cardiac comorbidities | 1.81 | 1.02-3.18 | 0.041 | 1.55 | 0.83-2.87 | 0.166 |
| Pulmonary comorbidities | 1.26 | 0.40-3.40 | 0.669 | |||
| Body mass index (≥ 22) | 1.62 | 0.94-2.81 | 0.084 | |||
| Preoperative PLR (< 0.71) | 2.94 | 1.66-5.35 | < 0.001 | 3.32 | 1.82-6.25 | < 0.001 |
| Tumor location (lower third) | 0.62 | 0.34-1.11 | 0.107 | |||
| Macroscopic tumor size (> 50 mm) | 1.19 | 0.69-2.05 | 0.534 | |||
| UICC cT (cT4) | 1.30 | 0.67-2.69 | 0.457 | |||
| UICC cN (cN1-3) | 1.75 | 1.01-3.08 | 0.045 | 1.69 | 0.93-3.08 | 0.083 |
| Operative procedure (total gastrectomy) | 2.42 | 1.39-4.23 | 0.002 | 2.43 | 1.35-4.43 | < 0.001 |
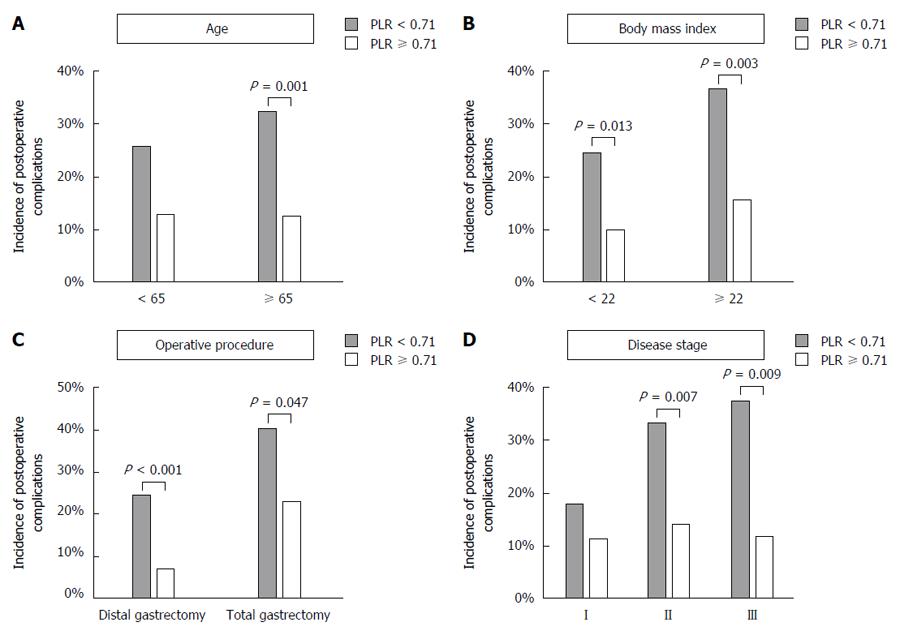
To further evaluate the clinical effect of the preoperative PLR on the postoperative course, subgroup analyses were conducted according to age (< 65 years or ≥ 65 years), preoperative BMI (< 22 or ≥ 22), operative procedure (distal or total gastrectomy) and clinical disease stage. The low PLR group exhibited an increased incidence of postoperative complications for all subgroup analyses (Figure 3).
The PLR, a combination of circulating platelet and lymphocyte counts, is a representative index of systemic inflammation and immune status[9,17]. Accordingly, a decreased PLR value indicate both systemic inflammation and compromised immune reaction. Although preoperative systemic inflammation has been reported as an underlying characteristic that predisposes the host to postoperative infection, only limited evidence is available[18]. In this study, a significant relationship between the preoperative PLR and postoperative complications was demonstrated. The PLR exhibited the highest AUC value for the incidence of postoperative complications among the candidate parameters obtained from routine preoperative blood tests. A low preoperative PLR was identified as an independent risk factor for postoperative complications in the multivariable analysis. Moreover, a low preoperative PLR was associated with an increased incidence of postoperative complications independent of age, BMI, operative procedure and disease stage, indicating that the preoperative PLR is applicable to various clinical settings.
The PLR can be determined in every hospital and is the subject of interest as a marker of systemic inflammation in various clinical circumstances. Several reports indicate that the PLR can serve as a prognostic factor for digestive malignancies[9,18]. Accumulating evidence indicates that a systemic inflammatory response could play an important role in the development and progression of cancer. Inflammation is closely related to different stages of tumor development, including initiation, invasion, metastasis, immune surveillance and responses to therapy[7,8,10,19,20]. However, the clinical impact of the preoperative PLR on postoperative short-term outcomes, particularly in patients undergoing a gastrectomy, remains unclear. Our results suggested that surgeons can provide precise informed consent information and identify high-risk patients to tailor perioperative care as an attempt to ultimately improve postoperative short-term outcomes for GC patients undergoing a gastrectomy using this prediction tool. Recently, preoperative risk calculation systems based on large-scale integrated databases, such as the Surgical Risk Preoperative Assessment System (SURPAS) and the Physiologic and Operative Severity Score for the enUmeration of Mortality and morbidity (POSSUM) model, have been widely used in clinical practice[5,21]. Our data suggest that the PLR could at least become a potential constituent of integrated scoring systems for surgical risk assessment.
The association between a decreased PLR and the development of postoperative infectious complication is likely complex and remains unclear. One plausible explanation is that a decreased PLR may reflect four disadvantages for the postoperative course including an inflammatory status, immune disorders, malnutrition and a tendency for micro-vessel thrombosis[10,18]. A decreased PLR collectively reflects a reduced TLC (compromised cell-mediated immunity and malnutrition) and an increased platelet count (inflammation and high thromphophilic diathesis). Lymphocytopenia is associated with malnutrition and cellular immunosuppression[22-24]. A pro-inflammatory status leads to compromised cell-mediated immunity and an impaired T-lymphocytic response via cytokines[25]. The ability of myeloid cells to synthesize pro-inflammatory and anti-inflammatory mediators is affected by their previous state[10,18]. Malnutrition is a major cause of delayed wound healing[11,15]. A decreased lymphocyte-mediated antibacterial immune reaction may weaken the lymphocyte-mediated antibacterial cellular immune response and contribute to increasing bacterial invasion and growth[10,18]. The platelet count is recognized as a marker of a systemic inflammatory response and potential micro-vessel thrombosis[7,9,17]. Formation of micro-vessel thrombosis inhibits wound healing via the deterioration of blood circulation in tissues. Eventually, the crosstalk of these complex factors increases the incidence of postoperative complications. In addition, our findings raised two questions. First, why is the PLR was more sensitive than the other indices, such as the PNI and GPS? We hypothesize that the PLR is indicative of four elements, including inflammatory status, immune disorders, malnutrition and a tendency for micro-vessel thrombosis, and therefore it was more closely associated with postoperative complications than other indices. Another question is whether preoperative modification of the PLR by anti-inflammatory treatment and nutritional support reduces the adverse effects on the postoperative clinical course. Further investigations are needed to answer these questions.
The current study also had several limitations that should not be ignored. This was a retrospective study of a limited number of patients from a single institute. Thus, our findings require validation by further large-scale prospective studies. To further evaluate the association of the PLR with the host inflammatory and nutritional status, information on levels of cytokines and rapid turnover proteins is required.
Taken together, the results indicate that the preoperative PLR is a simple and useful predictor of postoperative complications in patients who undergo a gastrectomy with systemic lymphadenectomy for GC. In the future, the development of an integrated risk stratification system using the PLR may facilitate physicians’ decision-making and contribute to the informed consent process before a gastrectomy.
Patients undergoing a gastrectomy with systemic lymphadenectomy for gastric cancer occasionally experience clinically relevant postoperative complications. Development of a prediction tool based on preoperatively determined factors would be helpful to identify individuals at risk of postoperative complications for whom precise informed consent and tailored perioperative management could be provided.
Platelet-lymphocyte ratio (PLR) showed the highest predictive value for postoperative complications among the 21 parameters. It was found to be an independent risk factor in a multivariate binomial logistic analysis.
The authors found that PLR served as a predictor of postoperative complications in all subgroups classified according to age, body mass index, operative procedure and clinical disease stage.
PLR is a simple and useful predictor of postoperative complications in patients who undergo a gastrectomy with systemic lymphadenectomy for gastric cancer. It may facilitate physicians’ decision-making and contribute to the informed consent process before a gastrectomy.
PLR is a platelet-lymphocyte ratio (total lymphocyte count/platelet count × 100) and is a representative index of systemic inflammation and immune status.
This is an interesting, single center retrospective study on clinical utility of preoperative predictors of postoperative complications for clinical T2-4 gastric cancer. The authors made a high quality statistical analysis and found a new statistical, easy, reproducible and overall available index, the PLR, to predict the postoperative complications after radical gastrectomy for clinical T2-4 gastric cancer.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eleftheriadis NP, Namikawa T S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 2. | Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Nomoto S. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer. 2016;19:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Kurita N, Miyata H, Gotoh M, Shimada M, Imura S, Kimura W, Tomita N, Baba H, Kitagawa Y, Sugihara K. Risk Model for Distal Gastrectomy When Treating Gastric Cancer on the Basis of Data From 33,917 Japanese Patients Collected Using a Nationwide Web-based Data Entry System. Ann Surg. 2015;262:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Kanda M, Tanaka C, Kobayashi D, Mizuno A, Tanaka Y, Takami H, Iwata N, Hayashi M, Niwa Y, Yamada S. Proposal of the Coagulation Score as a Predictor for Short-Term and Long-Term Outcomes of Patients with Resectable Gastric Cancer. Ann Surg Oncol. 2017;24:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Meguid RA, Bronsert MR, Juarez-Colunga E, Hammermeister KE, Henderson WG. Surgical Risk Preoperative Assessment System (SURPAS): III. Accurate Preoperative Prediction of 8 Adverse Outcomes Using 8 Predictor Variables. Ann Surg. 2016;264:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Tanaka Y, Kanda M, Tanaka C, Kobayashi D, Mizuno A, Iwata N, Hayashi M, Niwa Y, Takami H, Yamada S. Usefulness of preoperative estimated glomerular filtration rate to predict complications after curative gastrectomy in patients with clinical T2-4 gastric cancer. Gastric Cancer. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Neal CP, Mann CD, Garcea G, Briggs CD, Dennison AR, Berry DP. Preoperative systemic inflammation and infectious complications after resection of colorectal liver metastases. Arch Surg. 2011;146:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Moyes LH, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2009;100:1236-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, Wang T, Zhu W, Liu P. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 10. | Mohri Y, Tanaka K, Toiyama Y, Ohi M, Yasuda H, Inoue Y, Kusunoki M. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine (Baltimore). 2016;95:e3125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Kanda M, Mizuno A, Tanaka C, Kobayashi D, Fujiwara M, Iwata N, Hayashi M, Yamada S, Nakayama G, Fujii T. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine (Baltimore). 2016;95:e3781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Sobin LH, Gospodarowicz MK, C W. International Union Against Cancer, TNM Classification of Malignant Tumors. Seventh Edition. New York: Wiley-Blackwell 2009; . |
| 13. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1915] [Article Influence: 239.4] [Reference Citation Analysis (1)] |
| 14. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8632] [Article Influence: 539.5] [Reference Citation Analysis (0)] |
| 15. | Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 469] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 16. | Kanda M, Murotani K, Kobayashi D, Tanaka C, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Fujiwara M. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: A propensity score matching analysis. Surgery. 2015;158:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The Platelet-to-Lymphocyte Ratio Versus Neutrophil-to-Lymphocyte Ratio: Which is Better as a Prognostic Factor in Gastric Cancer? Ann Surg Oncol. 2015;22:4363-4370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Pang W, Lou N, Jin C, Hu C, Arvine C, Zhu G, Shen X. Combination of preoperative platelet/lymphocyte and neutrophil/lymphocyte rates and tumor-related factors to predict lymph node metastasis in patients with gastric cancer. Eur J Gastroenterol Hepatol. 2016;28:493-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 563] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 20. | Kanda M, Mizuno A, Fujii T, Shimoyama Y, Yamada S, Tanaka C, Kobayashi D, Koike M, Iwata N, Niwa Y. Tumor Infiltrative Pattern Predicts Sites of Recurrence After Curative Gastrectomy for Stages 2 and 3 Gastric Cancer. Ann Surg Oncol. 2016;23:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Chen T, Wang H, Wang H, Song Y, Li X, Wang J. POSSUM and P-POSSUM as predictors of postoperative morbidity and mortality in patients undergoing hepato-biliary-pancreatic surgery: a meta-analysis. Ann Surg Oncol. 2013;20:2501-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Watanabe M, Iwatsuki M, Iwagami S, Ishimoto T, Baba Y, Baba H. Prognostic nutritional index predicts outcomes of gastrectomy in the elderly. World J Surg. 2012;36:1632-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Song GM, Tian X, Liang H, Yi LJ, Zhou JG, Zeng Z, Shuai T, Ou YX, Zhang L, Wang Y. Role of Enteral Immunonutrition in Patients Undergoing Surgery for Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2015;94:e1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Wang F, Hou MX, Wu XL, Bao LD, Dong PD. Impact of enteral nutrition on postoperative immune function and nutritional status. Genet Mol Res. 2015;14:6065-6072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Okamura Y, Ashida R, Ito T, Sugiura T, Mori K, Uesaka K. Preoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinoma. World J Surg. 2015;39:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |